Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 16, Issue 6
Hybrid Liposomes of Nanoparticles Achieve Targeted Accumulation and Induce Apoptosis in TE-4 Esophageal Cancer Cells Without Antitumor Drugs
Junna Takai, Masaki Okumura, Koichi Goto, Yoko Matsumoto and Hideki Ichihara*Received: 12-Dec-2025, Manuscript No. JCM-25-30664; Editor assigned: 15-Dec-2025, Pre QC No. JCM-25-30664 (PQ); Reviewed: 29-Dec-2025, QC No. JCM-25-30664; Revised: 05-Jan-2026, Manuscript No. JCM-25-30664 (R); Published: 12-Jan-2026, DOI: 10.35248/2157-2518.25.16.490
Abstract
Nanoparticle Hybrid Liposomes (HL), composed of L-α-dimyristoylphosphatidylcholine (DMPC) vesicles and polyoxyethylene (25) dodecyl ether (C12 (EO)25 ) micelles, were prepared by ultrasonic irradiation in a 5% glucose solution. The HL remained stable for over one month, maintaining a hydrodynamic diameter below 100 nm. HL exhibited significant growth-inhibitory effects on esophageal cancer (TE-4) cells in the absence of antitumor drugs, as determined by the WST-8 assay. Flow cytometric analysis revealed an increase in apoptotic cells following HL treatment. Fluorescent substrate-based assays further demonstrated activation of caspase-3, -8, and -9, indicating that HL induced apoptosis via a caspase-dependent pathway in TE-4 cells. Accumulation of HL containing fluorescent probe (1-palmitoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-glycero-3-phosphocholine (NBDPC); HL/NBDPC)) was observed by fluorescence microscopy in vitro. Selective accumulation of HL containing near infrared fluorescent probe (indocyanine green (ICG); HL/ICG) within tumors in liver metastasis mouse models of TE-4 esophageal cancer was demonstrated by in vivo imaging. These results suggest that HL exert a potent growth inhibitory effects on TE-4 cells via apoptosis, selectively accumulate in TE-4 cells both in vitro and in vivo, and hold great promise as a potential cancer therapeutic drug.
Keywords
Hybrid liposome; Esophageal cancer; Apoptosis; Caspase; Cancer therapeutic agent
Abbreviations
C12(EO)25: polyoxyethylene(25)dodecyl ethers; DMPC: L-α-dimyristoylphosphatidylcholine; HL: Hybrid Liposomes; ICG: Indocyanine Green; NBDPC: 1-palmitoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino] dodecanoyl]-glycero-3-phosphocholine
Introduction
Esophageal cancer accounts for approximately 2.6% of all cancer cases in Japan; however, its 5-year relative survival rate is 41.5%, which is lower than that of many other cancers. Due to the absence of a serosal layer, the disease tends to spread easily and is associated with a high risk of developing multiple cancers [1-3]. Definitive diagnosis is typically made through endoscopic or pathological examination, followed by staging (0-IV) using imaging modalities such as ultrasonography and MRI. Consequently, multiple examinations are required, imposing both physical and economic burdens on patients. For advanced stages, surgical or palliative treatments are commonly employed, often resulting in a reduced Quality Of Life (QOL).
Liposomes are microscopic spherical vesicles primarily composed of phospholipids. Owing to their structural similarity to cell membranes and their ability to encapsulate or deliver bioactive substances, liposomes have been widely applied in both enzymology (e.g., as models for membrane-bound enzymes) and medicine (e.g., as drug delivery systems).
Hybrid Liposomes (HL) are a unique type of nanoparticle that can be readily prepared by ultrasonicating a mixture of vesicular and micellar molecules [3]. HL are free from organic solvent contamination and exhibit long-term stability. Their physicochemical properties-such as particle size, membrane fluidity, phase transition temperature, and hydrophobicity-can be precisely controlled by adjusting their composition. These characteristics have enabled HL to serve as versatile platforms for both fundamental studies and therapeutic applications [3-5].
Previous studies have demonstrated that HL containing antitumor drugs exert significant inhibitory effects on tumor growth in vitro and in vivo [6]. Remarkably, HL alone-without any anticancer drugs-have been shown strong growth-inhibitory effects on tumor cells, primarily through the induction of apoptosis [7-18]. In vivo experiments using mouse carcinoma models revealed substantial tumor volume reduction and prolonged survival following HL treatment, without observable toxicity in normal cells or adverse effects in healthy animals [12-17,19-26]. Furthermore, clinical studies approved by the bioethics committee have reported survival benefits and marked tumor regression in patients with malignant lymphoma [26]. These findings suggest that HL hold great promise as a novel chemotherapeutic approach, potentially offering an alternative to conventional anticancer drugs associated with severe side effects. However, the growth-inhibitory effect of HL on esophageal cancer cells has not yet been investigated.
