Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 16, Issue 2
Therapeutic Effects of Hybrid Liposomes against Triple Negative Mouse Breast Cancer in vitro and in vivo
Hideaki Ichihara*, Suguru Miyamoto, Junna Takai, Masaki Okumura, Koichi Goto and Yoko MatsumotoReceived: 17-Mar-2025, Manuscript No. JCM-25-28692; Editor assigned: 21-Mar-2025, Pre QC No. JCM-25-28692 (PQ); Reviewed: 05-Apr-2025, QC No. JCM-25-28692; Revised: 12-Apr-2025, Manuscript No. JCM-25-28692 (R); Published: 19-Apr-2025, DOI: 10.35248/2157-2518.25.16.470
Abstract
Hybrid Liposomes (HL), composed of L-α-dimyristoylphosphatidylcholine (DMPC) and polyoxyethylene (25) dodecyl ether (C12(EO)25), demonstrated significant therapeutic potential against breast cancer cells. HL formed a clear and stable solution with a hydrodynamic diameter of approximately 100nm, which remained unchanged for at least four weeks, suggesting high physicochemical stability suitable for clinical application. In vitro, HL exhibited a marked antiproliferative effect on 4T1-Luc murine breast cancer cells. Furthermore, flow cytometric analysis revealed a significant increase in apoptotic cell populations following HL treatment, indicating that HL effectively induces apoptosis in breast cancer cells. In vivo, administration of HL to 4T1-Luc tumor-bearing mice resulted in a significant reduction in tumor volume and tumor weight, without any observable adverse effects or changes in body weight throughout the treatment period. These findings collectively suggest that HL exert potent growth-inhibitory effects on breast cancer both in vitro and in vivo, and may serve as a promising drug delivery system with minimal toxicity for the treatment of breast cancer.
Abbrevations
C12(EO)25: Polyoxyethylene(25) Dodecyl Ether; DMPC: L-α-Dimyristoylphosphatidylcholine; HL: hybrid liposomes composed of DMPC, and C12(EO)25.
Keywords
Hybrid liposome; Chemotherapy; Breast cancer; Apoptosis
Introduction
Breast cancer is a malignant tumor that begins in the epithelial cells of the ducts or lobules of the mammary gland. Ductal carcinoma that begins in the ducts accounts for about 80% of all breast cancers. Breast cancer is likely to invade lymphatics and blood vessels, so it is likely to develop micrometastases from an early stage and spread to lymph nodes around the breast, brain, lung, liver and bone. Risk factors for breast cancer include lifestyle habits such as drinking and smoking, long-term estrogen exposure, and family and medical history [1-3].
Treatments for breast cancer include surgery, radiation therapy, and drug therapy. Surgery includes breast-conserving surgery, which is a partial mastectomy, and mastectomy, which removes all of the patient's breast. However, surgery has the disadvantage that it greatly affects the cosmetic aspect and that postoperative movement disorder occurs frequently. Radiation therapy irradiates cancer with high-energy x-rays to kill cancer cells and reduce their size. Side effects may include redness and itching of the irradiated skin and peeling of the skin surface. There are various approaches to medical therapy that aim to cure, prolong life, and relieve symptoms, including chemotherapy, hormonal therapy, and targeted therapies. The effects and side effects differ depending on the drug used, and for example, there are side effects that affect the life after treatment, such as hair loss symptoms due to damage to hair-producing cells and numbness of hands and feet.
Hybrid Liposomes (HL) [4-5] are prepared by ultrasonic irradiation of vesicle molecules and micelle molecules in a solvent solution. Unlike the conventional liposome preparation method, the liposome can be prepared without using an organic solvent, so that no residue of the organic solvent occurs. It is a medical material excellent in biocompatibility, in which the membrane diameter and membrane fluidity can be adjusted by selecting the material, composition ratio and ionic strength.
