Indexed In
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 13, Issue 4
Pharmacological Evaluations of Select Herbal Extracts on TLR7/8-induced Cytokine and Chemokine Production in Macrophage-like Cells
Manisha Dagar1,2, Kamala Priya1, Madhu Dikshit1,3* and Ajay Kumar1*2Department of Ophthalmology, University of California San Diego, San Diego, CA, USA
3Division of Pharmacology, CSIR-Central Drug Research Institute, Lucknow, Uttar Pradesh, India
Received: 08-Aug-2023, Manuscript No. CPECR-23-22514; Editor assigned: 10-Aug-2023, Pre QC No. CPECR-23-22514 (PQ); Reviewed: 24-Aug-2023, QC No. CPECR-23-22514; Revised: 31-Aug-2023, Manuscript No. CPECR-23-22514(R); Published: 07-Sep-2023, DOI: 10.35248/2161-1459.23.13.374
Abstract
Inflammation is an innate immune response triggered by harmful stimuli, such as pathogens, tissue injury, or toxins. The purpose is to eliminate the source of infection and initiate the healing process. However, an excessive acute inflammatory response can lead to severe and life-threatening complications, as seen during recent pandemics. In the context of viral infections, the activation of the TLR7/8 signaling pathway has been implicated in excessive cytokine secretion. In this study, we aimed to replicate the perturbed inflammatory environment resulting from the activation of the TLR7/8 specific agonists, imiquimod, and resiquimod.
We utilized macrophage-like cells, as macrophages are the first responders during infections and secrete key pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) to recruit immune cells to the site of infection. Herbal medicines have been traditionally used for centuries to enhance respiratory immune function. In the present study, we employed a prophylactic approach, where macrophage-like THP1 cells, differentiated with PMA, were pre-treated with select herbal extracts/formulations prior to TLR7/8 activation in the presence of agonists.
Several medicinal plants and formulations known for their therapeutic potential in respiratory ailments were investigated, including Withania somnifera, Tinospora cordifolia, Glycyrrhiza glabra, and AYUSH-64, an herbal formulation. The gene expression and corresponding secreted levels of various inflammatory mediators were measured using RT-PCR and ELISA methods, respectively, in treated and untreated differentiated THP1 cells induced with TLR7/8 agonists. Comparatively, the gene expression of inflammatory markers was significantly higher in resiquimod-induced cells than in imiquimod-treated cells. Notably, Withania somnifera demonstrated pronounced prophylactic efficacy compared to other herbs/formulations, as evidenced by reduction in expression of majority of investigated inflammatory marker genes.
Keywords
Imiquimod; Resiquimod; Aqueous herbal extracts; Withania somnifera; Inflammation; THP1 cells
Introduction
Excessive, acute, and uncontrolled release of pro-inflammatory cytokines into circulation during the recent COVID-19 pandemic resulted in severe life-threatening complications and even death in many cases. Such a storm of cytokines may lead to an increased flux of immune cells (macrophages, neutrophils, and T cells) to the site of infection eventually causing more damage than repair [1]. Herbal therapies have been used in traditional medicine for centuries and are also being explored for their potential benefits in cytokine storm management. While the research on specific herbal treatments for cytokine storm is limited, some herbs have shown immunomodulatory and anti-inflammatory properties that may help to alleviate the excessive immune response.
Administration of TLR7/8 agonists, such as imiquimod or resiquimod, has been associated with the development of cytokine storm [2,3]. Among others, TNF-α, IL-6, and IL-1β are the key pro-inflammatory cytokines which drive the excessive secretion of cytokines. Their increased levels are often correlated with the severity of cytokine storm which may eventually lead to tissue damage and organ dysfunction [4-6]. TNF-α has been correlated to pro-inflammatory response mediated by IL-1β and IL-6. It is found to be involved in the regulation of inflammatory responses, infectious diseases, and malignant tumors [7]. Higher levels of TNF-α were found in severe COVID-19 patients [4]. Among other immune cells, monocytes and macrophages are the main source of these pro-inflammatory cytokines [8]. Human THP1 cells are monocyte-derived macrophage-like cells which mimic the macrophage functions and therefore are routinely used in in-vitro cell models in basic research. Such cell-based models were instrumental during the recent pandemic where they not only helped in disease understanding but were also the first line preclinical models to screen a large number of drug candidates for their efficacy and safety. THP1 cells differentiate into macrophage-like cells when incubated in the presence of PMA. TLR7/8 specific signaling was induced in the presence of specific agonists and measured in terms of gene expression as well as circulating levels of various inflammatory genes.
We have evaluated a few select herbal extracts that have traditionally been used over centuries in therapeutics to cure different infectious as well as immunocompromised conditions. A similar line of investigations has been reported with other herbs or herbal oils using different disease models [9,10]. Syzygium aromaticum, Piper nigrum, Cimicifuga racemosa, Zanthoxylum asiaticum, Camellia sinensis, Curcuma longa, and Tribulus terrestris were found to be effective in COVID-19 therapeutics [11]. Similarly, the use of oil formulations such as Anu oil and Til tailya in the management of SARS-CoV-2 was explored where the authors observed a significant reduction in viral load as an effect of treatment with Anu oil [10].
In the present investigation, we have specifically examined the efficacy of Withania somnifera, Tinospora cordifolia, Glycyrrhiza glabra, and AYUSH-64 in mitigating the inflammatory response triggered by TLR-7/8 induction in the presence of the ligands imiquimod and resiquimod. While the first three are individual herbs, AYUSH-64 is an herbal formulation comprising a combination of four different herbs (Alstonia scholaris, Caesalpinia crista, Picrorhiza kurroa and Swertia chirata), each contributing to the overall therapeutic benefits. To assess the prophylactic effects of these herbs/formulations, we employed a macrophage-like cell line model. Our objective was to identify the herb or formulation that exhibited the highest efficacy in either suppressing the TLR7/8- induced inflammatory response by promoting an anti-inflammatory environment. We evaluated various inflammatory gene markers, including cytokines and chemokines, both at the gene expression level and their secreted levels in the supernatant. To analyse gene expression, we extracted RNA from the cell lysates and employed the RT-PCR method. Simultaneously, we measured the secreted cytokine levels in the culture supernatant using the ELISA method. These analyses enabled us to determine the impact of the tested herbs/formulations on the expression of inflammatory genes and the corresponding secretion of cytokines.
Methodology
Cell culture
THP1 is a spontaneously immortalized monocyte-like cell line, derived from the peripheral blood of a child suffering from acute monocytic leukemia [12]. THP1 cell line was purchased from ATCC (ATCC#TFB-202) and cultured as per ATCC recommendations. Briefly, RPMI 1640 (Gibco) supplemented with 10% FCS (Hyclone) and 1X penicillin (stock concentration=100 U/mL), and streptomycin (stock concentration=100 μg/mL) were used to culture the cell line. Cells were maintained in complete media at 37°C in humidified conditions with 5% CO2. Trypan blue was used to confirm cell viability. THP1 cells were seeded in the presence of PMA (5 ng/mL) and allowed to differentiate for 48 hours and subsequently used.
Preparation of the herbal extracts/formulations
Powdered form of well-characterized herbal extracts- W. somnifera, T. cordifolia, G. glabra and A64, prepared under the GMP conditions, were obtained from the National Medicinal Plants Board (NMPB), Ministry of AYUSH, Government of India. A stock solution of 4 mg/mL was prepared in the RPMI medium [13]. RPMI medium (1 mL) was added to 4 mg of each extract/formulation powder and kept in dark for overnight shaking at room temperature. Subsequently, the extract solutions were centrifuged at 10,000 rpm for 10 minutes and the supernatant was passed through a 0.22 μm syringe filter in sterile environment. This supernatant was assumed to be an absolute concentrate of the herbal extract and was used for investigations in this study. All stocks were prepared fresh for each experiment and the filtered stocks were saved at 4°C in dark.
Cell viability assay
Cell viability in the presence and absence of herbal extracts/ formulations was evaluated by MTT assay. Differentiated THP1 cells were treated with various concentrations of herbal extracts (100 μg/ mL, 300 μg/mL, and 1000 μg/mL). After 24 hours of treatment, cells were incubated in dark with 0.5 mg/mL MTT (Sigma#M2128) for 4 hours at 37°C. The culture supernatant was gently removed and replaced with 100 μL DMSO per well (Sigma#276855). The cells were allowed to incubate in dark at RT with constant shaking for 30 minutes. Subsequently, the absorbance was measured with a microplate reader (Molecular Devices, Spectramax M2) at 570 nm wavelength. Dexamethasone (Selleck Chemicals#S1322) (1 μM) was used as an internal reference. Latter is a steroidal drug with powerful anti-inflammatory properties.
qPCR to study gene expression of inflammatory mediators
Differentiated THP1 cells (1 million cells/well), seeded in a 6-well plate, were pre-treated for 60 minutes with herbal extracts/ formulations at different concentrations (3 μg/mL, 10 μg/ mL, 30 μg/mL, 100 μg/mL, and 300 μg/mL). Subsequently, TLR7/8 agonists (Imiquimod (Tocris#3700/50)/Resiquimod (Sigma#SML1096), respectively) were added to induce TLR signaling. Post induction, the cells, and culture supernatants were collected separately. Total RNA was extracted using a Trizol reagent (Sigma#T9424). Qualitative and quantitative RNA analysis was done on a Multiskan GO plate reader (Thermo Scientific). Reverse transcription of 250 ng RNA was done using the high-capacity cDNA reverse transcription kit (Applied Biosystems#4368814). For the real-time quantitative PCR, the cDNA was amplified using Power up SYBR Green mix (Applied Biosystems#A25742) following the standard protocol for the dye on QuantStudio 6 Real- Time PCR System (Applied Biosystems). Cycling conditions were UDG activation at 50°C for 2 minutes, Hold Dual-Lock™ DNA polymerase at 95°C for 2 minutes, and 40 cycles of Denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute each. For each reaction 300 nM of forward and reverse primer mix was used (Table 1). The relative mRNA expression data were analysed using the ΔΔCt method and the values were normalized to beta-actin mRNA levels. The data was expressed with respect to the fold change as compared to the untreated control.