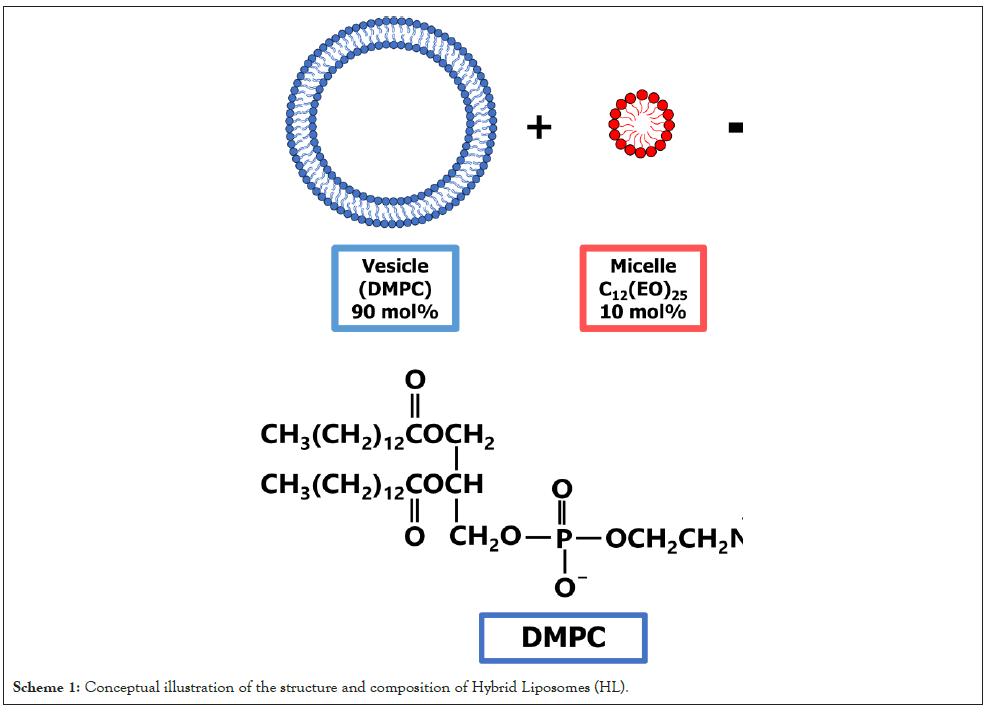
In this study, we investigated the growth-inhibitory effect of HL (Scheme 1) composed of L-α-dimyristoylphosphatidylcholine (DMPC) and micellar polyoxyethylene (25) dodecyl ether (C12(EO)25) on esophageal cancer (TE-4) cells, with a particular focus on apoptosis induction in vitro and selective accumulation in TE-4 cells both in vitro and in vivo.
Scheme 1: Conceptual illustration of the structure and composition of Hybrid Liposomes (HL).
Materials and Methods
Preparation of HL
Hybrid liposomes (HL) were prepared by sonicating a mixture of L-α-dimyristoylphosphatidylcholine (DMPC; NOF, Tokyo, Japan, 90 mol%) and polyoxyethylene (25) dodecyl ether (C12(EO)25; Nikko Chemicals, Tokyo, Japan, 10 mol%) in a 5% (w/v) glucose solution. Sonication was performed using a bath-type sonicator (Ultrasonic-cleaner ASU-10, as one, Tokyo, Japan) at 45°C and 240 W. The resulting dispersion was filtered through a 0.20 μm cellulose acetate membrane (DISMIC-13CP, ADVANTEC, Tokyo, Japan) to remove aggregates.
Dynamic light scattering measurements
The hydrodynamic diameter (dhy) of HL was measured by dynamic light scattering using a light scattering photometer (ELSZ-0, Otsuka Electronics, Osaka, Japan). A 660 nm He-Ne laser with an output power of 10 mW was used as the light source, and the scattering angle was set at 165°. The diffusion coefficient (D) was obtained from the autocorrelation function, and dhy was calculated using the Stokes-Einstein equation (Equation 1):
dhy=κT/(3πηD)……………(Equation 1)
Where κ is the Boltzmann constant, T is the absolute temperature, and η is the viscosity of the solvent.
Cell culture
Human esophageal squamous cell carcinoma TE-4 cells [27-28] (RIKEN, Ibaraki, Japan) were cultured in RPMI-1640 medium (Fujifilm Wako Pure Chemical Corporation, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS; Life Science Production, Sandy, UK). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Growth inhibition assay of HL in TE-4 cells
The growth-inhibitory effects of HL on TE-4 cells were evaluated using the WST-8 assay. Cells were seeded in 96-well plates at a density of 5.0 × 104 cells/mL and pre-incubated for 24 h. HL was added at the indicated concentrations, followed by incubation for an additional 48 h. WST-8 reagent [29-30] (Dojindo, Kumamoto, Japan) was then added, and absorbance was measured at 450 nm after 3 h. The cell viability was determined from the ratio of absorbance at the time of sample addition (Amean) to absorbance of control (AControl) (Amean / AControl).
Determination of DNA fragmentation by HL
TE-4 cells were seeded in 60 mm dishes at a density of 5.0 × 104 cells/mL and incubated for 24 h. Cells were then treated with DMPC (50-500 μM) or HL (50-500 μM, based on DMPC concentration) for 48 h. After treatment, cells were harvested, fixed, and stained with propidium iodide (PI; Molecular Probes, Eugene, OR, USA, 60 μM) in PBS containing RNase (1 mg/mL) and Triton X-100 (0.1%). Apoptotic cell populations were quantified using a flow cytometer (CytoFLEX, Beckman Coulter, CA, USA).