This HL can be used as a carrier for Drug Delivery System (DDS) [6], and the therapeutic effect of HL alone on cancer has been clarified [7-19]. In addition, correlation has been obtained between the anticancer effect of HL and cell membrane fluidity [20,21], and it has been clarified that HL specifically accumulates in cancer cells by recognizing the physical characteristics of cells, and that HL specifically fuses and accumulates only in cancer cells without affecting normal cells [22], and induces apoptosis [23-24]. Furthermore, in clinical studies after approval by the Bioethics Committee, the survival benefit in patients with malignant lymphoma and the remarkable reduction of solid lymphoma were confirmed [25], and it is expected to be a chemotherapeutic agent that replaces conventional anticancer drugs with large side effects.
In this study, we focused on the specific accumulation of HL in cancer cells and investigated the therapeutic effects of HL on mouse breast cancer (4T1-Luc [26]) cells in vitro and in vivo.
Materials and Methods
Preparation of HL
HL were prepared by sonication of a mixture containing 90 mol% L-α-dimyristoylphosphatidylcholine (DMPC; NOF, Tokyo, Japan) and 10 mol% polyoxyethylene(25) dodecyl ether (C12(EO)25; Nikko Chemicals, Tokyo, Japan) in a 5% glucose solution using a bath-type sonicator (VS-N300; VELVO-CLEAR, Tokyo, Japan) at 45°C and 300W and then filtering the mixture through a 0.20 μm cellulose acetate filter (Advantec, Tokyo, Japan).
Dynamic light scattering measurements
The diameter of the HL was measured with a light scattering spectrometer (ELSZ-0; Otsuka Electronics, Osaka, Japan) using a He-Ne laser (633 nm) at a 90° scattering angle. The hydrodynamic diameter (dhy) was calculated using the Stokes-Einstein formula (Equation 1), where κ is the Boltzmann constant, T is the absolute temperature, η is the viscosity, and D is the diffusion coefficient:
dhy = κT / 3πηD (Equation 1)
Cell culture: The cells used were 4T1-Luc cells (RIKEN, Ibaraki, Japan), a mouse mammary adenocarcinoma cell line expressing luciferase [26]. Complete medium consisting of RPMI1640 (Fujifilm Wako Pure Chemical Corporation, Tokyo, Japan) +10% Fetal Bovine Serum (FBS: Life Science Production, Sandy, UK) was used as the culture medium and incubated at 37°C, 5% CO2, and 95% humidity.
Cell proliferation inhibition assay: Cell growth inhibition was evaluated by the WST-8 method. 4T1-Luc cells were seeded (5.0 × 104 cells/mL) in 96-well plates, and HL was added after 24 hours of pre-culture. WST-8 (2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfophenyl]-2H-tetrazolium, monosodium salt, DOJINDO Laboratories, Kumamoto, Japan) solution was added 48 hours after addition, and absorbance was measured 3 hours later. The viability was determined from the ratio of absorbance at the time of sample addition (Amean) to absorbance of control (AControl) (Amean/AControl).
Analysis of apoptosis by flow cytometry: 4T1-Luc cells were seeded at a density of 5.0 × 104 cells/mL in 60-mm dishes and incubated in a humidified atmosphere of 5% CO2 at 37°C for 24 h. DMPC (100–500 μM), and HL (100–500 μM, on the basis of the DMPC concentration) were added to each dish and the dishes were further incubated for 48 h. The cells were harvested by centrifugation and then resuspended in PBS(-) containing 1 mg/mL RNase, 0.1% Triton X-100, and 40 μg/mL propidium iodide (PI, Molecular Probes, Eugene, OR, USA) in a dark room. The percentage of apoptotic cells was analyzed using a flow cytometer (Epics XL system II, Beckman Coulter, CA, USA).