| S.No. | Primer | Sequence |
|---|---|---|
| 1. | b-Actin | Forward: 5'-AGAGCTAGGAGCTGCCTGAC-3' Reverse: 5'-AGCACTGTGTTGGCGTACAG-3' |
| 2. | TNF-a | Forward: 5'-TGGGATCATTGCCCTCTTGAG-3' Reverse: 5'-TCTAAGCTTGGGTTCCGACC-3' |
| 3. | MCP1 | Forward: 5'-ACTGCACTCCTGGTTGTCCT-3' Reverse: 5'-CGGCACAGATCTCCTTATCC-3' |
| 4. | IL-6 | Forward: 5'-TACCCCCAGGAGAAGATTCC-3' Reverse: 5'-TTTTCTGCCAGTGCCTCTTT-3' |
| 5. | IL-8 | Forward: 5'-TCTGCAGCTCTGTGTGAAGG-3' Reverse: 5'-AATTTCTGTGTTGGCGCAGT-3' |
| 6. | CCL-5 | Forward: 5'-CGCTGTCATCCTCATTGCTA-3' Reverse: 5'-GAGCACTTGCCACTGGTGTA-3' |
| 7. | IL-10 | Forward: 5'-TGCCTTCAGCAGAGTGAAGA-3' Reverse: 5'-GGTCTTGGTTCTCAGCTTGG-3' |
| 8. | IL-1b | Forward: 5'-GGGCCTCAAGGAAAAGAATC-3' Reverse: 5'-TTCTGCTTGAGAGGTGCTGA-3' |
| 9. | CXCL-10 | Forward: 5'-CTGTACGCTGTACCTGCATCA-3' Reverse: 5'-TTCTTGATGGCCTTCGATT-3' |
Table 1: Human specific sequences of Forward and Reverse primers for various inflammatory genes
Cytokine measurement in the culture supernatants
Inflammatory cytokines/chemokines levels were determined in the culture supernatant using ELISA kits as per the manufacturer’s instructions (Invitrogen, Thermofisher Scientific). Sample dilutions were calibrated for respective analyte separately prior to analysing test samples. The reaction fluorescence was measured at 450 nm and 570 nm wavelength (Molecular devices, Spectramax M2).
Statistical analysis
All experiments were carried out independently in triplicate. The statistical analysis was done by one-way ANOVA with the Tukey test for multiple comparisons and a two-tailed unpaired Student’s t-test for comparisons between two groups on GraphPad Prism (Version 9). Data are expressed as mean ± SEM. A p-value of less than 0.05 between the compared groups was considered statistically significant.
Results
Given the pressing need for immunosuppressants derived from plants, which offer favorable safety profiles and the ability to regulate the inflammatory response without compromising overall immune function, our study aims to evaluate the efficacy of three herbal extracts (W. somnifera, T. cordifolia, G. glabra) and an herbal formulation (A-64) in restoring the dysregulated inflammatory environment within a TLR7/8-induced macrophage cell model. As a reference compound, dexamethasone was included to assess the sensitivity of our model. In our experimental approach, macrophage- like cells were differentiated and pre-treated with aqueous extracts of the selected herbs at various concentrations (ranging from 3 μg/mL to 300 μg/mL) prior to TLR induction using either IMQ or RSQ. The induced cells exhibited a significant increase in the expression of inflammatory markers. Conversely, the cells treated with dexamethasone demonstrated improvement in the expression of all the investigated inflammatory genes. This investigation aims to shed light on the potential of these herbal extracts and formulation to modulate the dysregulated inflammatory response observed in our TLR7/8-induced macrophage cell model. By assessing their impact on the expression of inflammatory markers, we can gain insights into their efficacy as immunosuppressants. Notably, our study highlights the promising role of plant-derived compounds in restoring the balance of the inflammatory milieu without compromising the immune system’s ability to mount an appropriate response.
Titration of agonist concentration and treatment duration
To determine the appropriate dose and induction duration for herbal evaluation, inflammatory genes (TNF-α, MCP1, IL-6, IL-1β) were examined in cells and culture supernatants treated with TLR agonists (IMQ and RSQ). Various concentrations (1 μg/mL, 5 μg/mL, and 10 μg/mL) of agonists were tested in differentiated macrophage-like cells for 8, 16, 24, and 36 hours. IMQ induced TLR7/8 signaling after 8 hours, while RSQ showed induction after 24 hours. IMQ had maximum induction at 5 μg/mL, whereas RSQ induced signaling at 1 μg/mL. Thus, separate induction profiles were designed for herbals: 8 hours with 5 μg/mL IMQ and 24 hours with 1 μg/mL RSQ. TNF-α, MCP1, and IL-1β showed significant induction with 5 μg/mL IMQ after 8 hours, while IL-6 showed induction after 16 hours (Supplementary Figures 1 and 2).
Before studying the effect of herbals on TLR signaling, their impact on cell viability was assessed using MTT assay, both in the absence and presence of TLR7/8 agonists. Even at a high concentration of 1000 μg/mL, none of the herbs showed any harmful effects on cell viability (Supplementary Figure 3). Subsequently, the efficacy of selected herbal extracts in mitigating IMQ/RSQ-induced inflammatory responses in macrophage cells was investigated. The study design involved inducing TLR signaling with agonists, resulting in increased expression/circulating levels of inflammatory markers, and assessing the effect of the herbals on these markers.
Prophylactic effect of W. somnifera on TLR7/8-induced inflammatory markers
W. somnifera, commonly known as Ashwagandha, is an Indian shrub used in Ayurvedic medicine for holistic health benefits, stress and anxiety management, reproductive health support, physical endurance enhancement, and immunomodulation [9,14,15]. W. somnifera showed suppression of pro-inflammatory cytokine-induced Th1, Th2, and Th17 differentiation. It created an immunosuppressive environment in hamster and hACE2 transgenic mice models of COVID-19, suggesting potential efficacy against acute viral infections. W. somnifera had a more pronounced prophylactic effect in RSQ-induced cells compared to IMQ-induced cells (Figure 1). Increasing W. somnifera concentration significantly corrected the expression levels of inflammatory genes (Figure 1B). W. somnifera effectively mitigated TNF-α, CXCL10, and IL-1β expression in both IMQ and RSQ-induced macrophage-like cells (Figure 1A). Interestingly, W. somnifera treatment decreased IL- 10 levels in RSQ-induced cells but had no such effect after IMQ induction. W. somnifera also significantly reduced MCP1 and IL-6 levels in RSQ-induced cells (Figure 1B). ELISA results showed significant reduction of MCP1 levels in culture supernatants of W. somnifera -treated IMQ-induced cells across all concentrations (Figure 2A). Treatment with 300 μg/mL W. somnifera mitigated the effect of RSQ induction on IL6 and IL10 (Figure 2B). No correction in levels of TNF-α was observed in the supernatant of W. somnifera -treated cells induced with either agonist.
