Indexed In
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Original Research Article - (2023) Volume 13, Issue 6
Herbal Constituents of AYUSH-64 Formulation Modulate Release of Cytokines in TLR7/8-Induced Macrophage-Like Cells
Kamala Priya1, Manisha Dagar1,2, Madhu Dikshit1,3* and Ajay Kumar1*2Department of Ophthalmology, University of California San Diego, San Diego, CA, USA
3Division of Pharmacology, CSIR-Central Drug Research Institute, Lucknow, Uttar Pradesh, India
Received: 11-Oct-2023, Manuscript No. CPECR-23-23531; Editor assigned: 13-Oct-2023, Pre QC No. CPECR-23-23531 (PQ); Reviewed: 27-Oct-2023, QC No. CPECR-23-23531; Revised: 03-Nov-2023, Manuscript No. CPECR-23-23531 (R); Published: 10-Nov-2023, DOI: 10.35248/2161-1459.23.13.394
Abstract
Recent insights into the pathophysiology of COVID-19 have revealed a significant relationship between Cytokine Release Syndrome (CRS) and the disease’s severity, characterized by elevated levels of cytokines such as TNF- α, IL- 1β, IL-6, and IL-10. In this study, we simulated virus recognition through TLR7/8 receptors and the subsequent inflammatory response using imiquimod and resiquimod within PMA-differentiated THP1 macrophage-like cells. We investigated the prophylactic potential of herbal constituents found in the AYUSH-64 formulation, including Alstonia scholaris, Picrorhiza kurroa, Swertia chirata, and Caesalpinia crista, to modulate gene expression and secretion of inflammatory cytokines in induced cells. Our findings were compared with the previously reported effects of the AYUSH-64 formulation in a similar TLR7/8-induced cell model.
Our results demonstrated concentration-dependent responses for all four herbal extracts in regulating TNF- α and IL-6 cytokines. Notably, Picrorhiza kurroa exhibited a concentration-dependent effect on both cytokine gene expression and secreted cytokine levels, highlighting its efficacy in ameliorating the induced inflammatory response. The positive impact of these investigated herbs in mitigating hyper-inflammatory responses complements the prophylactic efficacy previously observed with the AYUSH-64 formulation. This study contributes to the growing body of evidence supporting the use of herbal remedies in mitigating the inflammatory milieu associated with infectious conditions.
Keywords
AYUSH-64; Picrorhiza kurroa; TLR7/8; Imiquimod; Resiquimod; Inflammation; Cytokine; THP1
Introduction
Cytokines are the signaling molecules that arbitrate a sustained innate and adaptive immune response to an invading pathogen. Their secretion, during infection, is generally under tight regulation. However, the sudden release of too many cytokines into the blood is associated with severe immune suppression and may lead to multi-organ failure as was observed in SARS, MERS and SARS-CoV-2 infections [1-4].
The latter condition, better known as CRS is characterized broadly by similar cytokine profiles in different diseases [5]. Cytokines and chemokines are essential immune system mediators that play a significant role in maintaining anti-viral immunity. Pathogen Recognition Receptors (PRRs) like TLR7 and TLR8 are the only toll-Like Receptos (TLRs) that recognize an ssRNA virus. They also facilitate activating innate immune response and consequent expression of genes encoding pro-inflammatory cytokines and chemokines including TNF-α, IL-1β, and IL-6 [6]. TLR7/8 could therefore be a potential target in controlling the infection in the early stages of the disease [7].
Though the events involved in manifestation of a cytokine storm are not clearly understood at this point, targeting the storm to ameliorate the state of hyper inflammation could be a novel therapeutic approach [8,9]. Several synthetic drugs (hydroxychloroquine, dexamethasone), monoclonal antibodies (toclizumab, canakinumab) and stem cell therapies were explored to treat the cytokine storm during recent COVID-19 pandemic [10]. Though these synthetic immunomodulatory agents may have proven beneficial, their adverse side effects were often associated with their use [11,12].
Traditional forms of medicine like Ayurveda/herbal medicine are used along with allopathy in the treatment and management of various ailments. Whole plants or parts of plants are used as a phytopharmaceutical derivative in the treatment of respiratory illnesses [13]. Several plant derivatives such as homo-harringtonine, ouabain, lycorine, tylophorine, 7-methoxy-cryptopleurine, and silvestro have demonstrated significant anti-viral efficacy against inflammatory conditions at nanomolar concentrations [14]. Immunomodulatory agents from such plant sources offer enhanced bioavailability and are essentially devoid of toxic side effects. Such plants and their derivatives may present a novel approach to attenuate the CRS and the resulting hyper-inflammation [15,16].
Over the decades, remarkable success has been achieved in Western medicine with regard to the concept of drug combination. Drug combination therapies in infectious diseases as well as cancer have offered promise [17]. Similarly, naturally occurring herbs and their ingredients organized into certain formula have potential synergistic effects in disease therapeutics. During recent clinical trials, a polyherbal formulation called AYUSH-64 was readily used to treat asymptomatic and mild to moderate COVID-19 patients [18-20]. AYUSH-64 was originally developed by the Central Council for Research in Ayurvedic Sciences (CCRAS), Ministry of AYUSH to cure Malaria in 1980. Based on its anti-viral and immune–modulator activities, AYUSH-64 was repositioned for COVID-19 and did not disappoint [18,21-24]. Its adjunct administration alongside the standard medications in the treatment of mild to moderate COVID-19 resulted in early clinical recovery [20]. AYUSH-64 comprises of Alstonia scholaris; AS (aqueous bark extract), Picrorhiza kurroa; PK (aqueous rhizome extract), Swertia chirata; SC (aqueous extract of whole plant) and Caesalpinia crista; CC (fine-powdered seed pulp) [25]. While a significant amount of scientific information on anti-inflammatory efficacy of AYUSH-64 formulation was generated during the recent pandemic, similar scientific rationale for selecting the herbs to generate this formulation is missing. Also not many exploratory studies have attempted to compare the safety and efficacy of individual herbs as compared to the formulation with respect to its anti-inflammatory efficacy.
In this study, we aimed to assess the efficacy of individual herbal components (AS, PK, SC, CC) in resolving TLR7/8-induced inflammation and compare their performance with the complete AYUSH-64 formulation. We induced inflammatory responses in macrophage-like cells using TLR7/8 agonists (imiquimod and resiquimod) and examined the concentration-dependent prophylactic effects of aqueous herbal extracts. We measured gene expression and secretion of TNF-α, IL-1β, IL-6, and IL-10 cytokines to evaluate their potential in reducing excessive cytokine responses. We assessed the anti-inflammatory capabilities of AS, PK, SC, and CC individually and compared them with the previously published results on AYUSH-64 formulation [26].
Materials and Methods
Extract preparation
4 mg of each herbal extract powder of AYUSH-64 herbal constituents (AS, PK, SC, and CC) was weighed into pre-weighed and labelled sterile Eppendorf tubes. 1 ml of sterile RPMI media was added to each tube in sterile conditions and incubated overnight at room temperature on a rotary shaker to ensure thorough dissolution of extracts in aqueous media. Subsequently, the herbal mixture was centrifuged the following day at room temperature at 10,000 rpm for 10 minutes to remove any suspended extract powder as a pellet.
Supernatant from each tube was transferred into a fresh sterile tube and filtered using a 0.22 μm syringe filter. These filtered extract stocks were aliquoted and stored at 4°C in dark for further use.
Cell culture: Human monocytic THP-1 cells were purchased from ATCC and maintained in RPMI 1640 medium (Gibco) containing 10% FBS (Hyclone) and 1X penicillin (stock concentration=100 U/ml), and streptomycin (stock concentration=100 μg/ml) at 37°C in humidified conditions with 5% CO2. Prior to the start of the experiment, THP1 cells were maintained in culture in suspension form with passaging at regular intervals (every 2-3 days) based on cell confluence.
Cell viability assay
THP-1 monocytes were seeded in a 96-well plate and differentiated into macrophages in the presence of 5 ng/ml PMA over 48-hours incubation. Subsequently, differentiated cells were treated with 1000 μg/ml herbal extract concentration. After 24 hours of incubation with extracts, cells were incubated in the dark with 0.5 mg/ml MTT (Sigma, Cat no#M2128) for 4 hours at 37°C. The medium was then removed and 100 μl DMSO (Sigma, Cat no#276855) was added per well. Plates were incubated in the dark at RT with constant shaking for 30 minutes. Subsequently, the absorbance was measured with a microplate reader (Molecular Devices, Spectramax M2) at 570 nm wavelength. Dexamethasone (1 μM) (Selleck Chemicals,#S1322) was used as an internal reference.