Assessment of therapeutic effects in vivo: The mice were handled in accordance with the guidelines for animal experimentation set out in Japanese law. The animal studies were approved by the Committee on Animal Research of Sojo University. BALB/c-R/J mice were kindly provided by Prof. Okada (Kumamoto University, Japan) [27]. The mice were bred by 100% freshly ventilatory condition every 1 h for 14 times in room temperature at 25±1°C, humidity 50±10% and illumination cycle for every 12 h.
Breast cancer (4T1-Luc) cells were suspended in saline and transplanted subcutaneously (1.0 × 106 cells/body/0.05 mL) into combined immunodeficient mice (BALB/c-R/J) to produce a subcutaneous transplantation model mouse. The group was divided by the stratification random method based on the body weight of the mouse by the stratified randomization method and the transplantation was carried out. From the next day, HL (DMPC dose: 136 mg/kg) was administered daily for 2 weeks (14 times in total). Necropsy was performed on the day after the end of administration. During the treatment period, tumor volume was calculated based on the following equation (2). Tumors were removed from anaesthetized mice after completion of administration with HL and weighed.
Tumor volume (mm3) = 1/2 × major diameter (mm) × minor diameter (mm) × minor diameter (mm)
Statistical analysis: Results are presented as mean ± Standard Error (S.E.) and mean ± Standard Deviation (S.D.). Data were statistically analysed using Student’s t-test and the log-rank test. A p-value of less than 0.05 was considered to represent a statistically significant difference.
Results and Discussion
Physical properties of HL
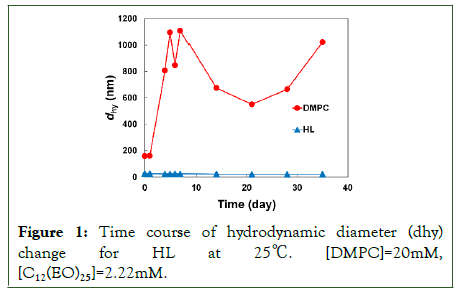
The physical properties of HL composed of DMPC and 10 mol% C12(EO)25 were examined. The diameter of the HL was measured using dynamic light scattering. The results are shown in Figure 1. The membrane size of DMPC liposomes was more than 200 nm and became more than 1000 nm with time, and was extremely unstable. In contrast, the hydrodynamic diameters of the HL were under 100 nm and were maintained for over one month [28]. HL maintained a membrane diameter that could avoid the Reticuloendothelial System (RES) for a long time.
Figure 1: Time course of hydrodynamic diameter (dhy) change for HL at 25â??. [DMPC]=20mM, [C12(EO)25]=2.22mM.
Inhibitory effects of HL on 4T1-Luc cell growth in vitro
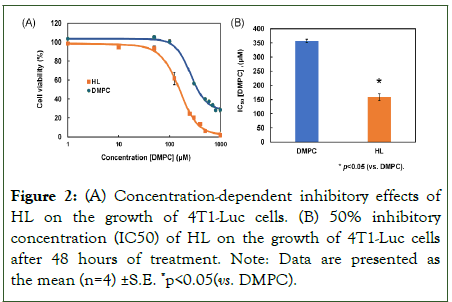
We investigated the inhibitory effects of Hybrid Liposomes (HL) on the growth of 4T1-Luc cells using the WST-8 assay, as shown in Figure 2. Both DMPC and HL inhibited the proliferation of 4T1-Luc cells in a concentration-dependent manner (Figure 2A). The 50% inhibitory concentration (IC50) of DMPC was 356.5 µM, whereas the IC50 of HL was 159 µM, which is less than half that of DMPC (p<0.05) (Figure 2B). These findings indicate that HL exhibits significantly stronger inhibitory effects on 4T1-Luc cell growth in vitro compared to DMPC.
Figure 2: (A) Concentration-dependent inhibitory effects of HL on the growth of 4T1-Luc cells. (B) 50% inhibitory concentration (IC50) of HL on the growth of 4T1-Luc cells after 48 hours of treatment. Note: Data are presented as the mean (n=4) ±S.E. *p<0.05(vs. DMPC).