Measurement of caspase activation by HL
Caspase-3, -8, and -9 activation were evaluated using fluorogenic substrates and flow cytometry. To evaluate caspase activation, TE-4 cells were treated with DMPC or HL (100 and 200 μM) for 48 h. Cells were then incubated with fluorogenic substrates specific for caspase-3, -8, and -9 for 1 h in the dark. The percentage of caspase-positive cells was determined by flow cytometry (CytoFLEX, Beckman Coulter, CA, USA).
Observation of HL/NBDPC accumulation in TE-4 cells
To visualize HL uptake and accumulation, TE-4 cells were seeded in glass-bottom dishes (35 mm) and incubated for 24 h. HL containing fluorescent probe (1-palmitoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-glycero-3-phosphocholine (NBDPC; Avanti Polar Lipids, Birmingham, USA)]) (HL/NBDPC, 200 μM) was then added, and cells were incubated for 24 h. Nuclei were counterstained with Hoechst 33342 (0.352 μM) for 30 min. Fluorescence images were acquired using a confocal all-in-one microscope (BZ-X810, Keyence, Tokyo, Japan).
Observation of HL/ICG fusion and accumulation in tumors of a liver metastasis mouse model of TE-4 esophageal cancer
HL containing indocyanine green (ICG; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) of near-infrared fluorescent probe were prepared similarly, using a composition of 89 mol% DMPC, 10 mol% C12(EO)25, and 1 mol% ICG (HL/ICG). All samples were stored at 4°C to minimize ICG degradation.
The animals were handled in accordance with the guidelines for animal experimentation under Japanese law. This study was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of Sojo University. Male BALB/c-R/J mice (8-10 weeks old) with severe immunodeficiency were used in this study. These mice, kindly provided by Professor Seiji Okada (Kumamoto University, Kumamoto, Japan), were bred in our laboratory. They are characterized by a double knockout of the Jak3 and Rag2 genes, resulting in a complete absence of T cells, B cells, and NK cells [31]. Mice were maintained under controlled environmental conditions (temperature: 24 ± 2°C; humidity: 55 ± 10%; 12-hour light/dark cycle). Sterilized water and autoclaved feed were provided ad libitum.
TE-4 cells were harvested and suspended in saline at a concentration of 6.0 × 107 cells/mL. Under anesthesia, mice underwent laparotomy, and 0.05 mL of the cell suspension (3.0 × 106 cells per mouse) was injected into the spleen using a 27-gauge needle. Mice were weighed and monitored daily starting the day after transplantation.
Twenty-eight days post-transplantation, animals were stratified by body weight using a serial randomization method and assigned to experimental groups. HL/ICG (DMPC dose: 203 mg/kg) was administered via the tail vein. In vivo imaging was performed at 24 hours post-injection using an imaging system (AEQUORIA, Hamamatsu Photonics; excitation: 775/50 nm, emission: 845/55 nm). On the day following the final imaging session, mice were euthanized, and organ weights were recorded.
Statistical analysis
Data are expressed as mean ± Standard Error (S.E.) or mean ± Standard Deviation (S.D.). Statistical significance was assessed using student’s t-test, with p<0.05 considered significant.
Results and Discussion
Hydrodynamic diameter of HL
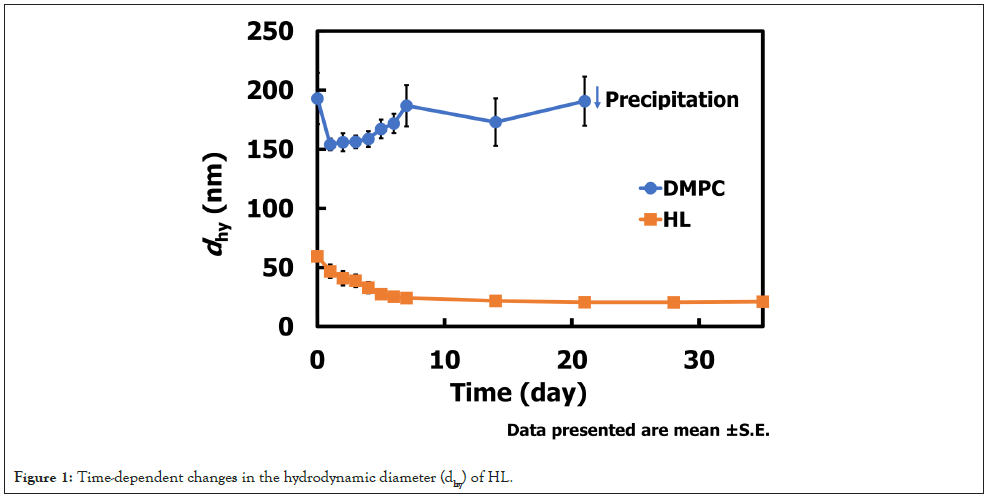
The hydrodynamic diameter (dhy) of HL (90 mol% DMPC/10 mol% C12(EO)25) was determined by dynamic light scattering to assess its stability over time. The results are shown in Figure 1. Immediately after preparation, HL exhibited a dhy of approximately 55 nm at 20 mM. Over the following seven days, the diameter gradually decreased to around 20 nm and remained stable for more than one month. In contrast, DMPC membranes displayed an initial dhy of approximately 200 nm at 20 mM, followed by marked size fluctuations and eventual precipitation. These results demonstrate that HL maintains a stable nanoscale size (<100 nm) for an extended period, a property advantageous for evading clearance by the Reticuloendothelial System (RES) [32,33] during intravenous administration.