Prophylactic treatment and induction
THP1 monocytes were differentiated into macrophage-like cells in the presence of 5 ng/ml phorbol 12-myristate 13-acetate (PMA) over a 48-hour incubation period. A total of 1-2 million cells per well were seeded in a 6-well plate in 3 ml complete culture medium for each assay. Upon differentiation, macrophage-like cells were pre-treated with aqueous herbal extracts at multiple concentrations (3 μg/ml, 10 μg/ml, 30 μg/ml, 100 μg/ml, and 300 μg/ml) for an hour. Subsequently, pre-treated cells were induced for TLR signalling by adding TLR7/8 agonists (Imiquimod- Tocris#3700/50); Resiquimod-Sigma#SML1096) and incubated for another 24 hours. Agonist concentrations and induction duration used here were as reported in a recent report from our group [26]. A set of untreated and uninduced control cells was included with each extract investigation along with a set of untreated but TLR7/8- induced cells as well as TLR7/8-induced cells treated with 30 nM dexamethasone as a positive control. After 24 hours of incubation, the culture supernatant, as well as treated cells, were collected separately for determination of secreted cytokine levels as well as the effect of herbal treatment on their gene expression, respectively.
Real-time PCR
Total RNA was extracted from the treated cell pellet using the trizol reagent (Sigma#T9424) and quantified on a Multiskan GO plate reader (Thermo Scientific). High-capacity cDNA reverse transcription kit (Applied Biosystems#4368814) was employed to reverse transcribe 250 ng RNA per reaction. Subsequently, qRT-PCR reactions were prepared using PowerUp SYBR Green mix (Applied Biosystems#A25742). Standard protocol on QuantStudio 6 Real-Time PCR System (Applied Biosystems) was used to run the qRT-PCR reactions. Primers to investigate the changes in gene expression of the targeted cytokine genes included–TNF-α Forward 5’-TGGGATCATTGCCCTCTTGAG-3’ and Reverse 5’- TCTAAGCTTGGGTTCCGACC-3’; IL- 1β Forward 5’- GGGCCTCAAGGAAAAGAATC-3’ and Reverse 5’- TTCTGCTTGAGAGGTGCTGA-3’; IL-6 Forward 5’- TACCCCCAGGAGAAGATTCC-3’ and Reverse 5’- TTTTCTGCCAGTGCCTCTTT-3’; IL-10 Forward
5’- TGCCTTCAGCAGAGTGAAGA-3’ and Reverse 5’- GGTCTTGGTTCTCAGCTTGG-3’ and β-actin Forward 5’- AGAGCTAGGAGCTGCCTGAC-3’ and Reverse 5’- AGCACTGTGTTGGCGTACAG-3’. Expression of the β-actin gene was used to normalize that of the targeted cytokine genes in the test samples. The data was expressed as fold change with respect to the β-actin gene and compared to the untreated control cells as well as induced but untreated macrophage-like cells.
ELISA
Secreted levels of the select key pro-inflammatory cytokines in the culture supernatant were assayed using ELISA kits as instructed by the kit manufacturer (Invitrogen, Thermofisher Scientific). Sample dilutions were standardized for each analyte. For TNF-α estimation, culture supernatant samples were diluted 50 times while the samples were used undiluted to determine secreted levels of other cytokines (IL-1β, IL-6, and IL-10). The reaction fluorescence was measured at 450 nm and 570 nm wavelengths using a multi-mode microplate reader.
Analysis: One‐way Analysis Of Variance (ANOVA) with the Tukey test for multiple comparisons and a two-tailed unpaired Student’s t-test for comparisons between two groups were used for statistical analysis and all graphs were generated using GraphPad Prism (Version 9). All data are expressed as mean ± SEM and the p-value<0.05 was considered statistically significant.
Results
Human PRRs recognize molecular patterns associated with different pathogens (Viruses, bacteria, fungi, or parasites) (PAMPs) and induce inflammatory mediators to initiate an immune response and contain infection. TLRs are a unique group of PRRs that primarily regulate inflammation during infection. TLR7/8 ligands like Imiquimod (IMQ) and Resiquimod (RSQ), that mimic PAMPs, may induce potent anti-viral response leading to secretion of cytokines/chemokines. IMQ is an immune modulator that acts as a TLR-7 agonist. It belongs to the class of aromatic heterocyclic compounds containing an imidazole ring fused to a quinoline ring system (imidazoquinolines). Resiquimod is a modified version of IMQ and is known for its immunomodulatory effects and has anti- viral and anti-tumor activity. It has several mechanisms of action, being both an agonist for toll-like receptors 7 and 8 and an up- regulator of the opioid growth factor receptor [27,28]. Animal studies have shown that IMQ may induce the expression of IL-6, IL-8, and TNF-α genes. Also, RSQ-loaded nanoparticles, when administered into circulation, improved response rates to cancer immunotherapy with a checkpoint inhibitor through stimulation of tumor-associated macrophages [29].
Dexamethasone is an anti-inflammatory drug known for its immune-suppressive properties. It was among the several drugs investigated in the RECOVERY trial during the recent viral pandemic [30]. Dexamethasone was specifically targeted against the overwhelmed immune response during SARS-CoV-2 infection to suppress it. Early use was recommended to minimize inflammation and hence benefit individuals with severe acute respiratory distress syndrome. Dexamethasone (30 nM) was used in this study as a reference standard for amelioration of IMQ/RSQ-induced cytokine expression/secreted levels in macrophage-like cells. As expected, expression as well as secreted levels of all investigated inflammatory cytokines (TNF-α, IL6 and IL-1β) were mitigated in cells treated with the corticosteroid as compared to their untreated but induced counterparts.
All 4 herbal extracts were investigated at 1000 μg/ml concentration for their effect on cell health of macrophage-like cells prior to efficacy studies (Figure 1). None of the extracts revealed any significant effect on cell viability. Similarly, dexamethasone- treated cells also showed no detrimental effect on the health of macrophage-like cells as compared to their untreated counterparts. Subsequently, efficacy studies were planned with multiple aqueous extract concentrations of all 4 herbal constituents of AYUSH-64 to determine their prophylactic effect on TLR7/8-induced inflammatory response in macrophage-like cells. Cells were pre-treated with herbal extracts for an hour prior to induction with TLR7/8 agonists for 24 hours. Subsequently, gene expression and secreted cytokine levels were monitored.
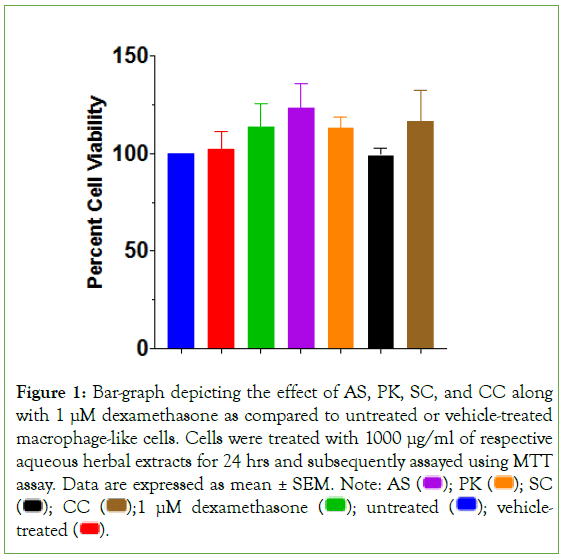
Figure 1: Bar-graph depicting the effect of AS, PK, SC, and CC along with 1 μM dexamethasone as compared to untreated or vehicle-treated
macrophage-like cells. Cells were treated with 1000 μg/ml of respective
aqueous herbal extracts for 24 hrs and subsequently assayed using MTT assay. Data are expressed as mean ± SEM. Note: AS  SC
SC  vehicle-treated
vehicle-treated  .
.
Prophylactic effect of Alstonia scholaris (AS) on TLR7/8- induced inflammatory response
Alstonia scholaris, commonly referred Saptaparni or Indian devil tree, is a tropical evergreen tree native to South Asia and Southeast Asia. It has demonstrated potent anti-viral properties and is used in the treatment of influenza. Its bark, leaves, and latex are used in traditional medicine systems including Ayurveda and traditional Chinese medicine. The bitter bark is used to treat fevers, malaria, and digestive disorders. Its latex is used for wound healing.
AS contains bioactive compounds with anti-inflammatory properties such as alkaloids, flavonoids, and terpenoids. Treatment with methanolic extract of stem bark of AS demonstrated its antioxidant and anti-inflammatory properties [31,32]. Total alkaloid extract from AS leaves has shown inhibitory activity against infections of Herpes Simplex Virus (HSV), adenovirus, Respiratory Syncytial Virus (RSV), and influenza virus [33]. It is also known to control malarial fever by its strong schizonticidal activity [34]. Anti-viral effects of alkaloids against the influenza virus were demonstrated when oral administration of alkaloids in infected mice showed protection from death against the virus [35]. Similarly, ameliorative prophylactic efficacy of AS in suppressing pro-inflammatory cytokines was demonstrated recently in a Syrian hamster SARS-CoV-2 infection model [36]. Robust anti-viral as well as immune-modulatory potential of AS was demonstrated.