Induction of apoptosis of HL for 4T1-Luc cells
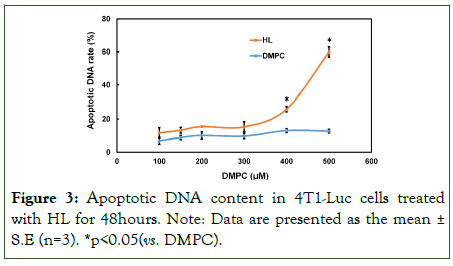
We evaluated the rate of apoptotic DNA fragmentation in 4T1-Luc cells treated with HL by flow cytometry analysis using a Propidium Iodide (PI) assay. Results are shown in Figure 3. No increase in apoptotic DNA fragmentation was observed in the DMPC-treated group. In contrast, treatment with HL resulted in a significant, concentration-dependent increase in apoptotic DNA fragmentation compared to DMPC (p<0.05). These findings suggest that HL induces apoptosis in 4T1-Luc cells.
Figure 3: Apoptotic DNA content in 4T1-Luc cells treated with HL for 48hours. Note: Data are presented as the mean± S.E (n=3). *p<0.05(vs. DMPC).
Therapeutic effects of HL in subcutaneous model mice of 4T1-Luc cells in vivo
We examined the inhibitory effects of HL on tumor growth in a mouse model subcutaneously inoculated with 4T1-Luc cells. Starting from the day of group assignment, DMPC and HL were administered intravenously via the tail vein at a volume of 10 mL/kg (corresponding to a DMPC dose of 136 mg/kg). The administration was carried out once daily for 14 consecutive days. Tumor volume was monitored over time in mice treated with HL. The results are presented in Figure 4. The subcutaneous tumor became visible seven days after implantation and was subsequently measured using a caliper. Compared with the control group, the HL-treated group showed a tendency to suppress tumor growth. The median tumor volumes in the control and HL-treated groups were 337 mm3 and 223 mm3 (p<.05 vs. control), respectively. This represents a 34% reduction in tumor volume in the model mice following intravenous administration of HL without any additional drugs, after subcutaneous inoculation of 4T1-Luc cells.
Figure 4: Suppression of tumor volume in breast cancer model mice intravenously treated with HL following subcutaneous inoculation of 4T1-Luc cells. Data are presented as the mean± S.D. (n=8). *p<0.05(vs. control).
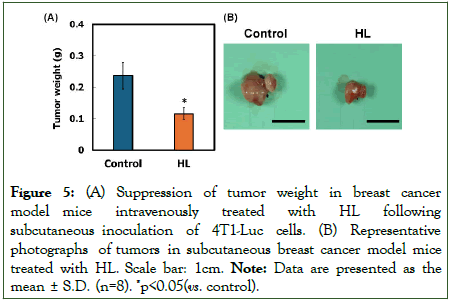
We next evaluated tumor weight in the HL-treated model mice. The results are shown in Figure 5. Mice treated with HL exhibited significantly lower tumor weights (0.04–0.19 g, p<0.05 vs. control) compared with those in the control group (0.05–0.43 g) (Figure 5A). To further assess the therapeutic effects of HL, an autopsy was performed (Figure 5B). Tumor reduction was observed in the HL-treated group, whereas tumor enlargement was evident in the control group.
Figure 5: (A) Suppression of tumor weight in breast cancer model mice intravenously treated with HL following subcutaneous inoculation of 4T1-Luc cells. (B) Representative photographs of tumors in subcutaneous breast cancer model mice treated with HL. Scale bar: 1cm. Note: Data are presented as the mean ± S.D. (n=8). *p<0.05(vs. control).
Furthermore, no significant changes in body weight were observed in either the control or HL-treated groups throughout the treatment period, and no adverse effects associated with HL administration were identified.
These results indicate that HL may be effective in the treatment of breast cancer model mice in vivo without side effects.