Figure 1: Time-dependent changes in the hydrodynamic diameter (dhy) of HL.
The ability of HL to maintain a hydrodynamic diameter below 100 nm for over one month suggests a significant advantage for systemic delivery, as nanoparticles within this size range are known to exhibit prolonged circulation and enhanced tumor accumulation via the Enhanced Permeability and Retention (EPR) effect [34,35]. The observed size reduction and subsequent stabilization may reflect structural rearrangements that lead to a more thermodynamically stable configuration, potentially improving colloidal stability and reducing aggregation risk. In contrast, the instability of DMPC vesicles highlights the critical role of incorporating C12(EO)25 in conferring long-term stability. These findings imply that HL could serve as a promising nanocarrier platform for intravenous administration, minimizing RES uptake and improving drug delivery efficiency.
Growth-inhibitory effects of HL without any anticancer drugs on TE-4 cells
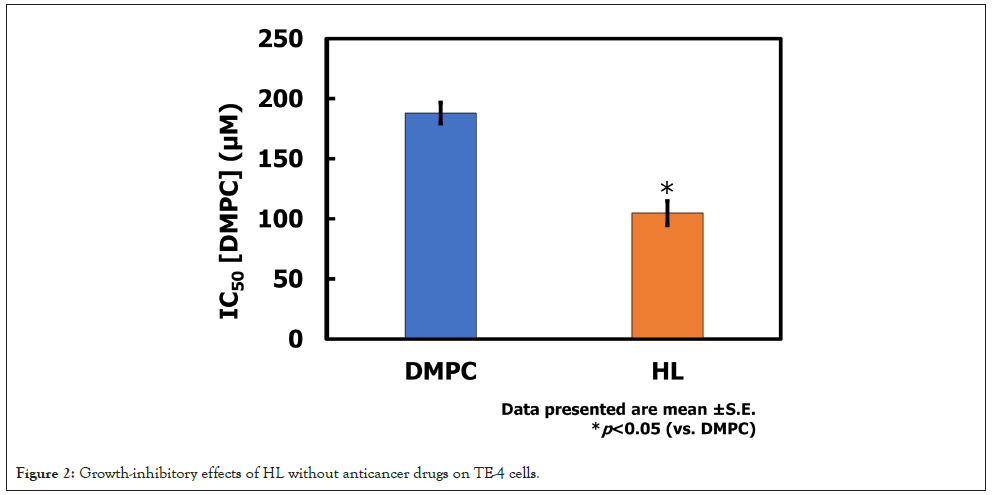
The growth-inhibitory effects of HL without anticancer drugs were evaluated against TE-4 cells using the WST assay. The results are shown in Figure 2. The IC50 value for DMPC was 188 μM, whereas that for HL was 105 μM. These findings indicate that HL exhibited a pronounced antiproliferative effect on TE-4 cells at approximately half the concentration required for DMPC.
Figure 2: Growth-inhibitory effects of HL without anticancer drugs on TE-4 cells.
The enhanced growth-inhibitory effect of HL compared to DMPC suggests that the incorporation of HL into cancer cell membranes alters their physicochemical properties, strengthens the interaction between HL and the membranes, and compromises membrane stability. PEG surfactant contained within HL may facilitate membrane fusion or destabilize cancer cell membranes, thereby inducing cytotoxic effects-such as apoptosis-even in the absence of conventional anticancer drugs. Such intrinsic activity of HL may provide a therapeutic advantage by reducing the required drug dose when used as a carrier, thereby minimizing systemic toxicity. Furthermore, the ability of HL to exert direct antiproliferative effects underscores its potential as a dual-functional nanocarrier, serving both as a drug delivery system and as an active therapeutic component.
Induction of apoptosis by HL in TE-4 cells
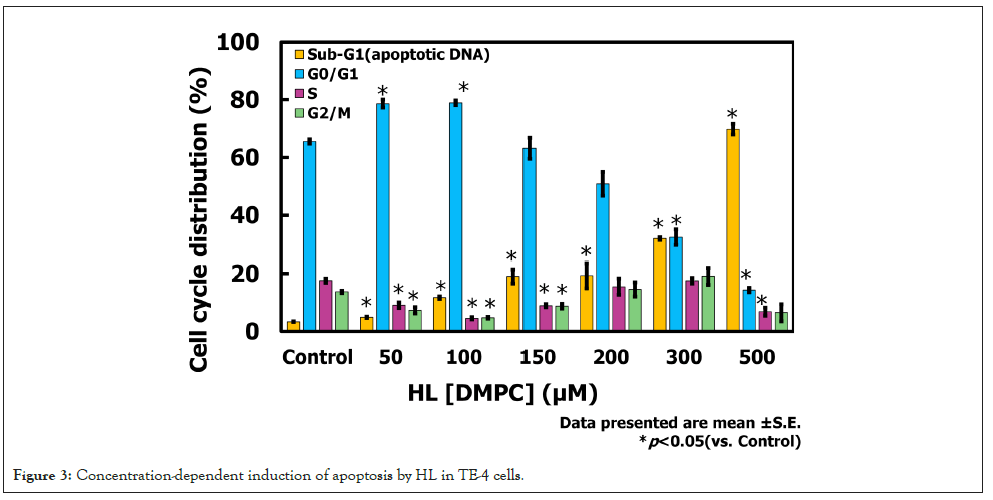
Cell death induced by HL in TE-4 cells was assessed based on the DNA fragmentation rate. The results are shown in Figure 3. In TE-4 cells treated with HL, the proportion of the DNA fragmentation (Sub-G1 (apoptotic DNA)) rate increased in a concentration-dependent manner, reaching 69.8% at 500 μM. In contrast, the DNA fragmentation rate in TE-4 cells treated with DMPC at 500 μM was 21.6%. Furthermore, a higher proportion of cells in the G0/G1 phase were observed at lower HL concentrations, suggesting the occurrence of G0/G1 arrest. These findings indicate that HL induces apoptosis in TE-4 cells in a concentration-dependent manner.