In IMQ-induced cells, the prophylactic effect of AS treatment was distinctly visible with respect to TNF-α and IL-6 cytokines (Figure 2). While IL-6 expression and secreted levels were significantly corrected at all investigated concentrations, AS treatment showed a distinct concentration-dependent decrease in TNF-α expression as well as its secreted levels (Figures 2A-2D) when compared to induced but untreated cells. Maximum reduction in TNF-α expression as well as its corresponding secreted protein levels was seen in cells treated with 300 μg/ml AS extract. Gene expression of IL-10 cytokine was significantly reduced as well at most of the investigated concentrations of A. scholaris. Interestingly, AS treatment revealed no distinct effect on the IL-1β gene or secreted levels in IMQ-induced macrophage-like cells.
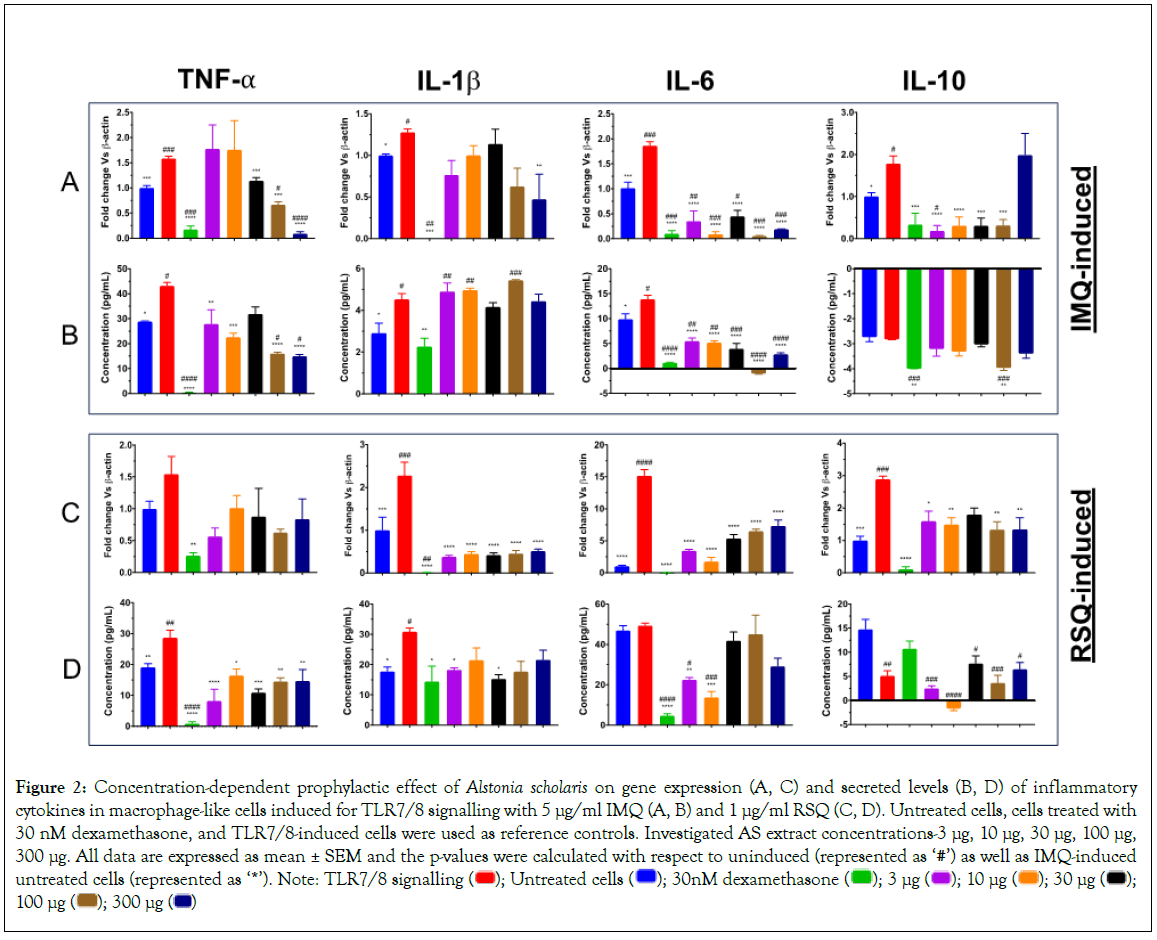
Figure 2: Concentration-dependent prophylactic effect of Alstonia scholaris on gene expression (A, C) and secreted levels (B, D) of inflammatory cytokines in macrophage-like cells induced for TLR7/8 signalling with 5 μg/ml IMQ (A, B) and 1 μg/ml RSQ (C, D). Untreated cells, cells treated with 30 nM dexamethasone, and TLR7/8-induced cells were used as reference controls. Investigated AS extract concentrations-3 μg, 10 μg, 30 μg, 100 μg,
300 μg. All data are expressed as mean ± SEM and the p-values were calculated with respect to uninduced (represented as ‘#’) as well as IMQ-induced untreated cells (represented as ‘*’). Note: TLR7/8 signalling  100 μg
100 μg 
The prophylactic efficacy of the herbal extracts in RSQ-induced cells was more pronounced than that seen in IMQ cells. As is evident for Figures 2C and 2D, AS treatment showed significant correction in gene expression levels of IL-1β, IL-6, and IL-10. While TNF-α expression was also corrected but was not found to be statistically significant. Corresponding secreted levels of TNF-α cytokine were significantly reduced at all investigated concentrations. Similarly, IL-1β and IL10 levels were significantly reduced after treatment with AS. Treatment with lower doses (3 μg/ml and 10 μg/ml) of AS extract revealed better efficacy in alleviating IL-6 secreted protein while no significant effect on IL-6 levels was seen in cells treated with higher concentrations (30 μg/ml, 100 μg/ml and 300 μg/ml) of AS extract.
With respect to TLR induction, the observed effect of AS treatment was more pronounced in RSQ-induced cells. A clear reduction in expression levels of most of the investigated cytokine genes was seen. However, the reduction in circulating levels of IL1-β and IL-6 proteins in AS-treated cells was consistent for both IMQ and RSQ induction.
Prophylactic effect of Picrorhiza kurroa (PK) on TLR7/8- induced inflammatory response
Picrorhiza kurroa or kutki is a perennial herbaceous plant found in the Himalayan region and other parts of Asia, including India, Nepal, and Bhutan. It is one of the popular medicinal plants known for its potential benefits for liver health, digestion, inflammation, and immune support and is widely used in the treatment of respiratory tract infections. Its bioactive compounds, including iridoid glycosides (such as picroside I and kutkoside), are believed to have anti-inflammatory properties. Latter was demonstrated when treatment of arthritic rats with rhizome extract of PK revealed a significant decrease in synovial expression of pro-inflammatory cytokines like IL-1β and IL-6 [24].
PK has been traditionally used to treat respiratory illness, chronic scorpion stings, allergies, inflammatory disorders, fever, diarrhea, and hepatic disease [24,37-40]. Numerous studies have shown that PK has antioxidant, anti-inflammatory, antiallergenic, hepatoprotective, choleretic, anti-asthmatic, and anti-cancerous qualities that have a therapeutic impact on several diseases. In RAW 264.7 murine macrophages, PK extract inhibited lipopolysaccharide-induced nuclear factor-kappa light chain enhancer of activated B cell (NF- κB) signalling (Figure 3) [24]. In IMQ-induced cells, PK treatment revealed a clear dose-response with respect to gene expression as well as secreted protein levels of TNF-α cytokine (Figures 3A and 3B). PK extract concentrations of 300 μg/ml and 100 μg/ml presented with the best reduction in TNF-α cytokine profiles. Similarly, IL- 1β expression was significantly reduced in cells treated with 300 μg/ml PK extract while at all other concentrations, there was only a corrective trend. Cells treated with increasing concentrations of PK extract again revealed a dose-dependent correction in secreted protein levels of IL-6. However, its corresponding gene expression was significantly reduced only in cells treated with PK extracts at 10 μg/ml and 300 μg/ml concentrations. Also, while the gene expression of IL-10 cytokine was significantly mitigated subsequent to treatment with PK extract at all concentrations, corresponding secreted protein levels in cells treated with 100 μg/ml 300 μg/ml PK extracts were also increased significantly.
