Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 12
Enhanced Production of α-Galactosidase from Novel Strain Bacillus megaterium VHM1 in Solid-State Fermentation by Using Citrus Waste
Nagangoud V Kote1, Veerappa H Mulimani1, Gurushantappa Kadakol2 and Aravind Goud G Patil3*2Department of Genetics and Anatomy, BLDE Deemed to be University, Karnataka, India
3Department of Allied Health Science, BLDE Deemed to be University, Karnataka, India
Received: 08-Nov-2023, Manuscript No. JFPT-23-23835; Editor assigned: 13-Nov-2023, Pre QC No. JFPT-23-23835 (PQ); Reviewed: 27-Nov-2023, QC No. JFPT-23-23835; Revised: 04-Dec-2023, Manuscript No. JFPT-23-23835 (R); Published: 11-Dec-2023, DOI: 10.35248/2157-7110.23.14.1074
Abstract
A high yield of α-galactosidase was achieved by citrus waste-based Solid State Fermentation (SSF) using a novel strain, Bacillus megaterium VHM1. The maximum production of α-galactosidase was obtained at 72 hours of fermentation. The optimal temperature and pH were 350°C 6.0, respectively. Higher enzyme production at 90% (58 U/g) was obtained with an increase in inoculum volume up to 100% (w/v). With the increase in moisture content of 50%- 100%, the production of α-galactosidase was concomitantly enhanced from 28 U/g to 56 U/g. Among the inorganic nitrogen sources tested, yeast extract yielded higher enzyme production (52 U/g). The enzyme production was maximum when raffinose was used as an additional carbon source. A forcefully aerated packed bed bioreactor was constructed for enhanced production of α-galactosidase. This enzyme could potentially be used for the processing of legumes in food processing industries to remove raffinose family oligosaccharides.
Keywords
Bacillus megaterium; Solid-State Fermentation (SSF); Citrus waste; α-galactosidase
Introduction
The enzyme α-galactosidase catalyzes the hydrolysis of α-1, 6-galactosidic bonds, releasing α-D-galactose, and is also called alpha Gal. The galactosidic bonds are found in oligosaccharides such as raaffinose, verbascose melibiose, and stachyose. These galactooligosaccharides are associated with flatulence in monogastric animals. The galactic-oligosaccharides, which are Non-Digestible (NDOs) in the large intestine, can be utilized by intestinal flora, which leads to flatulence and abdominal discomfort [1]. Other potential applications of α-galactosidase are in the processing of the beet sugar industry. The alpha-galactosidase enzyme hydrolyzes raffinose content in beet, avoiding crystallization inhibition in the beet sugar industry. In the paper industry, along with xylanase, adding α-galactosidase will help improve biobleaching. α-Galactosidase also plays an essential role in other industries, such as pharmaceutical and medicinal purposes. The enzyme α-galactosidases were present in all microorganisms, plants and animals.
Solid-state fermentation has many advantages over Sub Merged Fermentation (SMF), its simple technique, low capital investment, and low energy requirement, including superior productivity and better product recovery. SSF has gained importance because it utilizes cheap agricultural waste as raw material to produce enzymes. Enzyme production by SSF has advantages when its crude enzyme is used for several applications [2,3].
Bacillus megaterium is a Gram-positive, spore-forming bacterium found widely in diverse habitats, from seawater to soil. The organism is utilized for several industries as it possesses some beneficial enzymes and produces high-capacity exoenzymes [4]. Bacillus sp JF2 strain produced extracellular thermo-stable α-amylase (EC, 3.2.1.1) and intracellular α-galactosidase (EC, 3.2.1.22).
There are reports on the production of enzymes by SSF using fungi, but there are very few reports on the production of enzymes by SSF using bacteria. Bacteria are preferred over fungi as they can produce thermostable enzymes utilized in industry. Most of the bacteria used in SSF or from Bacillus Spp. Could be attributed to the ability to adhere to the substrate particles to produce filamentous cells for penetration and to their specific need for water activity. There are no reports in the literature on the production of α-galactosidase through SSF by bacterial cultivation. The aim of this work was to optimize the production of α-galactosidase by B. megaterium VHM1, under SSF conditions and to build a forcefully aerated packed bed bioreactor.