Figure 3: Concentration-dependent induction of apoptosis by HL in TE-4 cells.
The observed increase in DNA fragmentation (Sub-G1 (apoptotic DNA)) rate in a concentration-dependent manner strongly suggests that HL induces apoptosis in TE-4 cells. The markedly higher apoptotic rate compared with DMPC indicates that the incorporation of HL into the cancer cell membranes enhances the ability of HL to interact with cancer cell membranes, potentially triggering intrinsic apoptotic pathways. The additional observation of G0/G1 arrest at lower HL concentrations implies that HL may exert dual effects on cell cycle regulation-initially inhibiting cell cycle progression and subsequently inducing programmed cell death at higher concentrations. This dual mechanism could contribute to the overall antiproliferative activity of HL and highlights its potential as a multifunctional therapeutic platform, capable of both direct cytotoxicity and drug delivery.
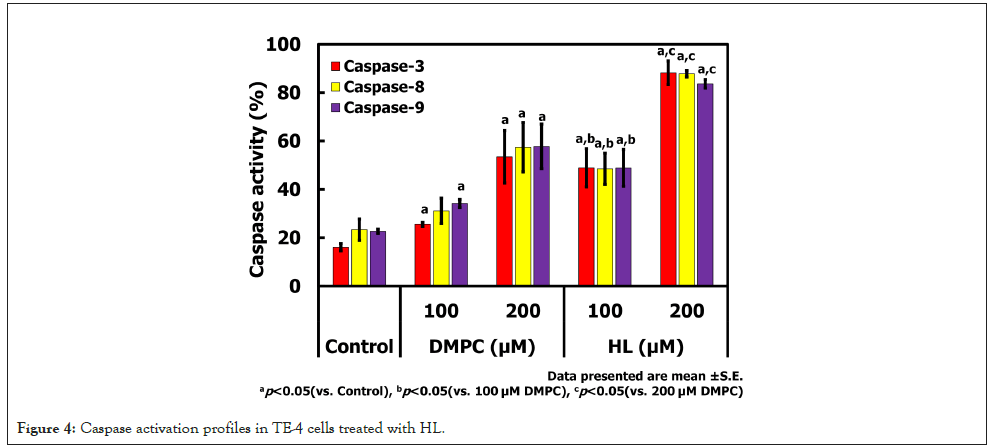
Activation of caspases by HL in TE-4 cells
Caspase-3, -8, and -9 activities in TE-4 cells treated with HL were measured using flow cytometry. The results are shown in Figure 4. Treatment with HL at 200 μM significantly activated caspases-3, -8, and -9. Significant differences compared with the control were observed for caspases-3 and -9 at 100 μM DMPC, and for caspases-3, -8, and -9 at 200 μM DMPC, as well as at 100 μM and 200 μM HL. Notably, caspase-3 activity at 100 μM HL was significantly higher than that at 100 μM DMPC, and caspase-8 and -9 activities at 200 μM HL were significantly higher than those at 200 μM DMPC. These findings indicate that HL induces apoptosis in TE-4 cells through activation of caspases-3, -8, and -9.
Figure 4: Caspase activation profiles in TE-4 cells treated with HL.
The activation of caspases-3, -8, and -9 by HL indicates that apoptosis in TE-4 cells occurs through both intrinsic and extrinsic pathways. Caspase-9 is a key initiator of the mitochondrial (intrinsic) pathway, whereas caspase-8 is associated with the death receptor (extrinsic) pathway, and caspase-3 acts as a common executioner caspase. The significant activation of these caspases at higher HL concentrations suggests that HL induces mitochondrial dysfunction and possibly engages death receptor signaling, leading to programmed cell death. The stronger activation observed with HL compared to DMPC further supports the hypothesis that the PEG surfactant contained within HL enhances its interaction with cancer cell membranes, triggering apoptotic signaling cascades. These findings highlight HL’s potential as a therapeutic platform capable of inducing apoptosis without conventional chemotherapeutic agents, which may contribute to reducing systemic toxicity in clinical applications.