Conclusion
In this study, we investigated the therapeutic effects of Hybrid Liposomes (HL) without the use of conventional anticancer drugs on breast cancer (4T1-Luc) cells and obtained the following notable findings:
1. HL formed a stable bilayer structure with a particle size of less than 100nm immediately after preparation, enabling prolonged circulation by avoiding rapid clearance by the Reticuloendothelial System (RES), thus suggesting potential for clinical application.
2. HL exhibited a significant antiproliferative effect on the growth of 4T1-Luc cells in vitro.
3. HL induced apoptosis in 4T1-Luc cells in vitro.
4. In in vivo experiments, HL significantly reduced tumor volume and weight in a breast cancer mouse model, without any observable side effects.
These results suggest that HL hold promise as a novel therapeutic agent for breast cancer, with minimal or no adverse effects.
Acknowledgments
We thank Kenta Tsujimura, and Yuki Katsuki for their technical assistance.
References
- Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134-1150.
[Crossref] [Google Scholar] [Pub Med]
- Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, et al. Breast cancer: Biology, biomarkers, and treatments. Int Immunopharmacol. 2020;84:106535.
[Crossref] [Google Scholar] [Pub Med]
- Katsura C, Ogunmwonyi I, Kankam HK, Saha S. Breast cancer: presentation, investigation and management. Br J Hosp Med (Lond). 2022;83:1-7.
[Crossref] [Google Scholar] [Pub Med]
- Ueoka R, Moss RA, Swarup S, Matsumoto Y, Strauss G, Murakami Y. Extraordinary micellar enantioselectivity coupled to altered aggregate structure. J Am Chem Soc. 1985;107:2185-2186.
- Ueoka R, Matsumoto Y, Moss RA, Swarup S, Sugii A, Harada K, Kikuchi J, Murakami Y. Membrane matrix for the hydrolysis of amino acid esters with marked enantioselectivity. J Am Chem Soc. 1988;110:1588-1595.
- Kitamura I, Kochi M, Matsumoto Y, Ueoka R, Kuratsu J, Ushio Y. Intrathecal chemotherapy with 1,3-bis(2-chloroethyl)-1-nitrosourea encapsulated into hybrid liposomes for meningeal gliomatosis: an experimental study. Cancer Res. 1996;56:3986-3992.
- Nagami H, Matsumoto Y, Ueoka R. Chemotherapy with hybrid liposomes for lymphoma without drugs in vivo. Int J Pharm. 2006;315:167-172.
[Crossref] [Google Scholar] [Pub Med]
- Shimoda S, Ichihara H, Matsumoto Y, Ueoka R. Chemotherapy with hybrid liposomes for human breast tumors along with apoptosis in vivo. Int J Pharm. 2009;372:162-168.
[Crossref] [Google Scholar] [Pub Med]
- Kuwabara K, Ichihara H, Matsumoto Y. Inhibitory effects and anti-invasive activities of trehalose liposomes on the proliferation of lung carcinoma cells. J. Carcinog. Mutagen.2017;8: 1000283-1-5.
- Ichihara H, Yamasaki S, Hino M, Ueoka R, Matsumoto Y. Hybrid liposomes inhibit the growth and angiogenesis in human breast cancer model. J Carcinog Mutagen. 2015;6:1000207-1-7.
- Ichihara H, Komizu Y, Ueoka R, Matsumoto Y. Inhibitory effects of hybrid liposomes on the growth of non-small cell lung carcinoma cells and anti-invasive activity by ceramide generation without any drugs. J Carcinog Mutagen. 2015;6:1000230-1-5.
- Ichihara H, Motomura M, Matsumoto Y. Negatively charged cell membranes-targeted highly selective chemotherapy with cationic hybrid liposomes against colorectal cancer in vitro and in vivo. J Carcinog Mutagen. 2016;7:1000267-1-8.