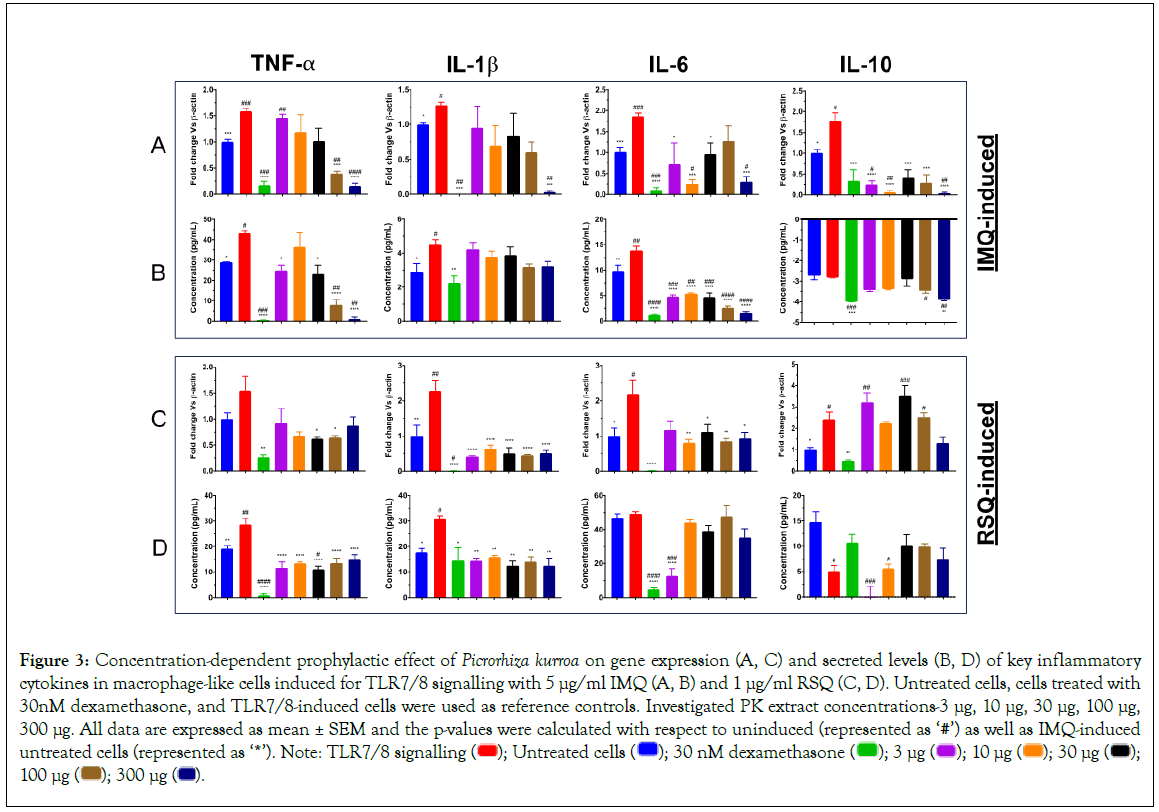
Figure 3: Concentration-dependent prophylactic effect of Picrorhiza kurroa on gene expression (A, C) and secreted levels (B, D) of key inflammatory cytokines in macrophage-like cells induced for TLR7/8 signalling with 5 μg/ml IMQ (A, B) and 1 μg/ml RSQ (C, D). Untreated cells, cells treated with 30nM dexamethasone, and TLR7/8-induced cells were used as reference controls. Investigated PK extract concentrations-3 μg, 10 μg, 30 μg, 100 μg,
300 μg. All data are expressed as mean ± SEM and the p-values were calculated with respect to uninduced (represented as ‘#’) as well as IMQ-induced untreated cells (represented as ‘*’). Note: TLR7/8 signalling  100 μg
100 μg 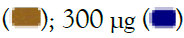
In contrast to the effect seen in IMQ-induced cells, PK treatment in RSQ-induced cells did not reveal dose response for any of the investigated inflammatory mediators. Nevertheless, PK extract treatment showed a significant correction of all cytokines at both gene expression in Figure 3C as well as secreted protein levels in Figure 3D at most or all of the investigated extract concentrations. Gene expression for IL-1β was significantly reduced in PK-treated RSQ-induced macrophage-like cells while that for other cytokines showed amelioration of RSQ-induced expression only at random PK extract concentrations. In line with gene expression, RSQ- induced IL-1β protein levels were also significantly mitigated in PK- treated cells. Also, protein levels of RSQ-induced TNF-α cytokine were significantly corrected in PK-treated macrophage-like cells at all concentrations. Interestingly, RSQ-induced IL-6 protein levels were only corrected in cells treated with 3 μg/ml PK extract, while all other PK concentrations showed no effect on IL-6 cytokine levels.
Prophylactic effect of Swertia chirata (SC) on TLR7/8- induced inflammatory response
Swertia chirata, commonly referred to as chirata, is a bitter herb native to the Himalayan region, including India, Nepal, and Bhutan, and is popularly used in traditional medicine to treat a number of ailments. It is rich in alkaloids and glycosides. Some of the identified plant compounds of chirata include swertanone, amarogenin, chiratol. It has potential anti-inflammatory properties that were exploited against malaria, diabetes, and liver disorders. Analgesic and anti-inflammatory activity of the ethanolic extract of SC may be mediated through the inhibition of bradykinins and prostaglandins [41]. Xanthones from SC demonstrated an inhibitory effect on inflammatory mediators by regulating COX-2/MAPK/Akt signaling pathways in mouse macrophage cells [22].
SC is a well-known anti-inflammatory agent and has proven to be efficient in the treatment of malaria, hepatitis B, gastritis, dyspepsia, bronchial asthma, and diabetes. Amarogentin is the active molecule present in S. chirata that has potential antiviral activity against the spike glycoprotein of SARS-CoV-2 [42]. Another successful formulation of S. chirata is Sudarshan Ghan Vati, which has been effective in the management of mild to moderate COVID-19 [43].
Increased expression of all the investigated cytokine genes as a consequence of TLR7/8-induction was significantly corrected in SC-treated cells (Figure 4). The dose-response of SC treatment was revealed on gene expression, as well as corresponding secreted protein levels in Figures 4A-4D of TNF-α cytokine in both IMQ and RSQ-induced conditions. Similarly, ELISA results from the IL-6 assay also showed decreasing IL-6 secreted levels with increasing SC extract concentration subsequent to IMQ-induction. Except for the reduction of IMQ-induced IL-1β gene expression at a few SC extract concentrations, no effect of SC treatment was seen on corresponding secreted protein levels. Interestingly, IL-10 protein levels in culture supernatants of IMQ-induced cells were significantly increased with increasing SC extract concentration, suggesting a role of SC in M2 response. Unlike IMQ-induced cells, no correction in IL-6 secreted levels was seen in SC-treated cells subsequent to RSQ induction.
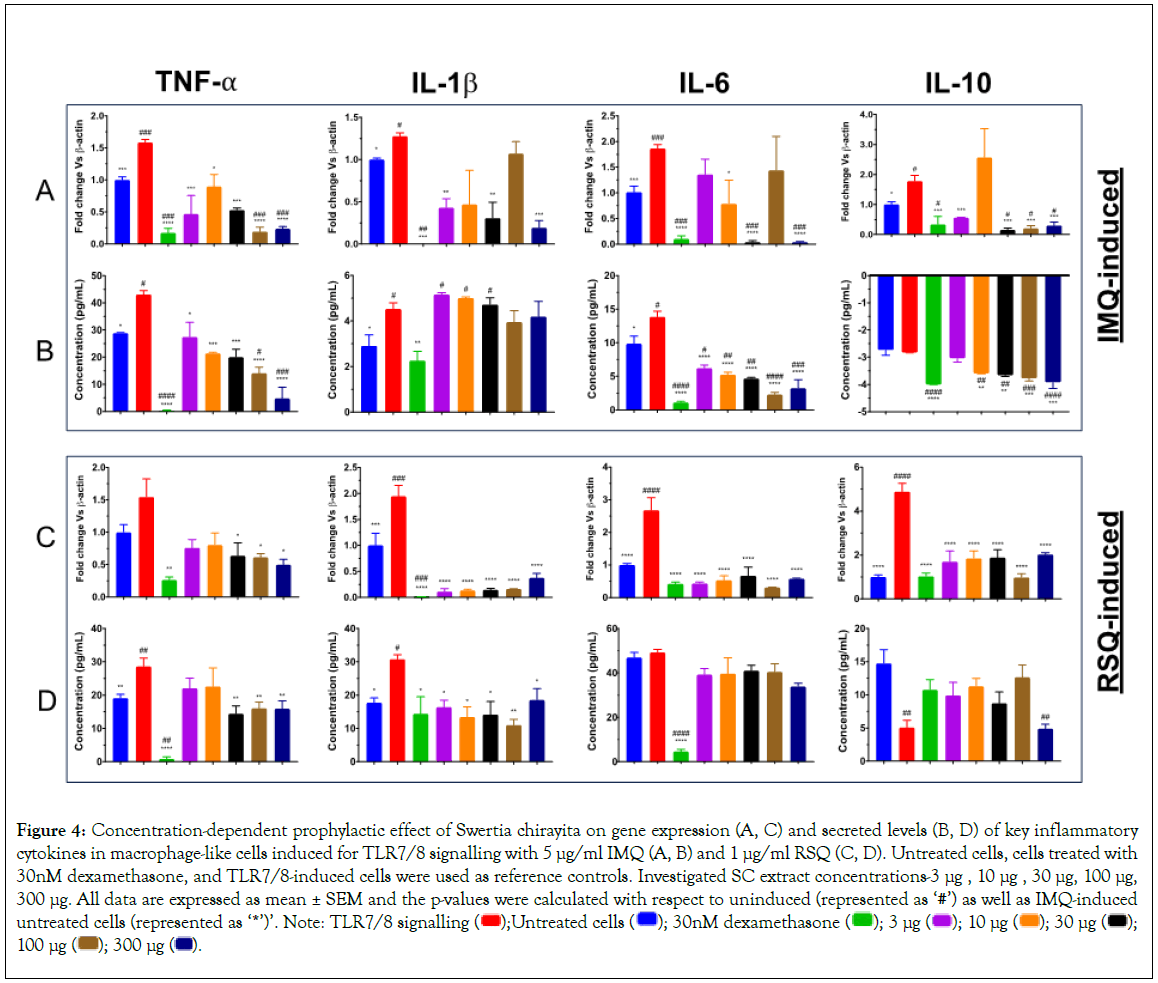
Figure 4: Concentration-dependent prophylactic effect of Swertia chirayita on gene expression (A, C) and secreted levels (B, D) of key inflammatory cytokines in macrophage-like cells induced for TLR7/8 signalling with 5 μg/ml IMQ (A, B) and 1 μg/ml RSQ (C, D). Untreated cells, cells treated with
30nM dexamethasone, and TLR7/8-induced cells were used as reference controls. Investigated SC extract concentrations-3 μg , 10 μg , 30 μg, 100 μg,
300 μg. All data are expressed as mean ± SEM and the p-values were calculated with respect to uninduced (represented as ‘#’) as well as IMQ-induced untreated cells (represented as ‘*’)’. Note: TLR7/8 signalling 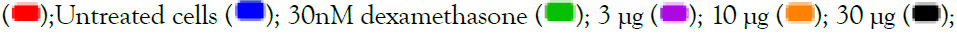 100 μg
100 μg 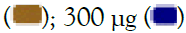 .