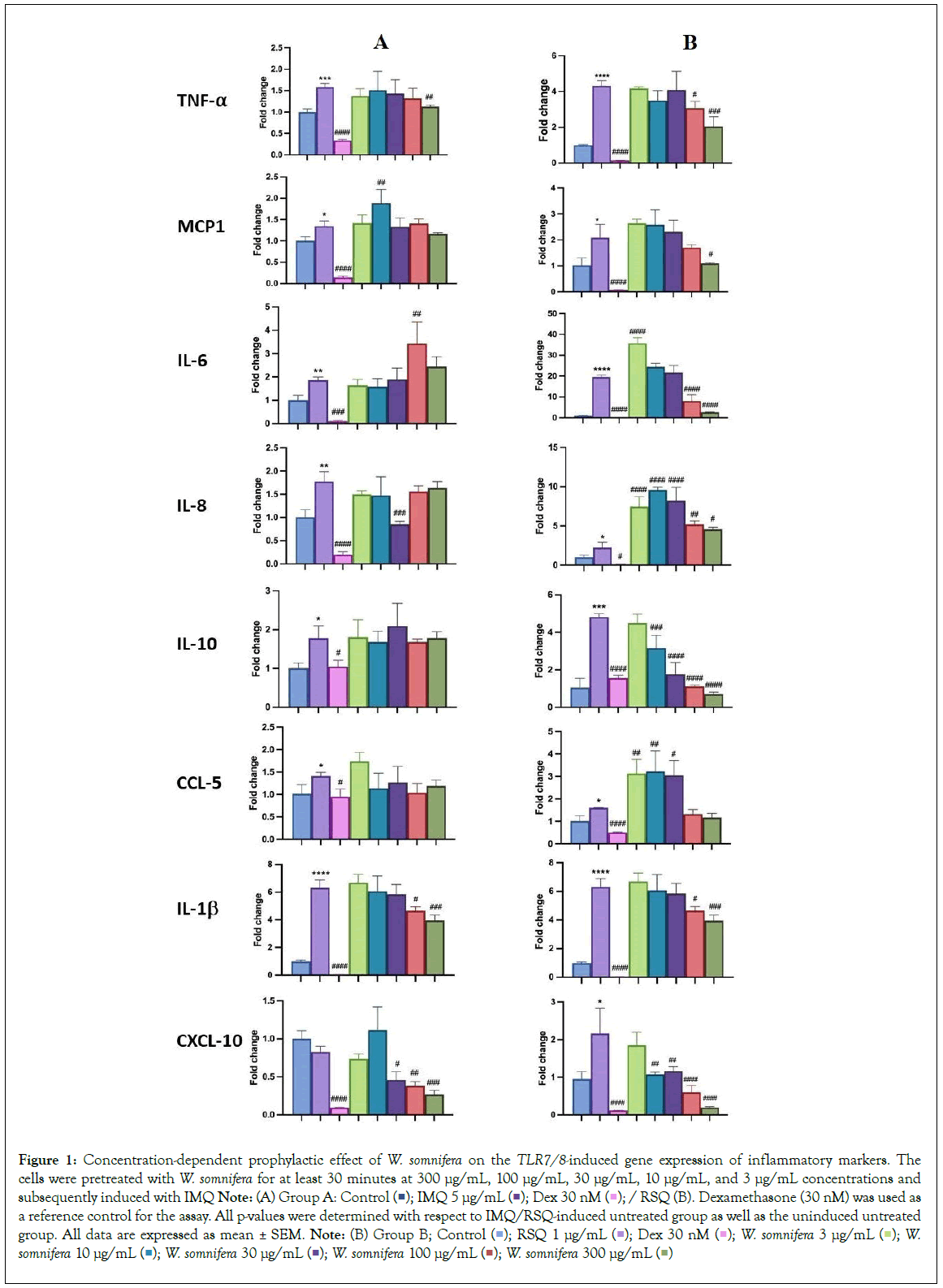
Figure 1: Concentration-dependent prophylactic effect of W. somnifera on the TLR7/8-induced gene expression of inflammatory markers. The
cells were pretreated with W. somnifera for at least 30 minutes at 300 μg/mL, 100 μg/mL, 30 μg/mL, 10 μg/mL, and 3 μg/mL concentrations and
subsequently induced with IMQ Note: (A) Group A: Control  / RSQ (B). Dexamethasone (30 nM) was used as a reference control for the assay. All p-values were determined with respect to IMQ/RSQ-induced untreated group as well as the uninduced untreated group. All data are expressed as mean ± SEM. Note: (B) Group B; Control
/ RSQ (B). Dexamethasone (30 nM) was used as a reference control for the assay. All p-values were determined with respect to IMQ/RSQ-induced untreated group as well as the uninduced untreated group. All data are expressed as mean ± SEM. Note: (B) Group B; Control  W. somnifera 10 μg/mL
W. somnifera 10 μg/mL 
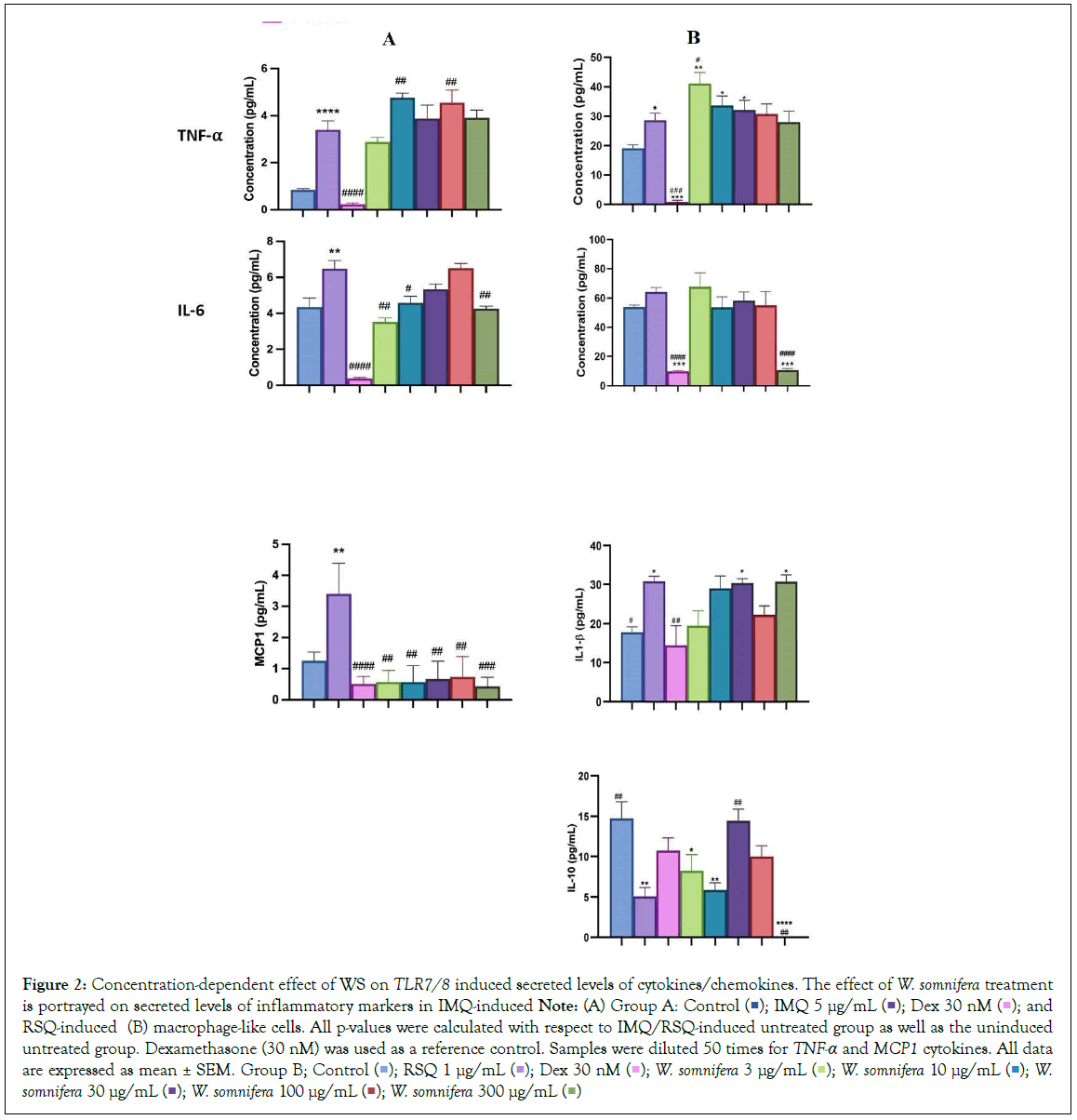
Figure 2: Concentration-dependent effect of WS on TLR7/8 induced secreted levels of cytokines/chemokines. The effect of W. somnifera treatment is portrayed on secreted levels of inflammatory markers in IMQ-induced Note: (A) Group A: Control  and RSQ-induced (B) macrophage-like cells. All p-values were calculated with respect to IMQ/RSQ-induced untreated group as well as the uninduced
untreated group. Dexamethasone (30 nM) was used as a reference control. Samples were diluted 50 times for TNF-α and MCP1 cytokines. All data are expressed as mean ± SEM. Group B; Control
and RSQ-induced (B) macrophage-like cells. All p-values were calculated with respect to IMQ/RSQ-induced untreated group as well as the uninduced
untreated group. Dexamethasone (30 nM) was used as a reference control. Samples were diluted 50 times for TNF-α and MCP1 cytokines. All data are expressed as mean ± SEM. Group B; Control  W. somnifera 30 μg/mL
W. somnifera 30 μg/mL 
TLR7/8-induced inflammatory markers in T. cordifolia pretreated cells
T. cordifolia, also known as Giloy or Guduchi, is a perennial shrub native to tropical regions of India and other neighboring countries. In Ayurveda, the stem, leaves, and roots of T. cordifolia have long been used for therapeutic purposes. It exhibits immunomodulatory properties, supporting the body’s defense against infection, and possesses antioxidant activity to reduce stress. Additionally, it offers anti-inflammatory, antipyretic, and hepatoprotective effects [16]. In silico studies suggest that its active phytoconstituents bind to RNA- dependent RNA polymerase [17].
The prophylactic effect of T. cordifolia on IMQ/RSQ-induced macrophage-like cells showed significant impact on inflammatory markers (Figures 3A and 3B). After 24 hours of treatment with 300 μg/mL T. cordifolia, differentiated macrophages exhibited a noteworthy reduction in RSQ-induced expression of TNF-α, MCP1, IL-6, IL-10, CCL-5, and CXCL-10 (Figure 3B). Similar to W. somnifera, T. cordifolia demonstrated a dose-response relationship with increasing T. cordifolia concentrations leading to significant reductions in IL-10 and CXCL-10 expression. Notably, ELISA results of T. cordifolia-treated cell culture supernatants revealed interesting findings (Figures 4A and 4B). While RSQ-induced IL-6 levels were mitigated at all investigated T. cordifolia doses, the maximum decrease was observed at a minimal T. cordifolia dose of 3 μg/mL (Figure 4B).