- Okumura M, Ichihara H, Matsumoto Y, Ueoka R. Hybrid liposomes showing enhanced accumulation in tumors as theranostic agents in the orthotopic graft model mouse of colorectal cancer. Drug Deliv. 2018;25:1192-1199.
[Crossref] [Google Scholar] [Pub Med]
- Kuwabara K, Ichihara H, Matsumoto Y. Inhibitory effect of hybrid liposomes on the growth of NP2 glioma cell. J Carcinog Mutagen. 2020;11:1000344-1-1000344-5.
- Kuwabara K, Ichihara H, Matsumoto Y. Novel therapy with hybrid liposomes for orthotopic graft mouse. J Carcinog Mutagen. 2021;12:1000360-1-1000360-5.
- Kuwabara K, Ichihara H, Matsumoto Y. Trehalose liposomes inhibit the growth of glioblastoma cell in vitro and in vivo. J Carcinog Mutagen. 2021;12:1000364-1-5.
- Ichihara H, Okumura M, MatsumotoY. Photodynamic therapy effects of hybrid liposomes including indocyanine green against a xenograft mouse model mice of colorectal cancer in vivo. J Carcinog Mutagen. 2022;13:1000395-1-5.
- Ichihara H, Matsumoto Y. Inhibitory effects of cationic hybrid liposomes against metastasis of bile duct cancer in vitro and in vivo. J Carcinog Mutagen. 2024;15:1000440(1-6).
- Ichihara H, Takaki H, Motomura M, Okumura M, Goto K, Matsumoto Y. Efficacy of cationic hybrid liposomes composed of L-α- dimyristoylphosphatidylcholine in targeting negatively charged cholangiocarcinoma cell membranes without any drug in vitro and in vivo. J Carcinog Mutagen. 2024;15,1000460.
- Komizu Y, Matsumoto Y, Ueoka R. Membrane targeted chemotherapy with hybrid liposomes for colon tumor cells leading to apoptosis. Bioorg Med Chem Lett. 2006;16:6131-6134.
[Crossref] [Google Scholar] [Pub Med]
- Komizu Y, Ueoka H, Ueoka R. Selective accumulation and growth inhibition of hybrid liposomes to human hepatocellular carcinoma cells in relation to fluidity of plasma membranes. Biochem Biophys Res Commun. 2012;418: 81-86.
[Crossref] [Google Scholar] [Pub Med]
- Towata T, Komizu Y, Suzu S, Matsumoto Y, Ueoka R, Okada S. Hybrid liposomes inhibit the growth of primary effusion lymphoma in vitro and in vivo. Leuk Res. 2010;34:906-911.
[Crossref] [Google Scholar] [Pub Med]
- Matsumoto Y, Iwamoto Y, Matsushita T, Ueoka R. Novel mechanism of hybrid liposomes-induced apoptosis in human tumor cells. Int J Cancer. 2005;115:377-382.
[Crossref] [Google Scholar] [Pub Med]
- Komizu Y, Nakata S, Goto K, Matsumoto Y, and Ueoka R. Membrane-targeted nanotherapy with hybrid liposomes for tumor cells leading to apoptosis. ACS Med Chem Lett. 2011;2:275-279.
[Crossref] [Google Scholar] [Pub Med]
- Ichihara H, Nagami H, Kiyokawa T, Matsumoto Y, Ueoka R. Chemotherapy using hybrid liposomes along with induction of apoptosis. Anticancer Res. 2008;28:1187-1195.
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399-405.
- Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, et al. Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol. 2011;539748.
[Crossref] [Google Scholar] [Pub Med]
- Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066:29-36.
[Crossref] [Google Scholar] [Pub Med]
Citation: Ichihara H, Miyamoto S, Takai J, Okumura M, Goto K, Matsumoto Y (2025). Therapeutic Effects of Hybrid Liposomes against Triple Negative Mouse Breast Cancer in vitro and in vivo. J Carcinog Mutagen. 16:469.
Copyright: © 2025 Ichihara H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.