.
Prophylactic effect of Caesalpinia crista (CC) on TLR7/8- induced inflammatory response
Caesalpinia crista, commonly referred to as fever nut or karanja, is native to South Asia and can be found in India, Sri Lanka, Nepal, and Myanmar. The tree contains various phytochemicals, including alkaloids, flavonoids, and tannins, which contribute to its medicinal properties. Its leaves and roots are generally used for their potential anti-inflammatory properties and to treat various inflammatory conditions. A potential antioxidant and anti-inflammatory role were demonstrated by the inhibition of 5-lipoxygenase in a dose- dependent manner using phenolic extracts of CC [44]. C. crista has demonstrated potent antiviral effects against paramyxovirus and orthomyxovirus [45]. The leaf extract of C. crista has potential anti-bacterial and anti-viral effects against Streptococcus aureus, paramyxovirus, and orthomyxovirus [46].
As is evident from Figure 5, gene expression in Figures 5A and 5C and secreted protein levels in Figure 5B and 5D of TNF-α in IMQ/RSQ-induced macrophage-like cells were significantly corrected after treatment with increasing doses of CC extract. Similarly, the effect of CC extract dosage on IMQ-induced IL-6 protein levels was seen, but a similar effect in RSQ-induced cells was missing. Nevertheless, CC-treated cells showed significant mitigation of RSQ-induced IL-6 gene expression at all CC concentrations. CC treatment revealed no effect on IMQ-induced IL-1β cytokine levels while RSQ-induced IL-1β gene expression was significantly ameliorated in CC treated cells at investigated extract concentrations. Secreted IL-10 levels in both IMQ and RSQ-induced cells appeared to improve in cells treated with CC extract.
Our findings clearly advocate that herbal extracts hold immense potential in the therapeutics of inflammatory disorders. Cell viability studies clearly highlight their safety thereby gauging them as a better alternative to conventional immunosuppressive agents. Overall, our study contributes to the growing research support in favor of plant-based therapeutics, particularly with respect to their anti-inflammatory and immune-modulatory properties.
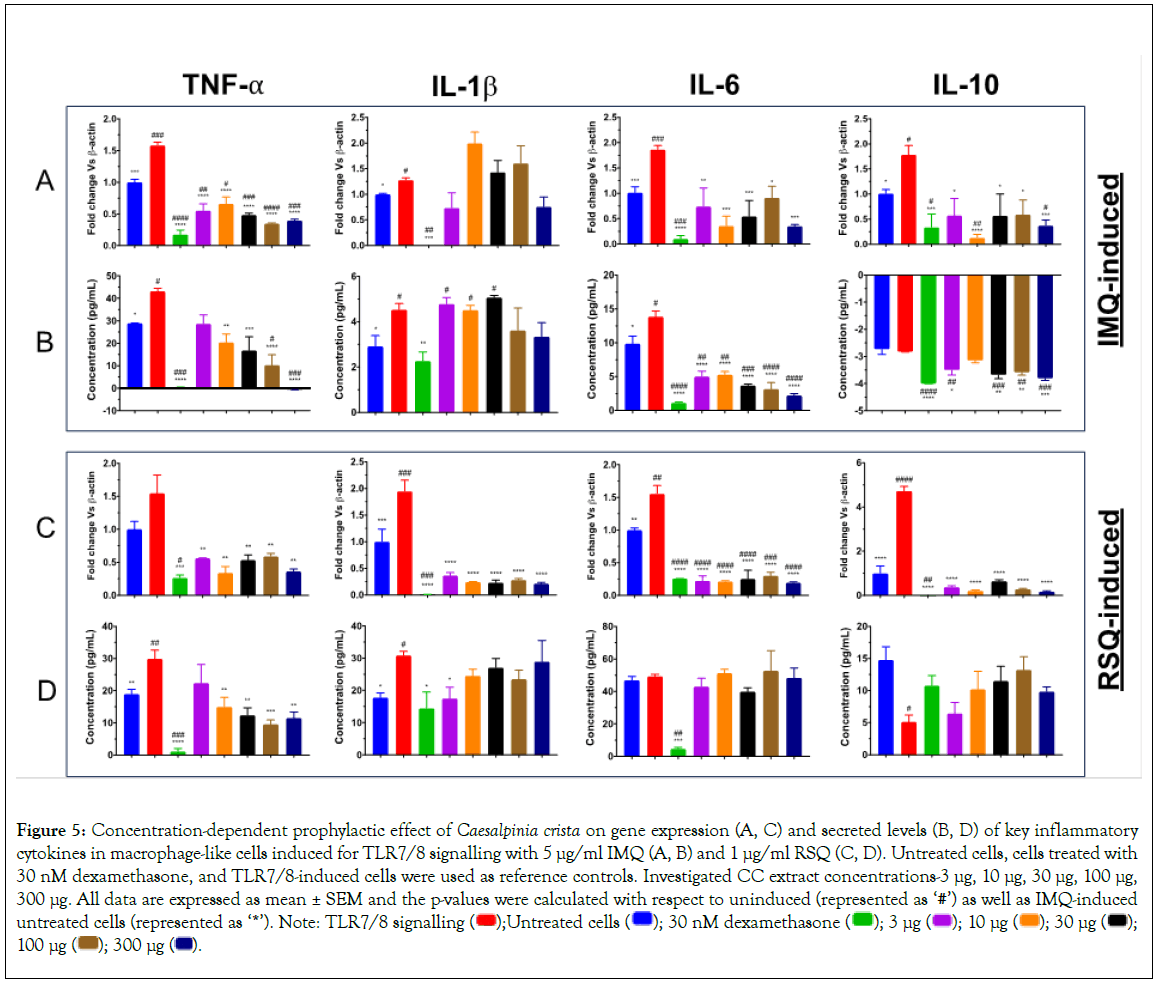
Figure 5: Concentration-dependent prophylactic effect of Caesalpinia crista on gene expression (A, C) and secreted levels (B, D) of key inflammatory cytokines in macrophage-like cells induced for TLR7/8 signalling with 5 μg/ml IMQ (A, B) and 1 μg/ml RSQ (C, D). Untreated cells, cells treated with
30 nM dexamethasone, and TLR7/8-induced cells were used as reference controls. Investigated CC extract concentrations-3 μg, 10 μg, 30 μg, 100 μg,
300 μg. All data are expressed as mean ± SEM and the p-values were calculated with respect to uninduced (represented as ‘#’) as well as IMQ-induced untreated cells (represented as ‘*’). Note: TLR7/8 signalling  100 μg
100 μg  .
.
Discussion
Inflammation is an important event in the defense system of a cell that may occur due to infection or injury to the cellular tissue. Though synthetic drugs like steroids, Nonsteroidal Anti- Inflammatory Drugs (NSAIDs), and immuno-suppressants are readily available and used in clinics for their anti-inflammatory properties, their prolonged use is limited by the associated side effects [47-49]. Hence, there is a need for safe, easily available, and economical treatment alternatives. According to a WHO report, around 80% of the developing world population still relies on traditional medicines for their healthcare [50]. Since ancient times, medicinal plants have been used to treat inflammation and are generally considered to be safer as compared to their synthetic counterparts [51-54]. Their phytochemical constituents like alkaloids, tannins, flavonoids, terpenoids, glycosides, carotenoids, and saponins possess anti-inflammatory properties [55].