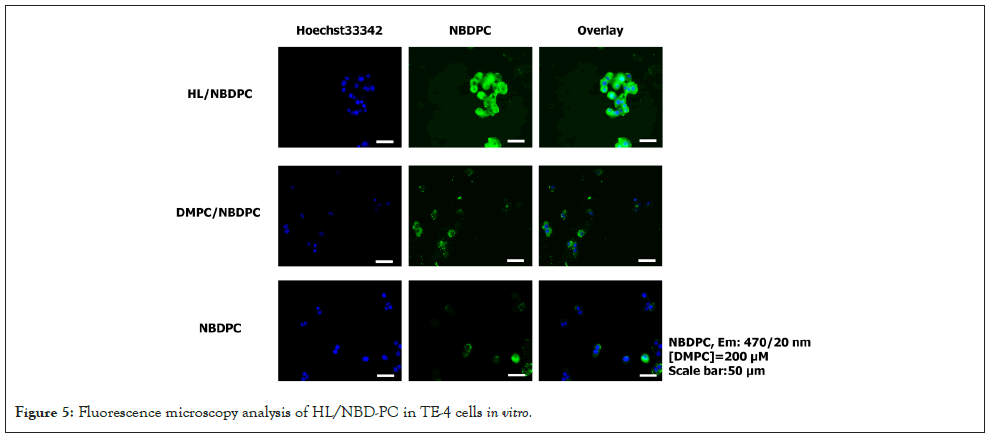
Fusion and accumulation of HL/NBDPC in TE-4 cells
The fusion and accumulation of HL/NBDPC in TE-4 cells were examined using a fluorescence microscope, and the results are presented in Figure 5. HL/NBDPC was found to fuse with and accumulate in TE-4 cells. Similarly, DMPC/NBDPC also fused with and accumulated in TE-4 cells; however, HL/NBDPC exhibited a greater degree of intracellular accumulation compared to DMPC/NBDPC.
Figure 5: Fluorescence microscopy analysis of HL/NBD-PC in TE-4 cells in vitro.
The present study demonstrated that HL/NBDPC exhibited a higher degree of fusion and intracellular accumulation in TE-4 esophageal cancer cells compared to DMPC/NBDPC. This enhanced accumulation suggests that the structural characteristics of HL may facilitate stronger interactions with the cellular membrane, thereby promoting more efficient incorporation into the cancer cell membranes. Increase in fluorescence intensity further indicates a progressive uptake process, which may be influenced by membrane fluidity and lipid composition of TE-4 cells. These findings imply that HL-based lipid assemblies could serve as a promising platform for targeted drug delivery in esophageal cancer therapy, owing to their superior cancer cellular affinity and retention. Future studies should investigate the underlying mechanisms of HL-mediated fusion, including the role of membrane microdomains and endocytic pathways, to optimize their therapeutic potential.
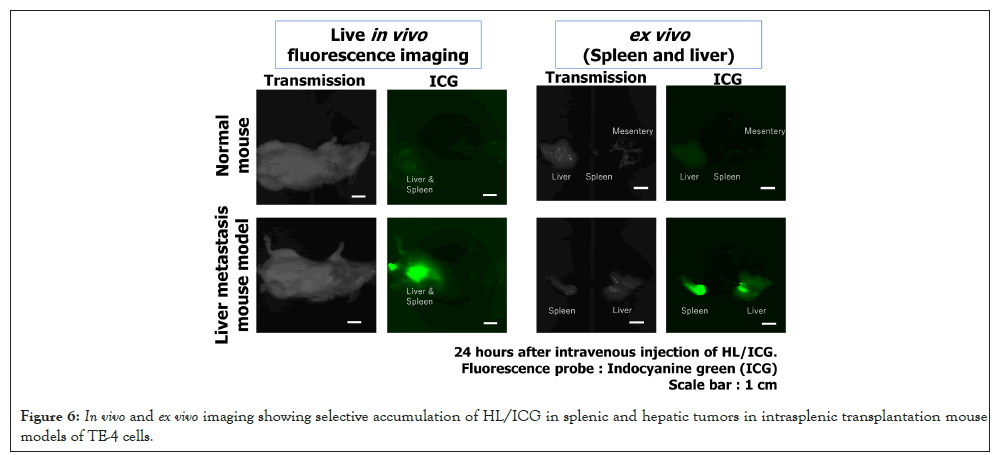
Selective arrival and accumulation of HL in tumors of a liver metastasis mouse model transsplenically transplanted with TE-4 cells
Using an in vivo imaging system, we evaluated the selective accumulation of HL in tumors of liver metastasis mouse models established by intrasplenic transplantation of TE-4 cells. The results are shown in Figure 6.
Figure 6: In vivo and ex vivo imaging showing selective accumulation of HL/ICG in splenic and hepatic tumors in intrasplenic transplantation mouse models of TE-4 cells.
HL/ICG including near-infrared fluorescent probe ICG was administered via the tail vein on day 28 after cell transplantation. 24 hours later, live in vivo imaging was performed on live animals from outside the body, revealing strong fluorescence localized around the liver region containing tumors. Following necropsy, ex vivo imaging of the excised spleen and liver confirmed HL/ICG fluorescence in both organs. These findings indicate that intravenously administered HL/ICG preferentially accumulates in splenic and hepatic tumors in this intrasplenic transplantation model.
The preferential accumulation of HL/ICG in splenic and hepatic tumors observed in this intrasplenic transplantation model suggests that HL possesses favorable biodistribution properties for targeting metastatic lesions. The strong fluorescence detected by in vivo imaging from outside the body indicates that HL remains stable in circulation and can reach tumor sites efficiently. Subsequent ex vivo imaging of the excised spleen and liver confirmed selective retention of HL/ICG in tumor-bearing organs, which may be attributed to the Enhanced Permeability and Retention (EPR) effect and the nanoscale size of HL (<100 nm), allowing it to evade rapid clearance by the reticuloendothelial system. These findings highlight the potential of HL as a nanocarrier for systemic delivery, particularly for treating metastatic tumors in the liver and spleen. Furthermore, the ability of HL to accumulate selectively in tumor tissues without additional targeting ligands underscores its promise for clinical applications in cancer therapy [36].