Materials and Methods
Isolation and identification of bacteria
A strain, B. megaterium VHM1, secreting extracellular α-galactosidase, was isolated from sugar cane industrial waste samples near Vijayapur (Karnataka, India). The isolation of the bacterium was done in nutrient broth supplemented with fat- free soya flour extract at 50°C, and pH. were adjusted to 7.5. The isolate was maintained on nutrient agar slants containing 2% guar gum. The isolate was identified by morphological, physiological, and biochemical tests described in Bergey’s manual of systematic bacteriology [5]. The partial 16S rDNA nucleotide sequences were determined. (Previously described by Kim et al., 2004). The sequences deposited in the NCBI nucleotide sequence database, No FJ 613521. The 16S rDNA was compared with the DNA sequences deposited in NCBI.
Solid State Fermentation (SSF)
Different agro-industrial waste materials, viz Red Gram Husk (RGH), Sugarcane Bagasse (SB), Wheat Bran Husk (WBH), Chickpea Husk (CKH), Rice Bran (RB), Pine Apple Waste (PW), Potato Peels (PP) Banana Fruit Waste (BFW), and Citrus Waste (CW) were collected from local place of Gulbarga Karnataka state, India. These wastes were cleaned with water, followed by distilled water to remove surface dust particles. Then, bleaching was carried out by immersing them in hot water (70°C-80°C) for 15 min, followed by drying at 450°C in Owen. The dried material was grounded and sterilized at 121°C, 15 lbs pressure for 15 min and stored at 40°C further use.
To screen the different substrates for α-galactosidase Production, initially, 5.0 g of the powdered substrate was taken in a 250 ml conical flask and to this add, a known quantity of mineral salt medium containing (grams per liter distilled water) K2HPO4, 6.3 g; KH2PO4, 1.8 g; NH4NO3, 1 g; MgSO4, 1 g; CaCl2, 0.1 g; FeSO4, 0.1 g; MnSO4, 0.1 g; NaMo7O24, 0.006 g.) pH 7.0 was added; the combination of other agro-industrial waste with citrus waste was also studied at 1:1(w/w) in the fermentation medium. The solid substrates were appropriately mixed and autoclaved at 121ºC, 15 lbs pressure for 15 min. The flasks were kept aside to attain a room temperature and then inoculated with 24-h grown bacterial culture of 2.0 ml (OD 600 nm between 0.49 and 0.51) and incubated at 400°C temperature for different periods (12 h, 24 h, 36 h, 48 h, 72 h, and 96 h). To investigate the influence of other culture parameters on α-galactosidase Production, initial moisture content (50%-100% w/v), inoculums volume (30% w/v-150% w/v), effect of initial temperature (25°C-500°C), effect of initial pH (3.5-8) co- carbon sources (glucose, galactose, fructose, xylose, sucrose, lactose, raffinose, stachyose, guar gum, locust bean gum at 2%, w/w) and co-nitrogen sources, (organic nitrogen sources, yeast extract, peptone, beef extract, soybean meal and gelatin were used in SSF medium. Inorganic nitrogen sources such as ammonium sulfate, ammonium acetate, urea and ammonium nitrate are also used. To determine the initial moisture content, the solid substrate was mixed with a known amount of mineral salt medium. One gram of substrate is added to 1 ml of water to achieve 100% miniaturization. Without having inoculums, flasks served as negative controls, whereas the inoculated flasks without any co-carbon or co-nitrogen source served as positive controls. The production of the enzyme is expressed as mean and standard deviations based on the results obtained with triplicate flasks.
Enzyme extraction
Fermented mass is added with sodium acetate buffer (0.2 M, pH 6; 1:10 w/v) for two h on an orbital shaker at 300 rpm, filtered with muslin cloth and centrifuged at 5000 rpm for 15 min. The supernatant was used to carry out the α-galactosidase assay.
Assay of α-galactosidase
The α-galactosidase assay was carried out according to the modified method of Dey and Pridham (1972). The reaction mixer contained 100 ul of enzyme+800 ul of 0.2 M phosphate buffer (pH 6.0)+1 ul of 2 mM PNPG. The reaction mixture was incubated at 500°C for 10 min. The reaction was stopped by adding 3 ml of 0.2 M Na2CO3 solution. The absorbance was taken at 405 nm in a spectrophotometer (Tecan). One unit of enzyme activity was defined as the amount of enzyme, which produced l μmol of para nitrophenol min-1 under standard conditions. The enzyme production under SSF was expressed as U/g dry fermented mass.