Polyherbalism or polyherbal formulation refers to the use of two or more medicinal plants to achieve extra-therapeutic effectiveness. Their use in traditional medicine has been practiced for centuries now, yet scientific evidence in support of their safety and therapeutic benefits is lacking [56]. As compared to conventional single-component drugs, botanicals may have the advantage of multiple active compounds that together can provide a synergistic effect which may not be achievable by any single compound. A combination of herbals may act on multiple targets at the same time to provide a complete therapy against a disease condition [57]. However, multiple constituents may lead to chemical incompatibility which may result in instability and therefore warrant caution while designing herbal formulations [58]. Therefore, chemical fingerprinting of the formulation along with safety evaluations to identify safe bioactive phytoconstituents that can work synergistically can significantly improve therapeutic effects. Proper characterization and toxicological evaluation ensure the safety, efficacy and quality of the preparations [59]. One such polyherbal formulation readily implicated in inflammatory pathology during the COVID pandemic was AYUSH-64. It exhibited promising results in mild COVID-19 patients due to its antipyretic properties [20,25]. While a number of clinical trials and studies targeting mild to moderate inflammatory pathologies have been conducted with AYUSH-64, there is a serious dearth of reports comparing its efficacy with its constituent herbal plants with comparable efficacy.
AYUSH-64 is a combination of 4 herbs–A. scholaris, P. kurroa, S. chirata and C. crista, known to possess anti-inflammatory, anti-oxidant, antipyretic, anti-viral, and immunomodulatory properties [22-24,60,61]. In-silico understanding of AYUSH-64 by molecular docking revealed that one of its constituents (MPro – Akuammicine N-Oxide) inhibited the replication of SARS-CoV-2 main protease enzyme [61]. In a recent report from our group evaluating the prophylactic efficacy of AYUSH-64 on TLR7/8- induced inflammatory response, significant alleviation of RSQ- induced IL-10 and CXCL-10 gene expression was demonstrated in AYUSH-64 treated cells. In addition to that, AYUSH-64 treatment revealed a concentration-dependent response relationship with RSQ-induced secreted levels of IL-1β cytokine [26]. Such a distinct effect of AYUSH-64 on inflammatory mediators prompted us to investigate and compare the effect on TLR7/8 signaling of its individual constituent herbs against its own and compare them to better understand it in the pathophysiology of CRS.
Macrophages secrete pro-inflammatory cytokines (TNF-α, IL- 1β, and IL-6) and upregulate inflammatory receptors, such as TNF-R1, during the inflammatory phase [62-65]. Macrophages also secrete regulatory anti-inflammatory cytokine IL-10 to mitigate the inflammatory state and reduce excess damage to tissues. In this study, PMA-differentiated THP1 macrophage-like cells were pre-treated with multiple concentrations of aqueous extracts of individual AYUSH-64 herbal constituents for at least an hour before inducing them for TLR7/8 signaling in the presence of IMQ and RSQ for another 24 hours. Thereafter, the cell culture supernatant, as well as cell pellets, were harvested and processed for determination of secreted cytokine levels as well as corresponding gene expression, respectively. TLR7/8 induction by agonists led to a significant change in expression as well as secreted levels of TNF-α, IL-1β, IL-6 cytokines. Even though IL-10 cytokine expression was observed to increase in induced cells corresponding change in secreted levels was either not seen in IMQ-induced cells or the levels were mitigated in RSQ-induced cells. As expected, treatment of induced cells with dexamethasone alleviated the changes observed in cytokine profiles as a consequence of TLR7/8 induction.
Interestingly, when the effect of AYUSH-64 herbs was explored with respect to their efficacy in mitigating IMQ/RSQ-induced inflammatory response, all 4 extracts showed an effect on most of the investigated inflammatory mediators. Particularly, a concentration- dependent response relationship on TNF-α cytokine was seen in cells treated with all AYUSH-64 herbs. The potential significance of TNF-α inhibitors is advocated in managing inflammation and prevention of excessive cytokine release associated with the immuno-pathogenesis of infection [66]. Likewise, a corrective effect on IMQ-RSQ-induced IL-6 cytokine was also seen. Our results support similar findings reported previously. Indole alkaloids of AS showed down-regulation of IL-6 and a balance of antioxidants. In bronchoalveolar lavage fluid and lung, total alkaloids, extracted from AS leaves by ethyl acetate, inhibited the production of TNF- α and IL-8 [21,67]. Picroliv, a PK derivative, showed a significant inhibition in the expression of IL-1β, and TNF-α in dextran sulfate sodium-induced mice which led to the pathogenesis of chronic inflammatory bowel disease and ulcerative colitis [68]. PK biopolymeric fraction RLJ-NE-205 showed improvement in the mouse immune system by increasing the proliferation of lymphocytes and cytokines levels of IL-4 and IFN-gamma in serum, CD4/CD8 population and phagocytic index [58]. Significant anti- inflammatory activity against oedema of a xanthone derivative (1,5-dihydroxy-3,8-dimethoxy xanthone) of SC was reported in a rat experimental model [69]. Similarly, gene expression of pro- inflammatory cytokines IL-1β, TNF-α, and IL-6 were significantly diminished in rat brain tissue while investigating its relevance for Alzheimer’s disease [70].
Our study clearly submits promising prophylactic efficacy of all the investigated herbal constituents of AYUSH-64 formulation in TLR7/8-induced macrophage-like cells. We not only concur with earlier studies supporting the role of the investigated herbs for their efficacy against inflammation but also provide support for the therapeutic use of AYUSH-64 as a formulation in inflammatory disorders [26,36]. We say this as all the investigated herbal extracts show comparable or even better prophylactic efficacy than the AYUSH-64 formulation to ameliorate TLR7/8- induced inflammatory response. Among the four herbs, Picrorhiza kurroa stands out as it showed concentration-dependent response relationship with alleviation of TLR7/8-induced TNF-α and IL-6 cytokines at both transcriptional stages as well as its secreted levels in the culture supernatant. Also, a similar concentration- dependent effect of PK on TLR7/8-induced cells was seen regarding the mitigation of IL-1β and IL-10 gene expression. Bioactive components such as picrosides I and II, apocynin and cucurbitacins from rhizome extracts of P. kurroa have earlier shown anticancer activity [71-75]. Its anti-inflammatory effects on granuloma formation caused by cotton pellet implantation and paw edema were tested and shown to be induced by carrageenan. Subsequent dose-dependent suppression was demonstrated in rats pre-treated with P. kurroa. Reduced levels of inflammatory cytokines (TNF-α, IL-1β, IL-6) accompanied by increased anti-inflammatory cytokine (IL-10) in the serum and peritoneal macrophages was reported. The herbal extract is also known to inhibit the proliferation of macrophages, neutrophils, and mast cells. Authors suggested that P. kurroa manifested its effect through the modulation of NF-κB signaling [24].
Conclusion
Our observations unequivocally demonstrate that all the herbal constituents of AYUSH-64 have no detrimental effects on the macrophage-like cells. Additionally, when these cells were subjected to prophylactic treatment with the aqueous extracts of these herbs, they exhibited promising efficacy against TLR7/8-mediated inflammatory pathology. Notably, Picrorhiza kurroa exhibited a concentration-dependent response with most of the investigated cytokines, suggesting its potential both as an individual herb and as a valuable component for the development of new and improved formulations, such as AYUSH-64.
The observed anti-inflammatory effects of individual herbs contribute to a synergistic effect that is reflected in the overall efficacy of the AYUSH-64 formulation. However, it is essential to exercise caution when interpreting our findings and avoid extrapolating them beyond the scope of our study design. To gain deeper insights into their effectiveness, further investigation of these herbal extracts in animal models is warranted, allowing for a more comprehensive comparison of their efficacy with the combined formulation, AYUSH-64.
Acknowledgments
The authors are thankful to the National Medicinal Plants Board (NMPB), Ministry of AYUSH, Government of India who provided Herbal extracts and Formulations for this study. The authors thank the Executive Director of THSTI for his generous overall support.
Author's Contributions
Manisha Dagar and Kamala Priya performed the in-vitro cell-based experiments as well as wrote the first draft of the manuscript. Ajay Kumar and Madhu Dikshit conceptualized the overall study design, interpretations, writing, and editing of the manuscript. All authors have read and accepted the manuscript.
Manisha Dagar and Kamala Priya contributed equally and are therefore sharing first authorship.
Funding
Authors express their gratitude to the Ministry of AYUSH and the Department of Biotechnology (DBT), Government of India for jointly funding the research work elaborated in this manuscript (Grant Nos.: BT/PR40738/TRM/120/486/2020 and A.11019/03/2020-NMPB-IV-A). Madhu Dikshit acknowledges the financial support from JBR/2020/000034.