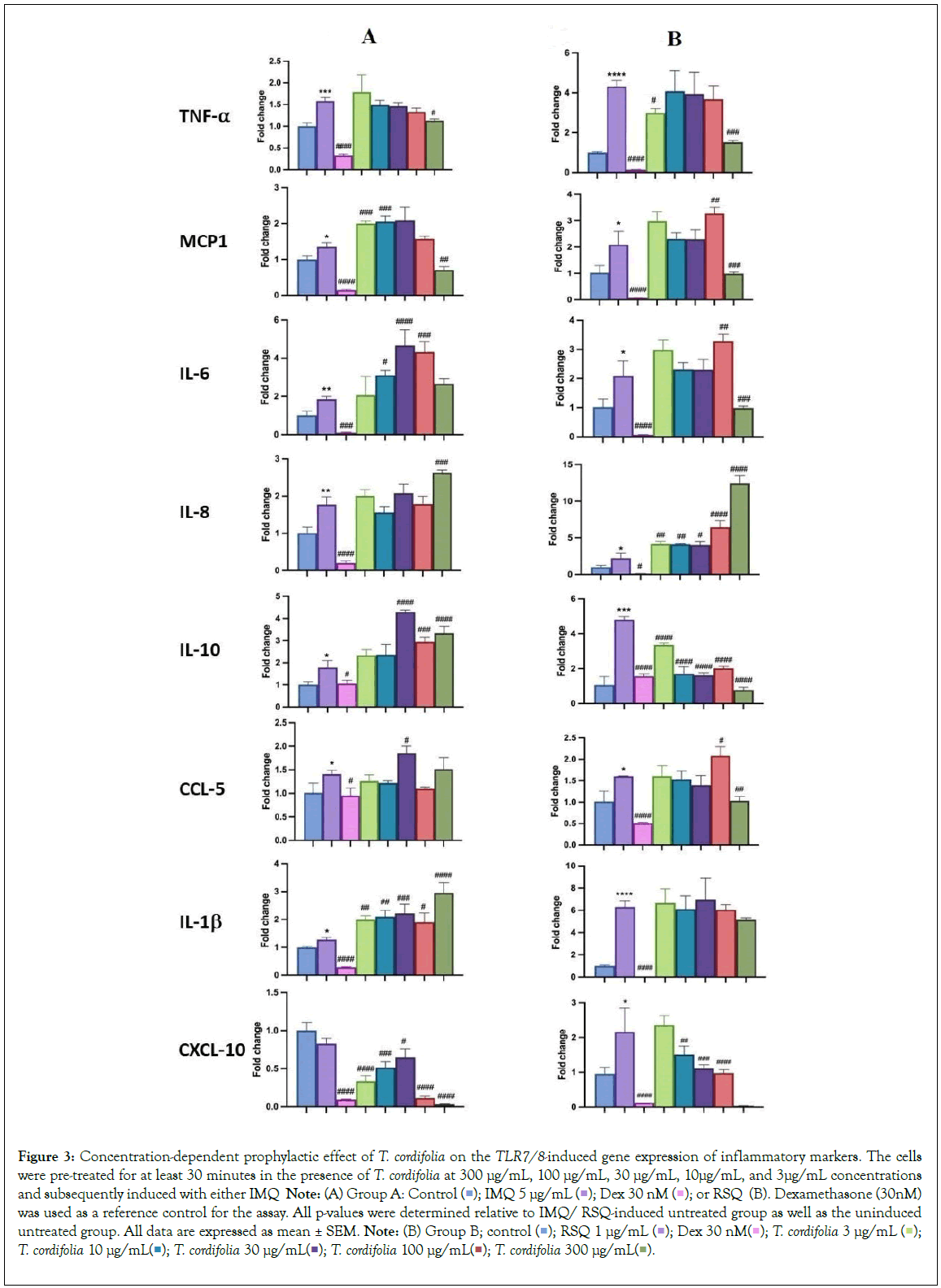
Figure 3: Concentration-dependent prophylactic effect of T. cordifolia on the TLR7/8-induced gene expression of inflammatory markers. The cells were pre-treated for at least 30 minutes in the presence of T. cordifolia at 300 μg/mL, 100 μg/mL, 30 μg/mL, 10μg/mL, and 3μg/mL concentrations and subsequently induced with either IMQ Note: (A) Group A: Control  or RSQ (B). Dexamethasone (30nM) was used as a reference control for the assay. All p-values were determined relative to IMQ/ RSQ-induced untreated group as well as the uninduced untreated group. All data are expressed as mean ± SEM. Note: (B) Group B; control
or RSQ (B). Dexamethasone (30nM) was used as a reference control for the assay. All p-values were determined relative to IMQ/ RSQ-induced untreated group as well as the uninduced untreated group. All data are expressed as mean ± SEM. Note: (B) Group B; control  T. cordifolia 10 μg/mL
T. cordifolia 10 μg/mL  .
.
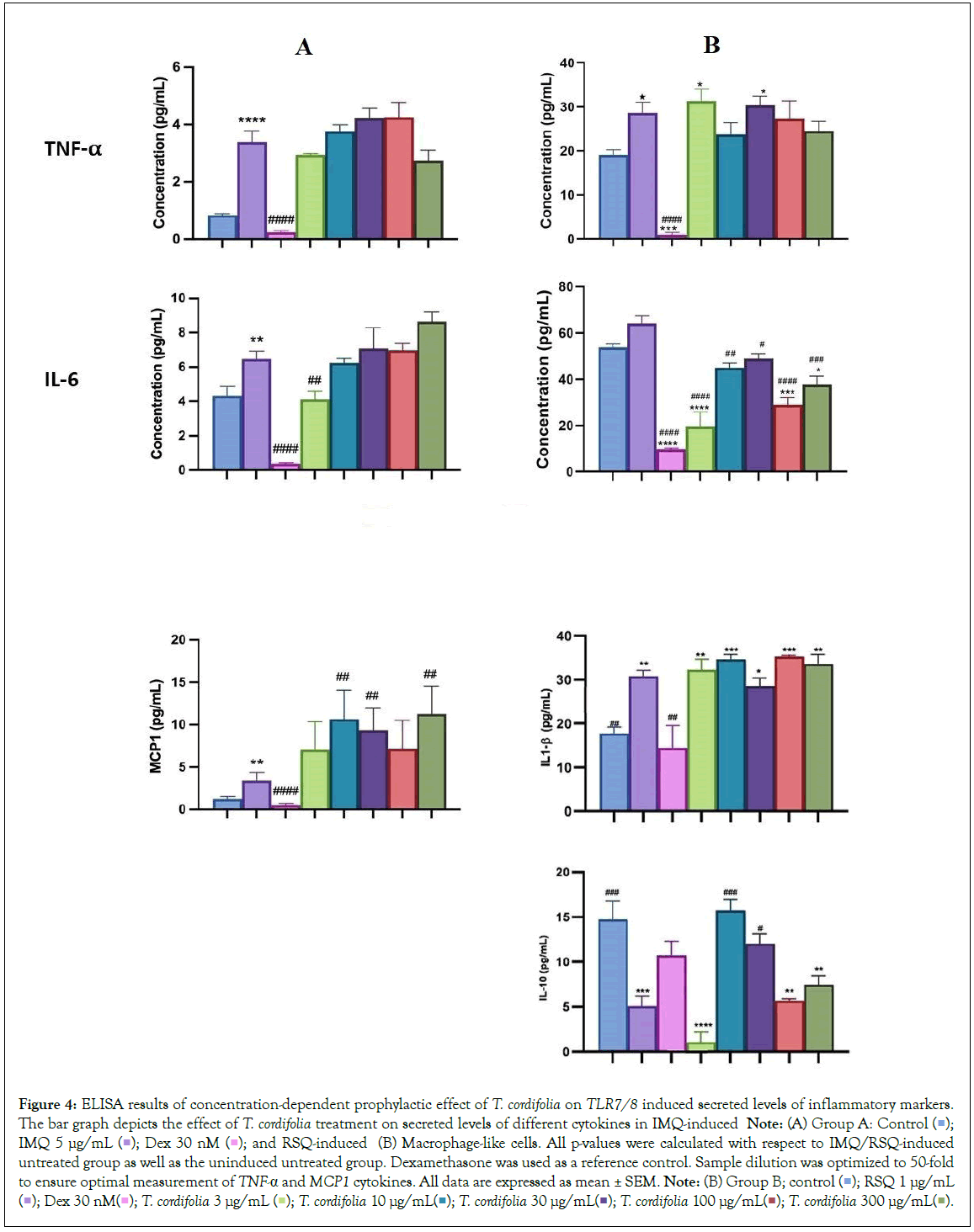
Figure 4: ELISA results of concentration-dependent prophylactic effect of T. cordifolia on TLR7/8 induced secreted levels of inflammatory markers. The bar graph depicts the effect of T. cordifolia treatment on secreted levels of different cytokines in IMQ-induced Note: (A) Group A: Control  IMQ 5 μg/mL
IMQ 5 μg/mL  and RSQ-induced (B) Macrophage-like cells. All p-values were calculated with respect to IMQ/RSQ-induced untreated group as well as the uninduced untreated group. Dexamethasone was used as a reference control. Sample dilution was optimized to 50-fold
to ensure optimal measurement of TNF-α and MCP1 cytokines. All data are expressed as mean ± SEM. Note: (B) Group B; control
and RSQ-induced (B) Macrophage-like cells. All p-values were calculated with respect to IMQ/RSQ-induced untreated group as well as the uninduced untreated group. Dexamethasone was used as a reference control. Sample dilution was optimized to 50-fold
to ensure optimal measurement of TNF-α and MCP1 cytokines. All data are expressed as mean ± SEM. Note: (B) Group B; control  RSQ 1 μg/mL
RSQ 1 μg/mL 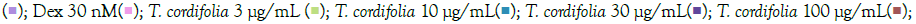 T. cordifolia 300 μg/mL
T. cordifolia 300 μg/mL 
Effect of G. glabra pretreatment on TLR7/8-induced inflammatory markers
Popularly known as liquorice or mulaithi, G. glabra is an ancient Ayurvedic herb widely used for its medicinal properties. G. glabra roots contain active compounds like glycyrrhizin, flavonoids, coumarins, and triterpenoids, contributing to its anti-inflammatory effects in arthritis, expectorant properties for respiratory congestion, gastric and digestive benefits, antimicrobial properties, and immune regulation during infections [18]. G. glabra administration in hamster models of COVID-19 demonstrated significant downregulation of pro-inflammatory cytokines TNF-α and IL-6 [19,20]. In our study, G. glabra treatment led to a notable reduction in TNF-α and IL-6 expression and levels in macrophage cells induced with TLR7/8 agonists. IL6 gene expression significantly decreased at 3 μg/mL G. glabra concentration but increased with higher G. glabra concentrations. IL-8 gene expression was inhibited at 300 μg/mL, 30 μg/mL, and 10 μg/mL, while CXCL10 gene expression decreased in a concentration-dependent manner in IMQ-induced cells, with the maximum reduction observed at 300 μg/mL G. glabra (Figure 5A). G. glabra treatment significantly reduced TNF-α expression at all investigated concentrations. Similarly, G. glabra treatment corrected IL-10 expression in RSQ- induced cells (Figure 5B). IL8 depletion was observed at 10 μg/mL G. glabra, while CCL5 gene expression was significantly corrected at lower G. glabra concentrations (3 μg/mL and 10 μg/mL) in RSQ- induced cells. Surprisingly, IL1-β gene expression increased with increasing G. glabra concentration in IMQ-induced cells. MCP1 gene expression showed an increasing trend in G. glabra -treated cells induced with both TLR7/8 agonists. Likewise, circulating MCP1 protein levels increased in culture supernatants with higher G. glabra concentrations (Figure 6A). However, IL6 levels were significantly reversed at most G. glabra concentrations investigated in RSQ-induced cells (Figure 6B).