Solid-state fermentation in forcefully aerated packed bed bioreactor
The glass column reactor (heat Sterilized, length 28 cm, inner diameter 6 cm) was aseptically filled with pre-inoculated citrus waste, leaving a headspace of 5 cm at the top of the column. Air from an aerator pump was filtered through a glass column filter with glass wool before entering the humidification flask. The saturated, moist air was then continuously supplied through the bottom of the column. The outlet air from the top of the column was directed to the air exit unit. For comparison, a similar glass column filled with pre-inoculated substrate but without aeration was carried out.
Result and Discussion
Screening of the substrates for α-galactosidase production
Production of α-galactosidase in SSF using various substrates and their combination with citrus waste. Table 1 shows the results of the production of enzymes by different agro industrial wastes. The CW was the best substrate for α-galactosidase production by B. megaterium VHM1 as it gave the highest enzyme titer (46.0 U/g), RGH (43.0 U/g), BGH (41.0 U/g) and CPH (40.0 U/g) are also utilized by A.VHM1 for enzyme production but relatively low enzyme titers were observed. However, other substrates, such as PW, PP, and SBG, were much less effective for enzyme production (Table 1).
| SI No | Substrates | α-Galactosidase activity |
|---|---|---|
| 1 | Chickpea husk | 40.0 ± 2.0 |
| 2 | Red gram husk | 43.0 ± 2.15 |
| 3 | Sugar cane bagasse | 29.0 ± 1.45 |
| 4 | Citrus waste | 46.0 ± 2.3 |
| 5 | Rice bran | 37.0 ± 1.85 |
| 6 | Wheat bran | 37.0 ±1.85 |
| 7 | Black gram husk | 41.0 ± 2.05 |
| 8 | Pineapple waste | 11.0 ±.055 |
| 9 | Potato peels | 22.0 ±1.10 |
| 10 | Banana fruit waste | 26.0 ±1.3 |
| 11 | Groundnut cake | 23.0 ±1.15 |
Note: ± Standard error.
Table 1: Production of α-galactosidase by B. megaterium VHM1 in SSF using various agro-industrial substrates.
Kotwal et al. reported that soy flour (44.6 U/g) and, coconut cake (41.5 U/g), wheat bran (22.8 U/g) are the best solid substrates for α-galactosidase production in SSF by a thermophilic fungus Humicola sp. This is the first report of α-galactosidase production using citrus waste as a solid substrate by B. megaterium VHM1 by SSF. Annunziato et al., reported wheat bran gave maximum for α-galactosidase production in SSF, but enzyme titer is much less compared to our results. Shankar et al. reported that red gram plant waste is the best solid substrate for α-galactosidase production by A. oryzae in SSF. The combination of CW (ratio 1:1 w/w) with RGH, CPH, RB, WB, PW, GNC, and BFW resulted in an increase in enzyme production (Table 2).
| S NO | Substrates (10 g) | α-Galactosidase activity (U/g) |
|---|---|---|
| 1 | Citrus waste | 47 ± 1.41 |
| 2 | CW+RGH | 49 ± 1.47 |
| 3 | CW+CPH | 47 ± 1.38 |
| 4 | CW+BGH | 46 ± 1.41 |
| 5 | CW+RB | 47 ± 1.38 |
| 6 | CW+WB | 46 ± 1.26 |
| 7 | CW+PW | 42 ± 1.35 |
| 8 | CW+PP | 45 ± 1.36 |
| 9 | CW+GNC | 46 ± 1.39 |
| 10 | CW + BFW | 48 ± 1.40 |
Note: ± Standard error; Citrus Waste (CW); Red Gram Husk (RGH); Chickpea Husk (CPH); Black Gram Husk (BGH); Rice Bran (RB); Wheat Bran (WB); Pineapple Waste (PAW); Potato Peals (PP); Ground Nut Cake (GNC); Banana Fruit Waste (BFW).
Table 2: Production of α-galactosidase by B. megaterium VHM1 in SSF using a combination of various substrates with citrus waste.