Conclusion
Hybrid Liposomes (HL), composed of the phospholipid DMPC and the PEG surfactant C12(EO)25, demonstrated significant antitumor activity against human esophageal cancer TE-4 cells. The following interesting findings were obtained:
1) HL maintained a stable membrane diameter of approximately 20 nm for over one month.
2) In growth inhibition assays, HL exhibited a potent inhibitory effect on TE-4 cell proliferation even at low concentrations.
3) HL induced apoptosis in TE-4 cells in a concentrationdependent manner via activation of caspases -3, -8, and -9.
4) HL/NBDPC were demonstrated to fuse with and accumulate in TE-4 cells under in vitro conditions.
5) Selective arrival and subsequent accumulation of HL/ ICG within tumors were observed in vivo in a mouse model established by intrasplenic transplantation of TE-4 cells.
These results demonstrate for the first time that HL composed of DMPC and C12(EO)25 exerts a remarkable antiproliferative effect on TE-4 cells. Furthermore, HL was found to selectively accumulate in TE-4 cells both in vitro and in vivo. Future studies will focus on evaluating the therapeutic efficacy of HL in TE-4-transplanted mice. Moreover, by employing not only the TE-4 cell line but also the TE-1 cell line (derived from primary esophageal tumors with epithelial characteristics) and the TE-8 cell line (moderately differentiated with mesenchymal features and higher malignancy than TE-4), it may be possible to tailor drug therapy according to the progression stage of each patient. This approach could contribute to improving the Quality Of Life (QOL) of esophageal cancer patients.
Acknowlegement
We thank Tomoaki Sakoda, Hinako Mori, Takato Onitsuka for technical assistance. We would like to thank Editage (www.editage. jp) for English language editing.
References
- Foundation for Promotion of Cancer Research (FPCR). Cancer statistics in Japan 2024. 2025.
- National cancer center Japan. Esophagus cancer statistics. 2025.
- Watanabe M. Recent topics and perspectives on esophageal cancer in Japan. JMA J. 2018;1(1):30-9.
[Crossref] [Google Scholar] [PubMed]
- Ueoka R, Moss RA, Swarup S, Matsumoto Y, Strauss G, Murakami Y. Extraordinary micellar enantioselectivity coupled to altered aggregate structure. J Am Chem Soc. 1985;107:2185-2186.
- Ueoka R, Matsumoto Y, Moss RA, Swarup S, Sugii A, Harada K, et al. Membrane matrix for the hydrolysis of amino acid esters with marked enantioselectivity. J Am Chem Soc. 1988;110:1588-1595.
- Ueoka R, Yamada E, Yamashita O, Matsumoto Y, Kato Y. Composition- and temperature-sensitive membranes composed of phosphatidylcholine and Triton X-100 for the remarkably enhanced stereoselective-hydrolysis. Tetrahedron Lett. 1991;32:6597-6600.
- Kitamura I, Kochi M, Matsumoto Y, Ueoka R, Kuratsu J, Ushio Y. Intrathecal chemotherapy with 1,3-bis(2-chloroethyl)-1-nitrosourea encapsulated into hybrid liposomes for meningeal gliomatosis: An experimental study. Cancer Res. 1996;56:3986-3992.
[Google Scholar] [PubMed]
- Matsumoto Y, Iwamoto Y, Matsushita T, Ueoka R. Novel mechanism of hybrid liposomes-induced apoptosis in human tumor cells. Int J Cancer. 2005;115(3):377-382.
[Crossref] [Google Scholar] [PubMed]
- Nagami H, Nakano K, Ichihara H, Matsumoto Y, Ueoka R. Two methylene groups in phospholipids distinguish between apoptosis and necrosis for tumor cells. Bioorg Med Chem Lett. 2006;16(4):782-785.
[Crossref] [Google Scholar] [PubMed]
- Kuwabara K, Ichihara H, Matsumoto Y. Inhibitory effects and anti-invasive activities of trehalose liposomes on the proliferation of lung carcinoma cells. J Carcinog Mutagen. 2017;8(1):1-5.
- Ichihara H, Komizu Y, Ueoka R, Matsumoto Y. Inhibitory effects of hybrid liposomes on the growth of non-small cell lung carcinoma cells and anti-invasive activity by ceramide generation without any drugs. J Carcinog Mutagen. 2015;6(4):1-5.
- Ichihara H, Motomura M, Matsumoto Y. Negatively charged cell membranes-targeted highly selective chemotherapy with cationic hybrid liposomes against colorectal cancer in vitro and in vivo. J Carcinog Mutagen. 2016;7(3):1-8.
- Kuwabara K, Ichihara H, Matsumoto Y. Inhibitory effect of hybrid liposomes on the growth of NP2 glioma cell. J Carcinog Mutagen. 2020;11(1):1-5.
- Kuwabara K, Ichihara H, Matsumoto Y. Trehalose liposomes inhibit the growth of glioblastoma cell in vitro and in vivo. J Carcinog Mutagen. 2021;12(4):1-5.