Availability and Data Materials
All data are included within the article. Any additional specific information may be made available if desired.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Institutional Biosafety Committee (IBSC) at THSTI (IBSC Approval No 221/2020) after a brief explanation of the study objectives and protocols. All experimental protocols were approved and carried out in accordance with relevant guidelines and regulations. Consent to participate is not applicable in this study.
Competing interests
The authors declare no competing interests.
References
- Himmerich H, Patsalos O, Lichtblau N, Ibrahim MAA, Dalton B. Cytokine research in depression: principles, challenges, and open questions. Front Psychiatry. 2019;10:30.
[Crossref] [Google Scholar] [PubMed]
- Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, et al. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75(2):185-94.
[Crossref] [Google Scholar] [PubMed]
- Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8-13.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Shen C, Li J, Yuan J, Wei J, Huang F, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020 Jul;146(1):119-127.e4.
[Crossref] [Google Scholar] [PubMed]
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16-32.
[Crossref] [Google Scholar] [PubMed]
- Dyavar SR, Singh R, Emani R, Pawar GP, Chaudhari VD, Podany AT, et al. Role of toll-like receptor 7/8 pathways in regulation of interferon response and inflammatory mediators during SARS-CoV2 infection and potential therapeutic options. Biomed Pharmacother. 2021;141:111794.
[Crossref] [Google Scholar] [PubMed]
- Khanmohammadi S, Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93(5):2735-2739.
[Crossref] [Google Scholar] [PubMed]
- Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: Immunology and treatment options. Clin Immunol. 2020 Jun;215:108448.
[Crossref] [Google Scholar] [PubMed]
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev. 2020;19(7):102569.
[Crossref] [Google Scholar] [PubMed]
- Colafrancesco S, Scrivo R, Barbati C, Conti F, Priori R. Targeting the immune system for pulmonary inflammation and cardiovascular complications in COVID-19 patients. Front Immunol. 2020;11:1439.
[Crossref] [Google Scholar] [PubMed]
- Atal S, Fatima Z. IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharmaceut Med. 2020 Aug;34(4):223-231.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Rai A, Khan MS, Kumar A, Haque ZU, Fazil M, et al. Role of herbal medicines in the management of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. J Tradit Complement Med. 2022 Jan;12(1):100-113.
[Crossref] [Google Scholar] [PubMed]
- Demeke CA, Woldeyohanins AE, Kifle ZD. Herbal medicine use for the management of COVID-19: A review article. Metabol Open. 2021 Dec;12:100141.
[Crossref] [Google Scholar] [PubMed]
- Grigore A. Plant phenolic compounds as immunomodulatory agents. 2017.
- Cena H, Chieppa M. Coronavirus disease (COVID-19-SARS-CoV-2) and nutrition: is infection in Italy suggesting a connection? Front Immunol. 2020 May 7;11:944.
[Crossref] [Google Scholar] [PubMed]
- Risberg K, Fodstad Ø, Andersson Y. Synergistic anticancer effects of the 9.2.27PE immunotoxin and ABT-737 in melanoma. PLoS One. 2011;6(9):e24012.
[Crossref] [Google Scholar] [PubMed]
- Chopra A, Chavan-Gautam P, Tillu G, Saluja M, Borse S, Lakdawala M, et al. Co-administration of AYUSH 64 as an adjunct to Standard of Care in mild and moderate COVID-19: A randomised, controlled, multicentric clinical trial. PLoS One. 2023;18(3):e0282688.
[Crossref] [Google Scholar] [PubMed]
- Reddy RG, Gosavi RV, Yadav B, Rai AK, Holay MP, et al. AYUSH- 64 as an add-on to standard care in asymptomatic and mild cases of COVID- 19: a randomized controlled trial. Ayu. 2020;41(2):107-116.
[Crossref] [Google Scholar] [PubMed]
- Singh H, Srivastava S, Yadav B, Rai AK, Jameela S, Sharma BS, et al. AYUSH-64 as an adjunct to standard care in mild to moderate COVID-19: An open-label randomized controlled trial in Chandigarh, India. Complement Ther Med. 2022;66:102814.
[Crossref] [Google Scholar] [PubMed]
- Zhao YL, Shang JH, Pu SB, Wang HS, Wang B, Liu L, et al. Effect of total alkaloids from Alstonia scholaris on airway inflammation in rats. J Ethnopharmacol. 2016 Feb 3;178:258-65.
[Crossref] [Google Scholar] [PubMed]
- Hu TY, Ju JM, Mo LH, Ma L, Hu WH, You RR, et al. Anti-inflammation action of xanthones from Swertia chirayita by regulating COX-2/NF-κB/MAPKs/Akt signaling pathways in RAW 264.7 macrophage cells. Phytomedicine. 2019;55:214-221.
[Crossref] [Google Scholar] [PubMed]
- Shukla S, Mehta A, Mehta P, Vyas SP, Shukla S, Bajpai VK. Studies on anti- inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food Chem Toxicol. 2010;48(1):61-4.
[Crossref] [Google Scholar] [PubMed]
- Kumar R, Gupta YK, Singh S, Raj A. Anti-inflammatory Effect of Picrorhiza kurroa in Experimental Models of Inflammation. Planta Med. 2016 Nov;82(16):1403-1409.
[Crossref] [Google Scholar] [PubMed]
- Panda AK, Kar S, Rai AK, Rao BCS, Srikanth N. AYUSH- 64: A potential therapeutic agent in COVID-19. J Ayurveda Integr Med. 2022 Apr-Jun;13(2):100538.
[Crossref] [Google Scholar] [PubMed]
- Dagar M, Priya K, Dikshit M, Kumar A. Pharmacological evaluations of select herbal extracts on TLR7/8-induced cytokine and chemokine production in macrophage-like cells. J Clin Exp Pharma. 2023; 13(4);1000374.
- Hurst J, Prinz N, Lorenz M, Bauer S, Chapman J, Lackner KJ, et al. TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells. Immunobiology. 2009;214(8):683-91.
[Crossref] [Google Scholar] [PubMed]
- Zagon IS, Donahue RN, Rogosnitzky M, McLaughlin PJ. Imiquimod upregulates the opioid growth factor receptor to inhibit cell proliferation independent of immune function. Exp Biol Med. 2008;233(8):968-79.
[Crossref] [Google Scholar] [PubMed]
- Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018 Aug;2(8):578-588.
[Crossref] [Google Scholar] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19-preliminary report. N Engl J Med. 2020; 384:693-704.
[Crossref] [Google Scholar] [PubMed]
- Subraya CK, Gupta D. Antioxidant anti-inflammatory activity of Alstonia scholaris R.Br. stem bark extract. Free Rad Ant. 2022;2(2): 55-57.
- Singh H, Arora R, Arora S, Singh B. Ameliorative potential of Alstonia scholaris (Linn.) R. Br. against chronic constriction injury-induced neuropathic pain in rats. BMC Complement Altern Med. 2017 Jan 19;17(1):63.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Zhang CJ, Zhang DB, Wen J, Zhao XW, Li Y, et al. An unusual indole alkaloid with anti-adenovirus and anti-HSV activities from Alstonia scholaris. Tetrahedron Lett. 2014; 55(5): 1815–1817.
- Bharat G, Farzin P. Evaluation of acetone extract of three Indian medicinal plants for schizonticidal properties in Plasmodium falciparum. Int J Pharm Tech. 2011;3(1):1373-84.
- Zhou HX, Li RF, Wang YF, Shen LH, Cai LH, Weng YC, et al. Total alkaloids from Alstonia scholaris inhibit influenza a virus replication and lung immunopathology by regulating the innate immune response. Phytomedicine. 2020;77:153272.
[Crossref] [Google Scholar] [PubMed]
- Rizvi ZA, Madan U, Tripathy MR, Goswami S, Mani S, Awasthi A, et al. Evaluation of Ayush-64 (a polyherbal formulation) and its ingredients in a Syrian hamster model for SARS-CoV-2 infection reveals the preventative potential of Alstonia scholaris. Pharmaceuticals. 2023;16(9):1333.
[Crossref] [Google Scholar] [PubMed]
- Dwivedi Y, Rastogi R, Garg NK, Dhawan BN. Picroliv and its components kutkoside and picroside i protect liver against galactosamine-induced damage in rats. Pharmacol Toxicol. 1992 Nov;71(5):383-7.
[Crossref] [Google Scholar] [PubMed]
- Luper S. A review of plants used in the treatment of liver disease: Part two. Altern Med Rev. 1999;4(3):178-88.
[Google Scholar] [PubMed]
- Sood H, Chauhan RS. Biosynthesis and Accumulation of a medicinal compound, Picroside-I, in cultures of Picrorhiza Kurroa royle ex benth. Plant Cell Tiss Organ Cult. 2010; 100: 113–117.