Maximum activity of α-galactosidase 49 U/g was produced CW+RGH (1:1; w/w). In most of the combinations tested, an increase in enzyme production was observed. This indicated that CW served as a good substrate for α-galactosidase production. However, CW+PW did not enhance the enzyme yield. Liu et al. reported maximum activity of α-galactosidase production when wheat bran (83.2%) along with soybean meal (16.64 w/w) in SSF by Aspergillus foetidus ZUG1 strain [6]. Shankar et al. reported the combination of red gram plant waste with wheat bran showed an increase in α-galactosidase production.
Effect of incubation period on α-galactosidase production
The time course of α-galactosidase production in a medium containing CW, RGH, and CPH is shown in Figure 1. The maximum production of α-galactosidase obtained at 72 hours of fermentation. After 72 hours, the α-galactosidase activity declines, possibly due to a shift of pH and catabolite activity of the organism. CW waste showed maximum activity compared to RGH and CPH. The α-amylase production using wheat bran and potato peels in SSF by Bacillus circulans was found to be maximum at 72 hours of fermentation [7]. Similarly, Srinivas et al. reported an optimum incubation period of 4 days-5 days for α-galactosidase production by Aspergillus niger in SSF using wheat bran as a solid substrate. Wang et al., reported 75 hours of incubation for α-galactosidase production by a novel strain of Penicillium sp in SSF by using wheat bran and soybean meal as a substrate [8]. Kotwal et al. reported six days for higher α-galactosidase production by thermophilic Humicola sp in SSF by using soybean flour and ground nut cake- based substrates.
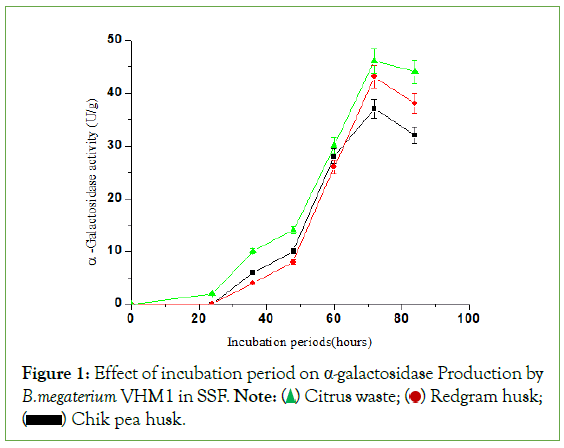
Figure 1: Effect of incubation period on α-galactosidase Production by B.megaterium VHM1 in SSF. Note:  Redgram husk;
Redgram husk;  Chik pea husk.
Chik pea husk.
Effect of temperature on α-galactosidase production
Production of α-galactosidase from B. megaterium was maximum active at 350°C, but enzyme activity decreased with a rise in temperature from 40 °C to 500 °C (Figure 2). Sodhi et al. reported 370°C temperature for production of α-amylase under SSF by Bacillus sp. Ps-7 [9]. Similarly, Babu et al. reported that 370°C was the optimal temperature for the production of α-amylase in SSF by Bacillus coagulans [10]. Jakubowski et al. reported that 390°C was the optimal temperature for the production of α-amylase in SSF by Bacillus lichiniformis [11]. Kotwal et al. reported that the thermophilic fungus Humicola sps required 450°C for growth as well as enzyme production in SSF. Liu et al. reported that the optimal temperature for α-galactosidase production in SSF by Aspergillus foetidus was 280°C. Anisha et al. reported that 350°C was the optimal temperature for α-galactosidase production in SSF by Sreptomyces griseolobus [12].
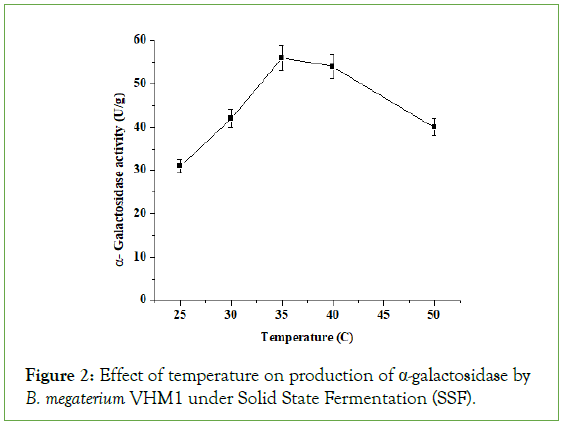
Figure 2: Effect of temperature on production of α-galactosidase by B. megaterium VHM1 under Solid State Fermentation (SSF).