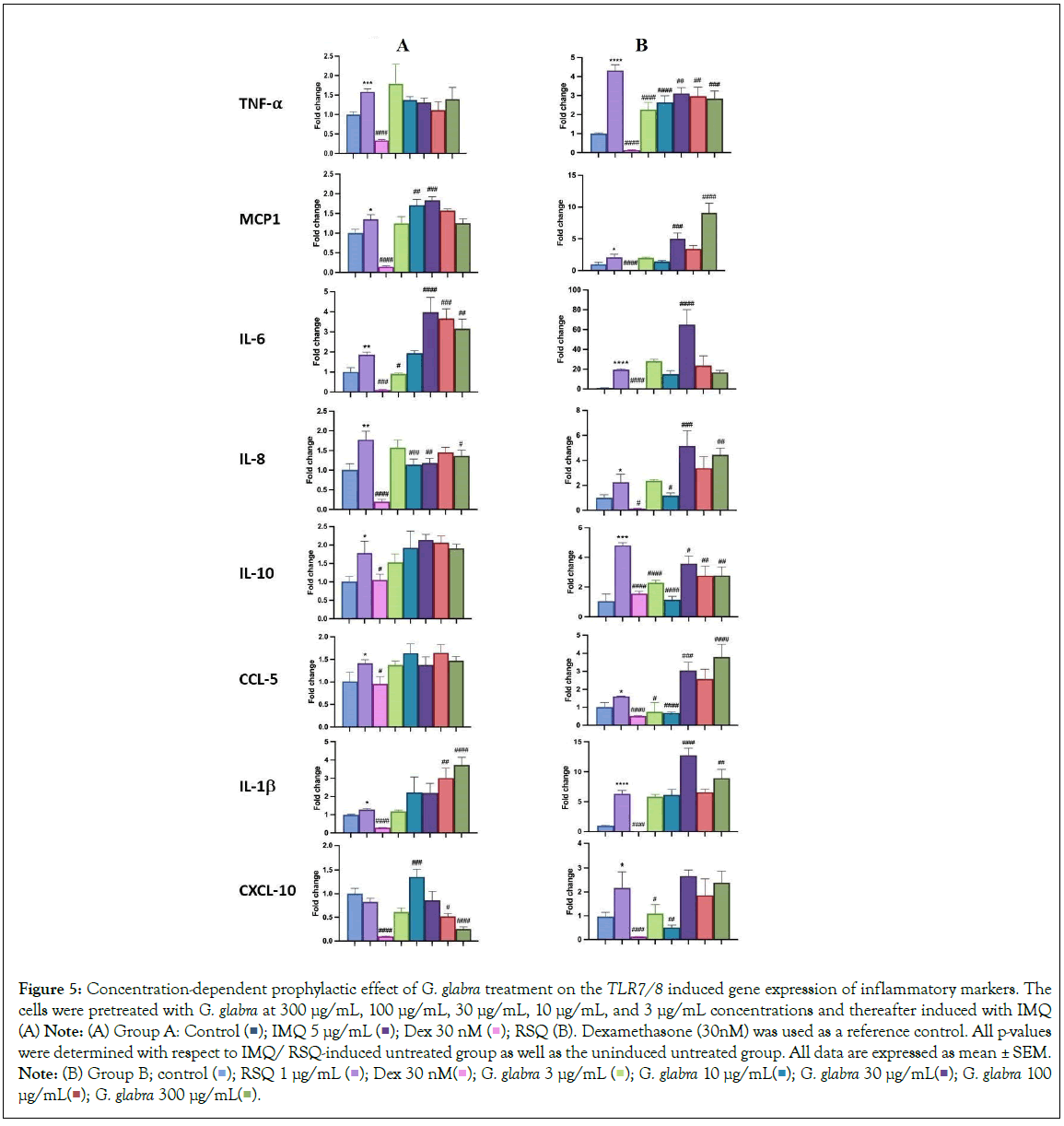
Figure 5: Concentration-dependent prophylactic effect of G. glabra treatment on the TLR7/8 induced gene expression of inflammatory markers. The cells were pretreated with G. glabra at 300 μg/mL, 100 μg/mL, 30 μg/mL, 10 μg/mL, and 3 μg/mL concentrations and thereafter induced with IMQ (A) Note: (A) Group A: Control 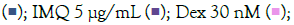 RSQ (B). Dexamethasone (30nM) was used as a reference control. All p-values were determined with respect to IMQ/ RSQ-induced untreated group as well as the uninduced untreated group. All data are expressed as mean ± SEM. Note: (B) Group B; control
RSQ (B). Dexamethasone (30nM) was used as a reference control. All p-values were determined with respect to IMQ/ RSQ-induced untreated group as well as the uninduced untreated group. All data are expressed as mean ± SEM. Note: (B) Group B; control  G. glabra 100 μg/mL
G. glabra 100 μg/mL  .
.
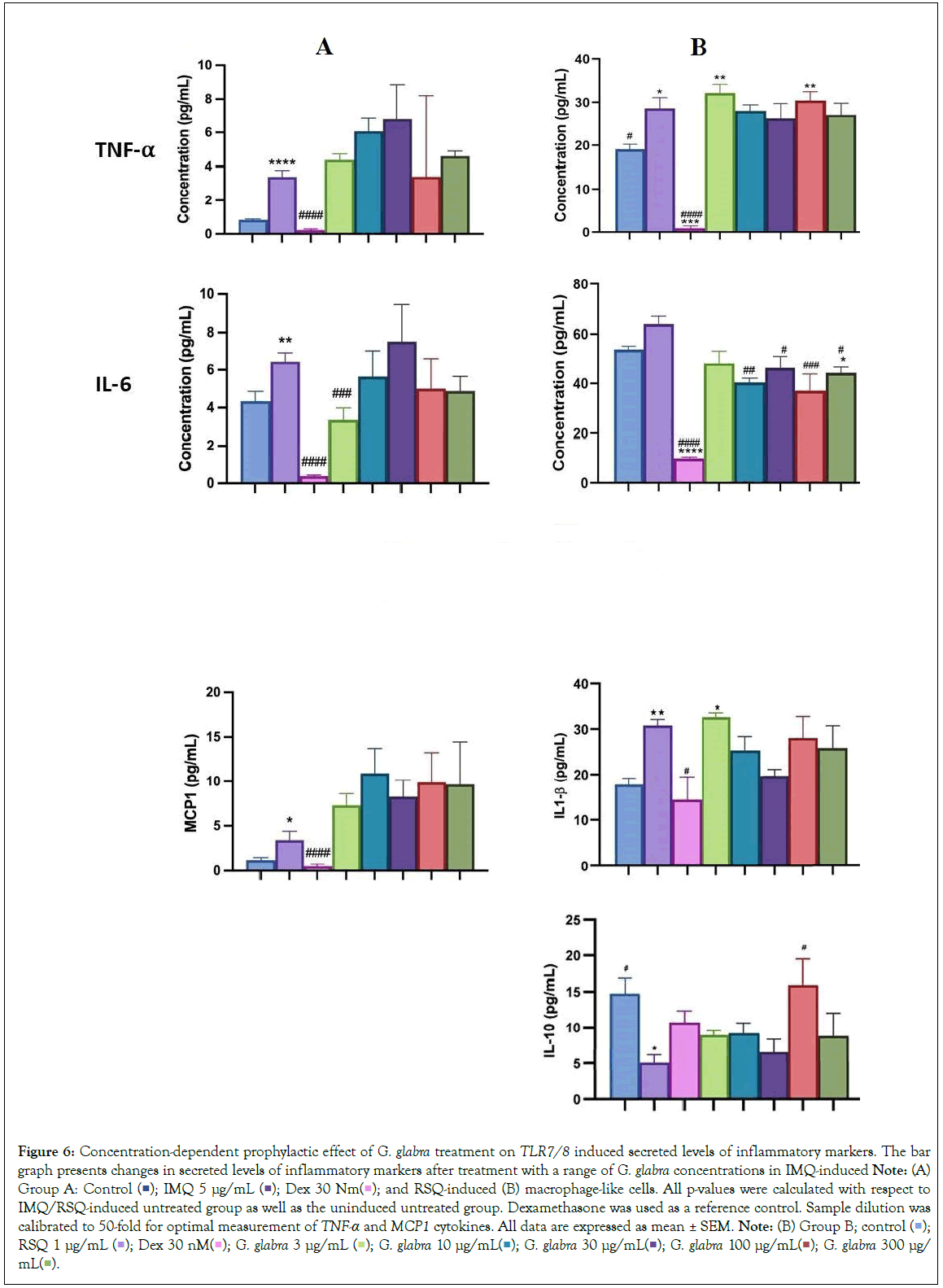
Figure 6: Concentration-dependent prophylactic effect of G. glabra treatment on TLR7/8 induced secreted levels of inflammatory markers. The bar graph presents changes in secreted levels of inflammatory markers after treatment with a range of G. glabra concentrations in IMQ-induced Note: (A) Group A: Control  and RSQ-induced (B) macrophage-like cells. All p-values were calculated with respect to IMQ/RSQ-induced untreated group as well as the uninduced untreated group. Dexamethasone was used as a reference control. Sample dilution was calibrated to 50-fold for optimal measurement of TNF-α and MCP1 cytokines. All data are expressed as mean ± SEM. Note: (B) Group B; control
and RSQ-induced (B) macrophage-like cells. All p-values were calculated with respect to IMQ/RSQ-induced untreated group as well as the uninduced untreated group. Dexamethasone was used as a reference control. Sample dilution was calibrated to 50-fold for optimal measurement of TNF-α and MCP1 cytokines. All data are expressed as mean ± SEM. Note: (B) Group B; control  RSQ 1 μg/mL
RSQ 1 μg/mL  G. glabra 300 μg/mL
G. glabra 300 μg/mL 
Effect of the pretreatment of cells with AYUSH-64 (A- 64) on the TLR7/8 induced expression and release of inflammatory markers
AYUSH-64 is an Ayurvedic formulation with immunomodulatory and anti-inflammatory properties. It contains herbs such as Alstonia scholaris, Caesalpinia crista, Picrorhiza kurroa, and Swertia chirata [21]. Safety studies have confirmed its tolerance up to 500 mg/kg body weight in rodents. A clinical trial conducted by the Ministry of AYUSH, India, and the Council of Scientific and Industrial Research, India, demonstrated its efficacy in managing mild to moderate COVID-19 patients, with a significant decrease in IL-6 levels [22-24]. In our study, we evaluated the effect of A-64 on the expression of inflammatory genes in the presence of TLR7/8 inducers (Figures 7A and 7B). We observed a significant reduction in TNF-α expression and levels in the supernatant of A-64 pre-treated cells. While, IL6 levels showed no overall change in expression or in the release in the supernatant, IL-1β levels decreased significantly with increasing A-64 concentration, indicating a concentration dependent-response relationship (Figures 8A and 8B). Moreover, IL-10 and CXCL10 expression were mitigated in a concentration- dependent manner, decreasing with increasing AYUSH-64 concentrations.