- Bhandari P, Kumar N, Singh B, Ahuja PS. Online HPLC-DPPH method for antioxidant activity of Picrorhiza kurroa Royle Ex Benth. and Characterization of Kutkoside by Ultra-Performance LC-Electrospray Ionization Quadrupole time- of-flight mass spectrometry. Indian J Exp Biol. 2010 Mar;48(3):323-8.
[Google Scholar] [PubMed]
- Das SC, Bhadra S, Roy S, Saha SK, Islam MS, Bachar SC. Analgesic and anti-inflammatory activities of ethanolic root extract of Swertia chirata (Gentianaceae). Jordan J Biol Sci. 2012; 5(1): 31-36.
- Maurya VK, Kumar S, Bhatt MLB, Saxena SK. Antiviral activity of traditional medicinal plants from Ayurveda against SARS-CoV-2 infection. J Biomol Struct Dyn. 2022;40(4):1719-1735.
[Crossref] [Google Scholar] [PubMed]
- Rastogi R, Singh B, Singhal A, Singh LR, Singh R. An analytical study of kalmegh (Andrographis Paniculata) and kiratikta (Swertia chirayita): an economic substitution for metro cities in global pandemic with healthcare 4.0. J Nat Ayu Med. 2022;6(2):000346.
[Crossref]
- Ramesh BN, Girish TK, Raghavendra RH, Naidu KA, Rao UJSP, Rao KS. Comparative study on anti-oxidant and anti-inflammatory activities of Caesalpinia crista and Centella asiatica leaf extracts. Pharm Bioallied Sci. 2014 Apr;6(2):86-91.
[Crossref] [Google Scholar] [PubMed]
- Nesari T, Kajaria D. Combating COVID-19 with holistic approach of Ayurveda. Indian J Trad Know 2020;19: 37-46.
- Chan EWC, Tangah J, Baba S, Chan HT, Kainuma M, Inoue T. Caesalpinia crista: A coastal woody climber with promising therapeutic values. J App Pharm Sci.2018;8(3): 133-140.
- Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal anti-inflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16(5):821-47.
[Crossref] [Google Scholar] [PubMed]
- Stanbury RM, Graham EM. Systemic corticosteroid therapy side effects and their management. Br J Ophthalmol. 1998;82(6):704-8.
[Crossref] [Google Scholar] [PubMed]
- Wiseman AC. Immunosuppressive medications. Clin J Am Soc Nephrol. 2016 Feb 5;11(2):332-43.
[Crossref] [Google Scholar] [PubMed]
- Mathew L, Babu S. Phytotherapy in India: Transition of tradition to technology. Curr Bot. 2011;2(5): 17–22.
- Furst R, Zundorf I. Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediators Inflamm. 2014;2014:146832.
[Crossref] [Google Scholar] [PubMed]
- Kazemi S, Shirzad H, Rafieian-Kopaei M. Recent findings in molecular basis of inflammation and anti-inflammatory plants. Curr Pharm Des. 2018;24(14):1551-1562.
[Crossref] [Google Scholar] [PubMed]
- Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H. Medicinal plants: Past history and future perspective. J Herbmed Pharm. 2018;7(1):1–7.
- Nasri H, Shirzad H. Toxicity and safety of medicinal plants. J HerbMed Plarmacol. 2013 May;2(2):21-2. [Crossref]
- Meena AK, Bansal P, Kumar S. Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J Tradi Med 2009;4(4): 152–70.
- Che CT, Wang ZJ, Chow MSS, Lam CWK. Herb-herb combination for therapeutic enhancement and advancement: Theory, practice and future perspectives. Molecules. 2013;18(5):5125-41.
[Crossref] [Google Scholar] [PubMed]
- Sarwar M, Attitalla IH, Abdollahi M. A review on the recent advances in pharmacological studies on medicinal plants: Animal studies are done but clinical studies needs completing. Asian J Ani Vet Adv 2011;6(8): 867-883.
- Kavitha AN, Deepthi V, Nayeem N. Design, formulation and evaluation of a polyherbal ointment for its wound healing activity. Pharmacophore 2013;4 (5): 175-180.
- Devipriya S, Ramesh NV, Vineeth PK, Mohanan A. A review on the inextricable relation of Ayurveda and Analytical chemistry. Mater Today Proc. 2021;46:3089-3095.
- Gupta A, Khajuria A, Singh J, Bedi KL, Satti NK, Dutt P, et al. Immunomodulatory activity of biopolymeric fraction RLJ-NE-205 from Picrorhiza kurroa. Inter Immunopharmacol. 2006;6 (10): 1543–1549.
[Crossref] [Google Scholar] [PubMed]
- Ram TS, Munikumar M, Raju VN, Devaraj P, Boiroju NK, Hemalatha R, et al. In silico evaluation of the compounds of the ayurvedic drug, AYUSH-64, for the action against the SARS-CoV-2 main protease. J Ayurveda Integr Med. 2022;13(1):100413.
[Crossref] [Google Scholar] [PubMed]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003 Feb;73(2):209-12.
[Crossref] [Google Scholar] [PubMed]
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537-45.
[Crossref] [Google Scholar] [PubMed]
- Shibata H, Yoshioka Y, Abe Y, Ohkawa A, Nomura T, Minowa K, et al. The treatment of established murine collagen-induced arthritis with a TNFR1-selective antagonistic mutant TNF. Biomaterials. 2009;30(34):6638-47.
[Crossref] [Google Scholar] [PubMed]
- Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2(3):189-202.
[Crossref] [Google Scholar] [PubMed]
- Guo Y, Hu K, Li Y, Lu C, Ling, K, Cai C, et al. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front Public Health. 2022 Feb 11;10:833967.
[Crossref] [Google Scholar] [PubMed]
- Zhao YL, Yang ZF, Shang JH, Huang WY, Wang B, et al. Effects of indole alkaloids from leaf of Alstonia scholaris on post-infectious cough in mice. Journal of Ethnopharmacology 218 (2018): 69-75.
[Crossref] [Google Scholar] [PubMed]
- Zhang DK, Yu JJ, Li YM, Wei LN, Yu Y, Feng YH, et al. APicrorhiza kurroa derivative, picroliv, attenuates the development of dextran-sulfate-sodium- induced colitis in mice. Mediators Inflamm. 2012;2012:751629.
[Crossref] [Google Scholar] [PubMed]
- Banerjee S, Kumar ST, Mandal S, Chandra DP, Sikdar S. Assessment of the anti-inflammatory effects of Swertia chirata in acute and chronic experimental models in male albino rats. Indian J Pharmacol. 2000;32 (1): 21-24.
- Ravi SK, Ramesh BN, Mundugaru R, Vincent B. Multiple pharmacological activities of Caesalpinia crista against aluminium-induced neurodegeneration in rats: Relevance for Alzheimer’s disease. Environ Toxicol Pharmacol. 2018;58:202-211.
[Crossref] [Google Scholar] [PubMed]
- Zhu JSS, Ouyang DYY, Shi ZJJ, Xu LHH, Zhang YTT, He XHH. Cucurbitacin B induces cell cycle arrest, apoptosis and autophagy associated with g actin reduction and persistent activation of cofilin in jurkat cells. Pharmacology. 2012;89(5-6):348-6.
[Crossref] [Google Scholar] [PubMed]
- Kong Y, Chen J, Zhou Z, Xia H, Qiu MH, Chen C. Cucurbitacin e Induces Cell Cycle G2/M Phase Arrest and Apoptosis in Triple Negative Breast Cancer. PLoS One. 2014;9(7):e103760.
[Crossref] [Google Scholar] [PubMed]
- Duangmano S, Saelim P, Suksamrarn A, Domann FE, Patmasiriwat P. Cucurbitacin B inhibits human breast cancer cell proliferation through disruption of microtubule polymerization and nucleophosmin/b23 translocation. BMC Complement Altern Med. 2012 Oct 12;12:185.
[Crossref] [Google Scholar] [PubMed]
- Aribi A, Gery S, Lee DH, Thoennissen NH, Thoennissen GB, Alvarez R, et al. The triterpenoid cucurbitacin b augments the antiproliferative activity of chemotherapy in human breast cancer. Int J Cancer. 2013 Jun 15;132(12):2730-7.
[Crossref] [Google Scholar] [PubMed]
- Alghasham AA. Cucurbitacins: A promising target for cancer therapy. Int J Health Sci (Qassim). 2013;7(1):77-89.
[Crossref] [Google Scholar] [PubMed]
Citation: Priya K, Dagar M, Dikshit M, Kumar A (2023) Herbal Constituents of AYUSH-64 Formulation Modulate Release of Cytokines in TLR7/8- Induced Macrophage-Like Cells. J Clin Exp Pharmacol. 13:394.
Copyright: © 2023 Priya K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : Authors express their gratitude to the Ministry of AYUSH and the Department of Biotechnology (DBT), Government of India for jointly funding the research work elaborated in this manuscript (Grant Nos.: BT/PR40738/TRM/120/486/2020 and A.11019/03/2020-NMPB-IV-A). Madhu Dikshit acknowledges the financial support from JBR/2020/000034