Effect of pH on α-galactosidase production
The optimum pH of the medium for α-galactosidase production was 6.0 pH (Figure 3). Agarwal et al. reported an optimal initial pH of 5.8 for the production of α-amylase in SSF by Bacillus sp KCA 102 [13]. It is speculated that solid substrates contribute to a better buffering capacity. The range of pH 5.5 to 6.5 was favorable for α-galactosidase biosynthesis from a novel strain, Penicillium sps, in SSF [14]. The α-galactosidase activity is strictly dependent upon the pH of the incubation medium. Pencillum ochrochloron showed maximal α-galactosidase at pH 4.5 [15]. Liu et al. reported that 5.0-6.0 initial pH of the medium for optimum α-galactosidase production by a novel strain of Aspergillus foetidus Zu-G1 in SSF.
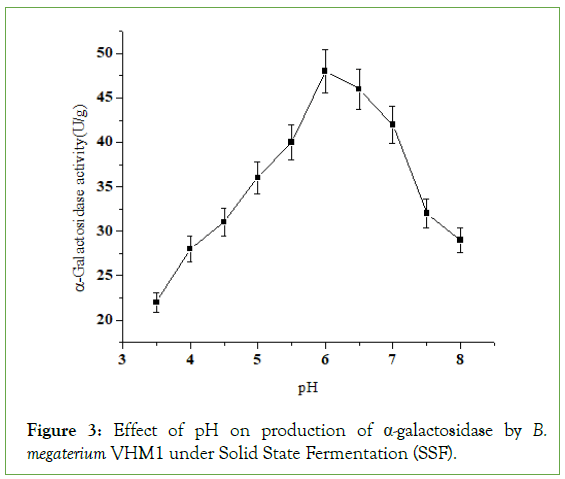
Figure 3: Effect of pH on production of α-galactosidase by B. megaterium VHM1 under Solid State Fermentation (SSF).
Effect inoculum volume on α-galactosidase production
The inoculum concentration plays an important role in enzyme production. With the increase in inoculum volume up to 90% (w/v), there is an increase in α-galactosidase production by B. megaterium (Figure 4). However, with an increase in inoculum level beyond 90% (w/v), the production of α-galactosidase by B. megaterium declined, which might be due to exhaustion of nutrients in the fermentation mash. Callahan et al., 2006, reported that 35% inoculum volume (v/w) is optimal for the production of protease from Engoyodiantum album. Ramachandran et al. reported that 100% (v/w) inoculum size was optimal for the production of thermostable α-amylase by Bacillus sps [16]. Mukherjee et al. reported that 100% inoculum size was optimal for the production of protease by Bacillus sps [7]. Liu et al. reported maximal production of α-galactosidase at 10% (v/w) inoculum size by a novel strain Aspergillus foetidus Zu-G1-Strain.
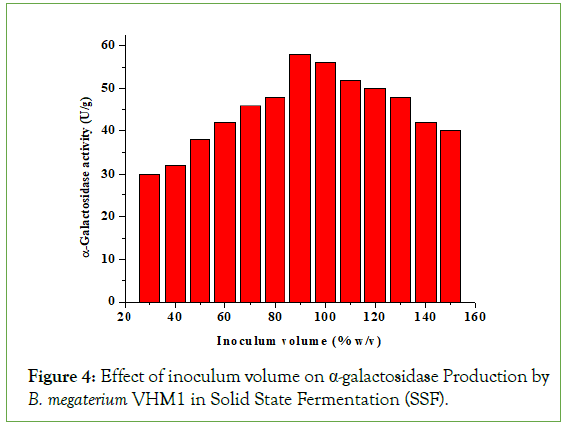
Figure 4: Effect of inoculum volume on α-galactosidase Production by B. megaterium VHM1 in Solid State Fermentation (SSF).
Effect of moisture content on α-galactosidase production
The moisture content is the major factor for microbial growth in SSF, especially the initial moisture content of the substrate, which will play an important role in production and yield [16,17].