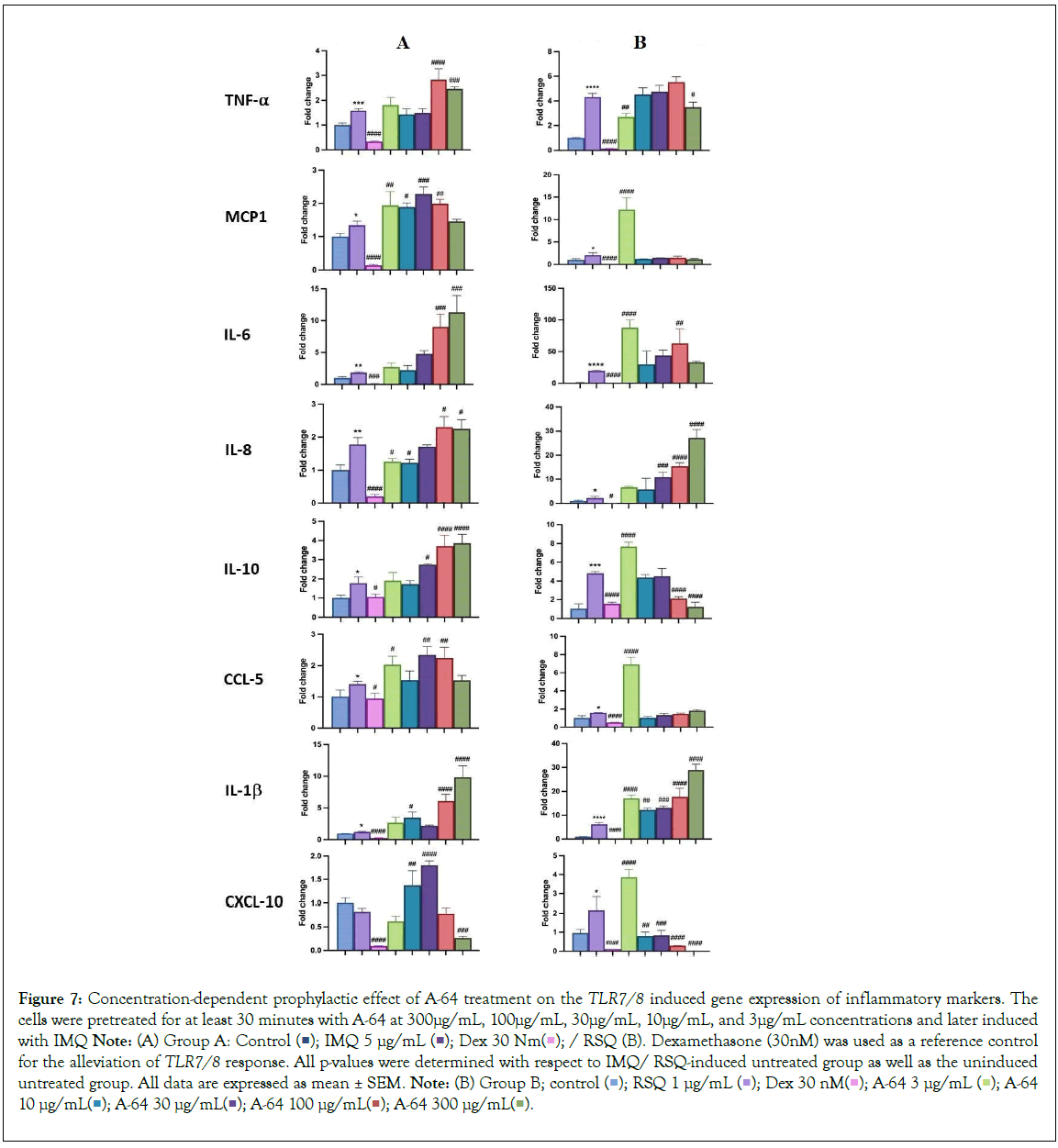
Figure 7: Concentration-dependent prophylactic effect of A-64 treatment on the TLR7/8 induced gene expression of inflammatory markers. The cells were pretreated for at least 30 minutes with A-64 at 300μg/mL, 100μg/mL, 30μg/mL, 10μg/mL, and 3μg/mL concentrations and later induced with IMQ Note: (A) Group A: Control  / RSQ (B). Dexamethasone (30nM) was used as a reference control for the alleviation of TLR7/8 response. All p-values were determined with respect to IMQ/ RSQ-induced untreated group as well as the uninduced untreated group. All data are expressed as mean ± SEM. Note: (B) Group B; control
/ RSQ (B). Dexamethasone (30nM) was used as a reference control for the alleviation of TLR7/8 response. All p-values were determined with respect to IMQ/ RSQ-induced untreated group as well as the uninduced untreated group. All data are expressed as mean ± SEM. Note: (B) Group B; control  A-64 10 μg/mL
A-64 10 μg/mL 
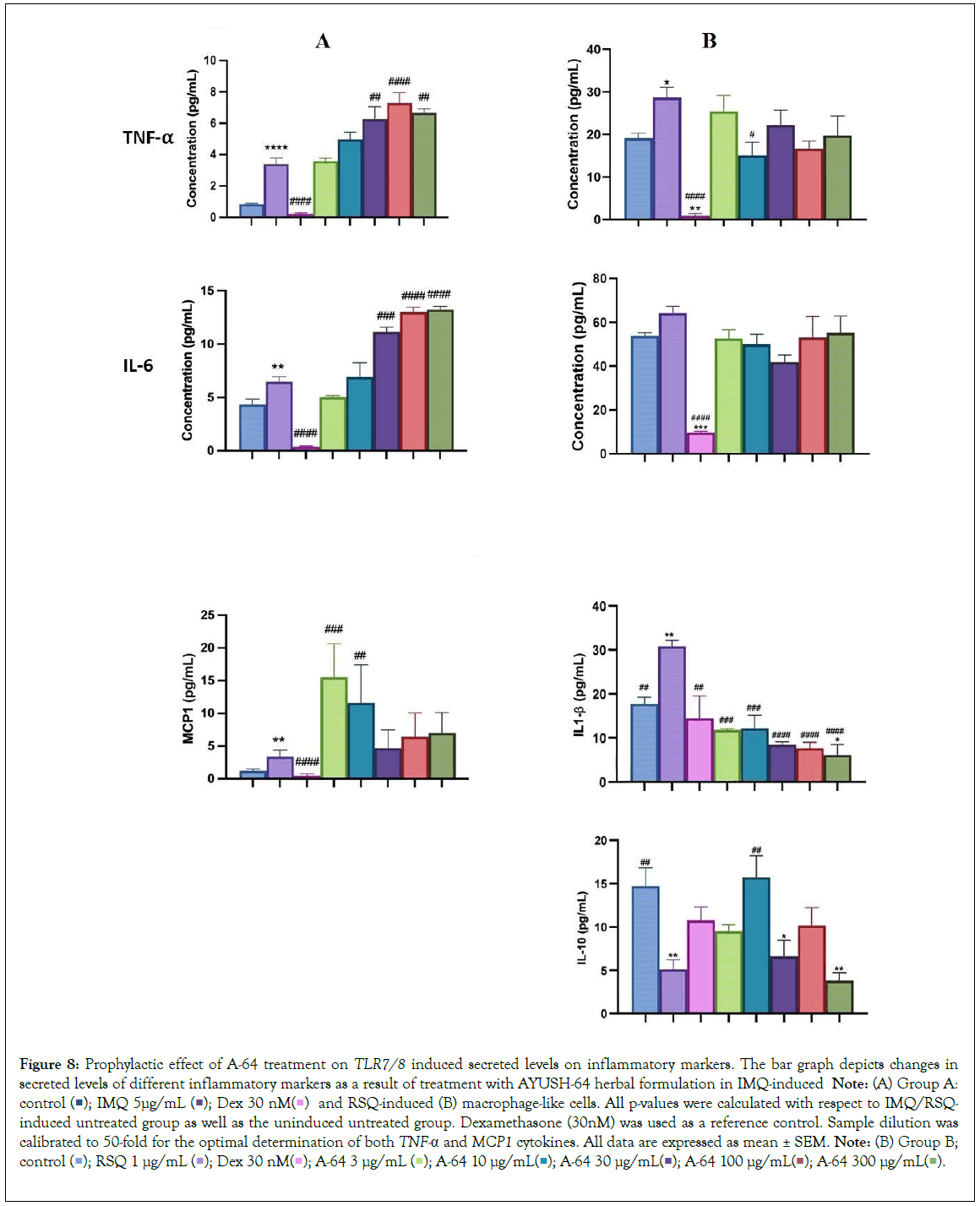
Figure 8: Prophylactic effect of A-64 treatment on TLR7/8 induced secreted levels on inflammatory markers. The bar graph depicts changes in secreted levels of different inflammatory markers as a result of treatment with AYUSH-64 herbal formulation in IMQ-induced Note: (A) Group A: control 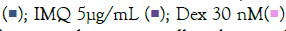 and RSQ-induced (B) macrophage-like cells. All p-values were calculated with respect to IMQ/RSQ-induced untreated group as well as the uninduced untreated group. Dexamethasone (30nM) was used as a reference control. Sample dilution was calibrated to 50-fold for the optimal determination of both TNF-α and MCP1 cytokines. All data are expressed as mean ± SEM. Note: (B) Group B; control
and RSQ-induced (B) macrophage-like cells. All p-values were calculated with respect to IMQ/RSQ-induced untreated group as well as the uninduced untreated group. Dexamethasone (30nM) was used as a reference control. Sample dilution was calibrated to 50-fold for the optimal determination of both TNF-α and MCP1 cytokines. All data are expressed as mean ± SEM. Note: (B) Group B; control 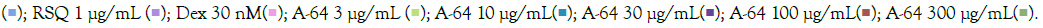
Overall, our findings contribute to the growing body of research on plant-based therapeutics, particularly in the context of immunomodulation. The use of herbal extracts and formulations holds great potential in the development of effective treatments for inflammatory disorders, offering a safe and well-tolerated alternative to conventional immunosuppressive agents.
Discussion
The dysregulated inflammatory response has emerged as a prominent pathology during the recent COVID-19 pandemic. To gain a comprehensive understanding of the disease, cell-based models have played a crucial role in elucidating the mechanisms behind various candidate molecules in COVID-19 therapeutics. Specifically, the activation of Toll-like receptor 7/8 (TLR7/8) signaling pathway in response to the presence of viral RNA within host cells has been implicated in the release of pro-inflammatory cytokines, essential for mediating the anti-viral immune response. In our study, we employed macrophage-like differentiated THP1 cells as a model system to mimic TLR7/8 induction. This was achieved by treating the cells with imidazoquinolinamines, namely imiquimod (IMQ) and resiquimod (RSQ). IMQ and RSQ are well- known TLR7/8 agonists that have demonstrated potent ability to induce the production of Interferon-α (IFN-α) and other cytokines [2,25].
By utilizing this experimental setup, we aimed to gain mechanistic insights into the TLR7/8-mediated inflammatory response and its potential implications in immune-compromised pathologies including COVID-19. The use of cell-based models has proven to be invaluable in unravelling the complex interplay between viral components and the host immune system, paving the way for the development of novel therapeutic strategies. By elucidating the specific mechanisms underlying herbal modulation of TLR7/8 signaling and subsequent cytokine release, our study contributes to a better understanding of the dysregulated inflammatory response observed during COVID-19. These findings may have implications for the development of targeted therapeutics aimed at modulating the TLR7/8 pathway to mitigate the excessive inflammation associated with the disease.