- Ichihara H, Matsumoto Y, Inhibitory effects of cationic hybrid liposomes against metastasis of bile duct cancer in vitro and in vivo. J Carcinog Mutagen. 2024;15(2):1-6.
- Ichihara H, Takaki H, Motomura M, Okumura M, Goto K, Matsumoto Y. Efficacy of cationic hybrid liposomes composed of L-α- dimyristoylphosphatidylcholine in targeting negatively charged cholangiocarcinoma cell membranes without any drug in vitro and in vivo. J Carcinog Mutagen. 2024;15(6):1-5.
- Ichihara H, Miyamoto S, Takai J, Okumura M, Goto K, Matsumoto Y, Therapeutic effects of hybrid liposomes against triple negative mouse breast cancer in vitro and in vivo. J Carcinog Mutagen. 2025;16(2):1-5.
- Okumura M, Otsuka H, Takai J, Goto K, Matsumoto Y, Ichihara H. Growth suppression of triple-negative breast cancer cells by the photodynamic therapy effect of hybrid liposomes containing indocyanine green. J Carcinog Mutagen. 2025;16(2):1-5.
- Ichihara H, Yamasaki S, Hino M, Ueoka R, Matsumoto Y. Hybrid liposomes inhibit the growth and angiogenesis in human breast cancer model. J Carcinog Mutagen. 2015;6(1):1-7.
- Kuwabara K, Ichihara H, Matsumoto Y. Novel therapy with hybrid liposomes for orthotopic graft mouse. J Carcinog Mutagen. 2021;12(2):1-5.
- Ichihara H, Okumura M, Matsumoto Y, Photodynamic therapy effects of hybrid liposomes including indocyanine green against a xenograft mouse model mice of colorectal cancer in vivo. J Carcinog Mutagen. 2022;13(5):1-5.
- Ichihara H, Funamoto K, Matsushita T, Matsumoto Y, Ueoka R. Histological bioanalysis for therapeutic effects of hybrid liposomes on the hepatic metastasis of colon carcinoma in vivo. Int J Pharm. 2010;394(1-2):174-178.
[Crossref] [Google Scholar] [PubMed]
- Ichihara H, Hino M, Umebayashi M, Matsumoto Y, Ueoka R. Intravenous injection of hybrid liposomes suppresses the liver metastases in xenograft mouse models of colorectal cancer in vivo. Eur J Med Chem. 2012;57:143-148.
[Crossref] [Google Scholar] [PubMed]
- Kitajima H, Komizu Y, Ichihara H, Goto K, Ueoka R. Hybrid liposomes inhibit tumor growth and lung metastasis of murine osteosarcoma cells. Cancer Med. 2013;2(3):267-276.
[Crossref] [Google Scholar] [PubMed]
- Okumura M, Ichihara H, Matsumoto Y. Hybrid Liposomes showing enhanced accumulation in tumors as theranostic agents in the orthotopic graft model mouse of colorectal cancer. Drug Deliv. 2018;25(1):1192-1199.
[Crossref] [Google Scholar] [PubMed]
- Ichihara H, Nagami H, Kiyokawa T, Matsumoto Y, Ueoka R. Chemotherapy using hybrid liposomes along with induction of apoptosis. Anticancer Res. 2008;28(2B):1187-1195.
[Google Scholar] [PubMed]
- Bao XH, Takaoka M, Hao HF, Fukazawa T, Yamatsuji T, Sakurama K, et al. Antiproliferative effect of the HSP90 inhibitor NVP-AUY922 is determined by the expression of PTEN in esophageal cancer. Oncol Rep. 2013;29(1):45-50.
[Crossref] [Google Scholar] [PubMed]
- Wuu KD, Wang-Wuu S. Karyotypic analysis of seven established human esophageal carcinoma cell lines. J Formos Med Assoc. 1994;93(1):5-10.
[Google Scholar] [PubMed]
- Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44(7):1299-1305.
[Crossref] [Google Scholar] [PubMed]
- Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, et al. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1999;36:47-50.
- Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, et al. Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol. 2011;2011:539748.
[Crossref] [Google Scholar] [PubMed]
- Gregoriadis G, Swain CP, Wills EJ, Tavill AS. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet. 1974;1(7870):1313-1316.
[Crossref] [Google Scholar] [PubMed]
- Proffitt RT, Williams LE, Presant CA, Tin GW, Uliana JA, Gramble RC, et al. Liposomal blockade of the reticuloendothelial system: Improved tumor imaging with small unilamellar vesicles. Science. 1983;220(4596):502-505.
[Crossref] [Google Scholar] [PubMed]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12):6387-6392.
[Google Scholar] [PubMed]
- Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J Control Release. 2012;164(2):138-144.
[Crossref] [Google Scholar] [PubMed]
- Tsuchihashi K, Hirata Y, Yamasaki J, Suina K, Tanoue K, Yae T, et al. Presence of spontaneous epithelial-mesenchymal plasticity in esophageal cancer. Biochem Biophys Rep. 2022;30:101246.
[Crossref] [Google Scholar] [PubMed]
Citation: Takai J, Okumura M, Matsumoto Y, Ichihara H. (2025). Hybrid Liposomes of Nanoparticles Achieve Targeted Accumulation and Induce Apoptosis in TE-4 Esophageal Cancer Cells without Antitumor Drugs. J Carcinog Mutagen. 16:500.
Copyright: © 2025 Takai J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.