The growth of microbes and product formation takes place near the surface of the most solid substrate. Therefore, achieving the maximum yield of the desirable product is the most crucial step in optimizing the moisture content of the substrate [17]. In the present study, with an increase in moisture content of 50%-100%, the production of α-galactosidase was concomitantly enhanced from 38 U/g to 56 U/g, and a further increase in moisture content resulted in a steady decline in the production of α-galactosidase (Figure 5).
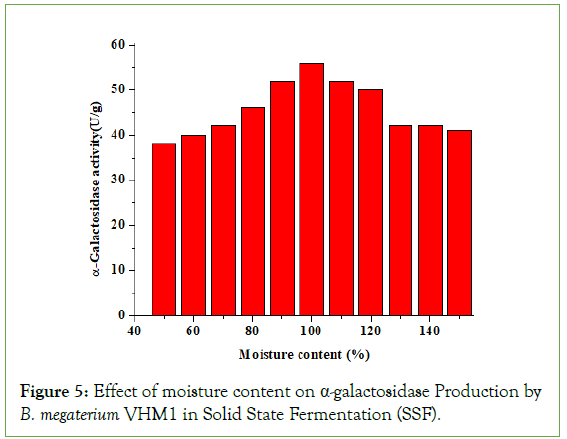
Figure 5: Effect of moisture content on α-galactosidase Production by B. megaterium VHM1 in Solid State Fermentation (SSF).
Das et al. reported that 150% (v/w) moisture content was optimal for the production of α-amylase by Bacillus sp-7 [18]. Prakasham et al. reported that 100% initial moisture content of solid substrate was optimal production of protease by Bacillus subtilis DM04 [17]. Kotwal et al. reported the maximum production of α-galactosidase when the moisture content was 86% (v/w). Shankar et al. reported that 89% (v/w) initial moisture content was optimal for α-galactosidase production by Aspergillus oryzae [1]. The moisture content beyond the optimum level decreases the porosity as well as changes the structure of the particles of the fermentable solid substrates that subsequently promote the development of sickness, reduce gas volume and exchange and lower oxygen transfer, which in turn interfere with the microbial activity [19].
Effect of various nitrogen sources on α-galactosidase production
The effect of nitrogen on enzyme production is shown in Table 3. Among the organic sources tested, yeast extract yielded higher enzyme production (52 U/g), whereas beef extract, peptone, gelatin, and tryptone resulted from lower yield compared to yeast extract. The inorganic nitrogen sources did not show much increase in α-galactosidase activity. Kotwal et al. reported that beef extract acted as the best nitrogen source for maximum α-galactosidase production by Humicola sps in SSF. Zaprometova et al. reported that 2% of ammonium sulfate acted as the best nitrogen source for maximal production of α-galactosidase by Cephalosporium acremonium. Gindy et al. reported beef extract was the best nitrogen source for optimal production of α-galactosidase in SSF by Aspergillus awamori and A. corbanarians [20].
| Nitrogen sources (2% w/w) |
α-Galactosidase activity (U/g) |
|---|---|
| Control | 43 ±2.12 |
| Peptone | 48±2.6 |
| Yeast extract | 52±2.86 |
| Gelatin | 43±2.12 |
| Soybean meal | 48±2.6 |
| Tryptone | 46±2.44 |
| Beef extract | 45±2.40 |
| Ammonium sulphate | 44±2.36 |
| Ammonium nitrate | 42±2.18 |
| Ammonium acetate | 44±2.01 |
| Urea | 41±2.12 |
Note: ± standard error.
Table 3: Effect of various nitrogen sources on the production of α-galactosidase by B. megaterium VHM1.
Effect of various sugars on α-galactosidase production
The enzyme production was maximum when raffinose was used as an additional carbon source (Table 4). Galactose and melibiose also induced the maximum production of α-galactosidase, whereas glucose and sucrose were much less effective for α-galactosidase Production as well as growth of the organism. Raffinose enhanced the α-galactosidase activity (176%) in the SSF medium. Liu et al. reported maximal α-galactosidase production when glucose was added to the wheat bran in solid-state fermentation by Aspergillus foetidus. Anisha et al. reported that the addition of lactose to the solid substrates medium induced the maximal production of α-galactosidase from Streptomyces griseolobus [12].
| Sugars (2% w/w) | α-Galactosidase activity (%) |
|---|---|
| None | 100 |
| Glucose | 123 |
| Galactose | 176 |
| Sucrose | 120 |
| Lactose | 103 |
| Melibiose | 171 |
| Raffinose | 182 |
Table 4: Effect of sugars on α-galactosidase Production by B. megaterium VHM1 in solid-state fermentation.