Excessive and dysregulated release of pro-inflammatory cytokines, known as cytokine storm, can occur during severe infections and autoimmune disorders [26]. This uncontrolled cytokine release was observed in the recent COVID-19 pandemic, leading to life- threatening systemic inflammation. Immunomodulatory therapies targeting specific cytokine pathways, such as TNF-α, IL-6, and IL- 1β, are used to manage cytokine storm [27]. Tocilizumab, an IL-6 receptor inhibitor, has shown efficacy in this regard [28]. IL-1β, on the other hand, induces IL-6 and TNF-α production and activates immune cells [29]. Elevated levels of TNF-α, IL-6, and IL-1β are associated with severe symptoms and tissue damage. Blocking IL-1 signaling with antagonists like anakinra has potential in managing the inflammatory response [30]. However, synthetic immunomodulatory agents often come with adverse side effects [31]. In contrast, the herbs investigated in this study (W. somnifera, T. cordifolia, G. glabra, A-64) have a long history of traditional use. Their anti-inflammatory and immunomodulatory properties offer a potential approach to manage the hyper inflammatory response without toxic side effects [32,33]. These botanical sources of immunomodulatory agents provide a novel avenue to moderate the exaggerated cytokine response and restore immune balance [32,33].
TLR7/8 activation plays a crucial role in recruiting immune cells to the site of viral infection by triggering pro-inflammatory cytokine production [34,2,3]. However, the administration of TLR7/8 agonists like imiquimod or resiquimod can lead to cytokine storms in certain clinical scenarios. Macrophage-like THP1 cells, which differentiate from monocytes when exposed to PMA, provide a relevant model to study the dysregulated cytokine response induced by TLR7/8 agonists and evaluate the prophylactic effect of herbal extracts. The study model was optimized for agonist dosage and treatment duration to achieve optimal TLR signaling induction. RSQ showed a higher induction rate of cytokine expression compared to IMQ, and the concentration of RSQ required for TLR7/8 induction (1 μg/mL) was five times lower than that of IMQ (5 μg/mL). Similar observations were reported by another research group using a similar model [8].
Induction of TLR7/8 signaling with agonists increased TNF-α expression, which was moderated in presence of dexamethasone. Treatment with herbal extracts significantly improved TNF-α gene expression in macrophage-like cells, with expression generally decreasing as extract concentration increased. Among the extracts, W. somnifera and T. cordifolia showed significant correction of IL-6 gene expression, while all treatment groups exhibited perturbed levels of IL-6 in the culture supernatant. W. somnifera treatment also corrected TLR7/8-induced IL-1β levels, whereas other extracts had no effect on IL-1β gene levels. The interplay between IL-1β and IL-6 regulation is crucial for understanding inflammatory processes and developing therapeutic strategies [35]. A-64 inhibition of IL- 1β resulted in suppressed IL-6 release. Additionally, the selected herbs significantly corrected CXCL-10 expression, an immune cell trafficking and activation chemokine. CXCL-10 levels are associated with disease severity and can serve as a biomarker in inflammatory conditions [36].
Among all the investigated herbal extracts, W. somnifera showed remarkable efficacy in mitigating the increase in expression of inflammatory markers induced by TLR7/8 activation. W. somnifera exhibited its effects by attenuating excessive cytokine release and regulating the production and activity of pro-inflammatory cytokines. W. somnifera contains bioactive compounds, including with anolides, known for their anti-inflammatory and immunomodulatory properties. Previous studies have demonstrated the ability of W. somnifera to suppress TNF-α, IL-6, and IL-1β cytokines in various inflammatory conditions, such as LPS-induced neuroinflammation [37]. Furthermore, W. somniferahas shown a protective effect against COVID-19 by modulating the inflammatory response and inhibiting pro-inflammatory cytokine- induced differentiation of Th1, Th2 and Th17 cells [38].
It is essential to acknowledge that although these botanical drugs have demonstrated promising results in our preclinical model, further research is warranted to comprehensively understand their mechanisms of action. Additionally, optimizing dosages and conducting thorough evaluations of their safety and efficacy in animal models are crucial steps to advance their potential as therapeutic interventions. While our study model exhibits sensitivity and robustness, rigorous clinical validation is imperative to establish the viability and effectiveness of these botanical drugs as potential treatment options.
Conclusion
Based on our study, it is evident that herbal therapeutics hold promise in regulating dysregulated inflammatory responses. Resiquimod exhibited better efficacy as an agonist for activating the TLR7/8 signaling pathway in our experimental model. Among the evaluated herbs/formulations, Withania somnifera demonstrated significant prophylactic efficacy by effectively mitigating the upregulation of numerous inflammatory genes in the PMA-differentiated THP1 cells activated via the TLR7/8 signaling pathway. These findings provide a basis for exploring the potential of herbal interventions in modulating inflammatory cascades, but additional research is required to fully understand their mechanisms of action and therapeutic applications.
Acknowledgments
We acknowledge THSTI Small Animal Facility (SAF) for its services. The authors are thankful to NMPB for providing Herbal extracts and Formulations for the study. The authors thank the Executive Director of THSTI for his generous overall support. We also appreciate the technical assistance provided by Ms. Deepika Tuli from THSTI during in-vitro experimental procedures.
Author's Contributions
Manisha Dagar and Kamala Priya performed the in vitro cell-based experiments, writing, and interpretation. Ajay Kumar and Madhu Dikshit conceptualized the overall study design, interpretations, writing, and editing of the manuscript. All authors have read and accepted the manuscript.
Manisha Dagar and Kamala Priya contributed equally and are therefore sharing first authorship.
Funding
The authors express their gratitude to the Ministry of AYUSH and the Department of Biotechnology (DBT), Government of India for jointly funding the research work presented in this manuscript (Grant Nos.: BT/PR40738/TRM/120/486/2020 and A.11019/03/2020-NMPB-IV-A). Madhu Dikshit also acknowledges the financial support from JBR/2020/000034.
Availability and Data Materials
All data are included within the article. Any additional specific information may be made available if desired.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from Institutional Biosafety Committee (IBSC) at THSTI (IBSC Approval No 221/2020) after a brief explanation about the study objectives and protocols. All experimental protocols were approved and carried out in accordance with relevant guidelines and regulations. Consent to participate is not applicable in this study.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
References
- Shimizu M. Clinical features of cytokine storm syndrome. Cytokine storm syndrome. 2019:31-41.
- Dyavar S, Singh R, Emani R, Pawar GP, Chaudhari VD, Podany AT, et al. Role of toll-like receptor 7/8 pathways in regulation of interferon response and inflammatory mediators during SARS-CoV-2 infection and potential therapeutic options. Biomed Pharmacother. 2021;141:111794.
[Crossref] [Google Scholar] [PubMed]
- Manik M, Singh RK. Role of toll like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J Med Virol. 2022;94(3):869-877.
[Crossref] [Google Scholar] [PubMed]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506.
[Crossref] [Google Scholar] [PubMed]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: Consider cytokine storm syndromes and immunosuppression .Lancet. 2020;395(10229):1033-1034.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17-41.
[Crossref] [Google Scholar] [PubMed]
- Pasquereau S, Kumar A, Herbein G. Targeting TNF and TNF receptor pathway in HIV-1 infection: from immune activation to viral reservoirs. Viruses 2017;9(4):64.
[Crossref] [Google Scholar] [PubMed]
- Deshmane SL, Kremlev S, Amin S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res. 2009;29(6):313-326.
[Crossref] [Google Scholar] [PubMed]
- Budhiraja RD, Sudhir S. Review of biological activity of withanolides. J Sci Ind Res.1987;11:488-491.
- Rizvi ZA, Tripathy MR, Sharma N, Goswami S, Srikanth S, Sastry JLN, et al. Effect of prophylactic use of intra-nasal oil formulations in the hamster model of COVID-19. Front Pharmacol. 2021;12:746729 (1-11)
[Crossref] [Google Scholar] [PubMed]
- Mukherjee PK, Efferth T, Das B, Kar A, Ghosh S, Singha S, et al. Role of medicinal plants in inhibiting SARS-CoV-2 and in the management of post-COVID-19 complications. Phytomed. 2022;98:1-33.
[Crossref] [Google Scholar] [PubMed]
- Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980;26:171-176.
[Crossref] [Google Scholar] [PubMed]
- Kasarla SS, Borse S, Kumar Y, Sharma N, Dikshit M. In vitro effect of Withania somnifera, AYUSH-64, and remdesivir on the activity of CYP-450 enzymes: Implications for possible herb-drug interactions in the management of COVID-19. Front Pharmacol. 2022;13:973768.
[Crossref] [Google Scholar] [PubMed]
- Ingawale DSM, Namdeo AG. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J Diet Suppl. 2020;18(2):183-226.
[Crossref] [Google Scholar] [PubMed]
- Vellingiri B, Jayaramayya K, Iyer M, Narayanasamy A, Govindasamy V, Giridharan B, et al. COVID-19: A promising cure for the global panic. Sci Total Environ. 2020;725:138277.
[Crossref] [Google Scholar] [PubMed]
- Sharma U, Bala M, Kumar N, Singh B, Munshi RK, Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J Ethnopharmacol. 2012;141:918-926.
[Crossref] [Google Scholar] [PubMed]
- Varshney D, Spiegel J, Zyner K, Tannahill D, Balasubramanian S. The regulation and functions of DNA and RNA G-quadruplexes. Nat Rev Mol Cell Biol. 2020;21:459-474.
[Crossref] [Google Scholar] [PubMed]
- Ming LJ, Yin AC. Therapeutic effects of glycyrrhizic acid. Nat Prod Commun. 2013;8:415-418.
[Crossref] [Google Scholar] [PubMed]
- Rizvi ZA, Babele P, Sadhu S, Madan U, Tripathy MR, Goswami S, et al. Prophylactic treatment of Glycyrrhiza glabra mitigates COVID-19 pathology through inhibition of pro-inflammatory cytokines in the hamster model and NETosis. Front Immunol. (Viral Immunology). 2022;13:945583.
[Crossref] [Google Scholar] [PubMed]
- Yang R, Yuan BC, Ma YS, Zhou S, Liu Y. The anti-inflammatory activity of liquorice, a widely used Chinese herb. Pharm Biol. 2017;55(1):5-18.
[Crossref] [Google Scholar] [PubMed]
- Panda AK, Kar S, Rai AK, Rao BCS, Srikanth N. AYUSH- 64: A potential therapeutic agent in COVID-19. J Ayurveda Integr Med. 2022;13(2):100538.
[Crossref] [Google Scholar] [PubMed]
- Reddy RG, Gosavi RV, Yadav B, Rai AK, Holay MP, Talekar M, et al. AYUSH-64 as an add-on to standard care in asymptomatic and mild cases of COVID-19: A randomized controlled trial. Ayu. 2020;41(2):107-116.
[Crossref] [Google Scholar] [PubMed]
- Singh H, Srivastava S, Yadav B, Rai AK, Jameela S, Muralidharan S, et al. AYUSH-64 as an adjunct to standard care in mild to moderate COVID-19: An open-label randomized controlled trial in Chandigarh, India. Complement Ther Med. 2022;66:102814.
[Crossref] [Google Scholar] [PubMed]
- Thakar A, Goyal M, Bhinde S, Chhotala Y, Panara K, Chaudhari S. Impact of AYUSH 64 as an adjunctive to standard of care in mild COVID 19-An open-label randomized controlled pilot study. J Ayurveda Integr Med. 2022;13(3):100587.
[Crossref] [Google Scholar] [PubMed]
- Dockrell DH, Kinghorn GR. Imiquimod and resiquimod as novel immunomodulators. J of Antimicrob Chemother .2001;48(6):751-755.
[Crossref] [Google Scholar] [PubMed]
- Falcone M, Sarvetnick N. Cytokines that regulate autoimmune responses. Curr Opin Immunol. 1999;11:670-676.
[Crossref] [Google Scholar] [PubMed]
- Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Wu Z, Li J, Zhao H, Wang G. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954.
[Crossref] [Google Scholar] [PubMed]
- Clinical features of cytokine storm syndrome
- Jesus AA, Goldbach-Mansky R. IL-1 blockade in auto inflammatory syndromes. Annu Rev Med. 2014;65:223-244.
[Crossref] [Google Scholar] [PubMed]
- Colafrancesco S, Alessandri C, Conti F, Roberta Priori R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020;7:102573.
[Crossref] [Google Scholar] [PubMed]
- Cena H, Chieppa M. Coronavirus disease (COVID-19–Sars-Cov-2) and nutrition: is infection in Italy suggesting a connection? Front Immunol. 2020;11:944.
[Crossref] [Google Scholar] [PubMed]
- Grigore A. Plant phenolic compounds as immunomodulatory agents. Phenolic Compounds. 2017;75-98.
- Ahmadpour E, Safarpour H, Xiao L, Zarean M, Hatam-Nahavandi K, Barac A, et al. Cryptosporidiosis in HIV-positive patients and related risk factors: A systematic review and meta-analysis. Parasite. 2020;27:27.
[Crossref] [Google Scholar] [PubMed]
- Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283(38):25900-25912.
[Crossref] [Google Scholar] [PubMed]
- Jiang Y, Xu J, Zhou C, Wu Z, Zhong S, Liu J, et al. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171(8):850-857.
[Crossref] [Google Scholar] [PubMed]
- Gupta M, Kaur G. Withania somnifera as a potential anxiolytic and anti-inflammatory candidate against systemic lipopolysaccharide-induced neuroinflammation. Neuromolecular Med. 2018;3:343-362.
[Crossref] [Google Scholar] [PubMed]
- Rizvi ZA, Babele P, Madan U, Sadhu S, Tripathy MR, Goswami S, et al. Pharmacological potential of Withania somnifera (L.) Dunal and Tinospora cordifolia (Willd.) Miers on the experimental models of COVID-19, T cell differentiation, and neutrophil functions. Front Immunol. 2023;14:1138215.
[Crossref] [Google Scholar] [PubMed]
Citation: Dagar M, Priya K, Dikshit M, Kumar A (2023) Pharmacological Evaluations of Select Herbal Extracts on TLR7/8-induced Cytokine and Chemokine Production in Macrophage-like Cells. J Clin Exp Pharmacol. 13:374.
Copyright: © 2023 Dagar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : Ministry of AYUSH and the Department of Biotechnology (DBT), Government of India for jointly funding the research work presented in this manuscript (Grant Nos.: BT/PR40738/TRM/120/486/2020 and A.11019/03/2020-NMPB-IV-A). Madhu Dikshit also acknowledges the financial support from JBR/2020/000034