Enhanced production of α-galactosidase in forcefully aerated packed bed bioreactor
Enhanced production of α-galactosidase was carried out using forcefully aerated packed bed bioreactor. In SSF, the most limiting factor is the aeration. The metabolic heat produced by the microorganisms limits the growth and production of α-galactosidase. This can be overcome by constructing the forcefully aerated packed bed bioreactor. The schematic diagram of the bioreactor is shown in Figure 6. Alpha-galactosidase production by B.megaterium in packed bed with aeration and without aeration is shown in Table 5. The forceful aeration resulted in the highest α-galactosidase yield of 105 U/g, which was approximately twice the yield obtained in flasks. In a packed bed bioreactor, most of the metabolic heat and CO2 released from the fermented mash could be removed by forced aeration with humidified air, thus minimizing the rise in temperature of the fermenting substrate. The packed bed bioreactor offers several advantages over tray fermentation previously reported by several workers for α-galactosidase production in SSF. It allows better control of fermentation parameters than possible in trays [21-26]. The production of α-galactosidase tested at different hours of incubation in a forcefully aerated packed bed bioreactor (Figure 7). Maximal production of α-galactosidase was observed at 60 hours of incubation. After that, enzyme production gradually decreased. Anisha et al. similarly reported a fold increase in α-galactosidase production using forcefully aerated packed bed bioreactor by Streptomyces griseoloalbus [12].
| Bacillus megaterium VHM1 | α-Galactosidase activity (U/g) |
|---|---|
| Without aeration | 72 ± 3.6 |
| With aeration | 105 ±5.25 |
Table 5: Effect of aeration on packed bed bioreactor for production of α-galactosidase by B. megaterium VHM1.
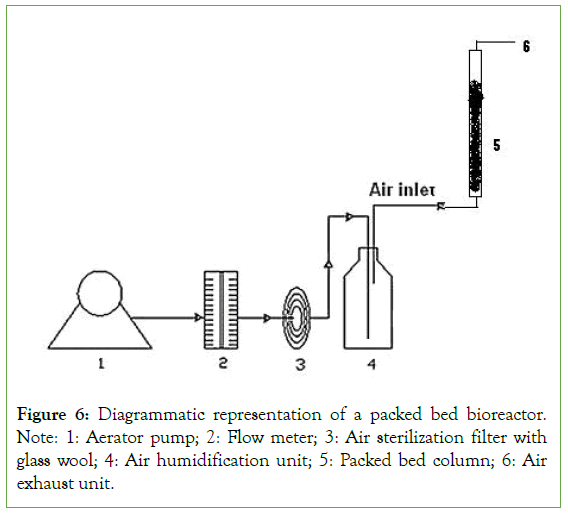
Figure 6: Diagrammatic representation of a packed bed bioreactor. Note: 1: Aerator pump; 2: Flow meter; 3: Air sterilization filter with glass wool; 4: Air humidification unit; 5: Packed bed column; 6: Air exhaust unit.
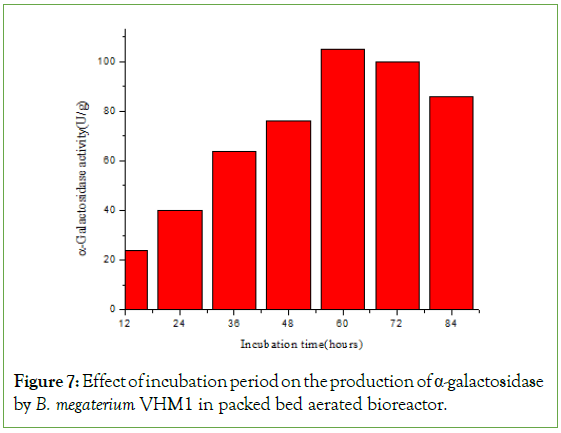
Figure 7: Effect of incubation period on the production of α-galactosidase by B. megaterium VHM1 in packed bed aerated bioreactor.
Conclusion
The reports reveal the α-galactosidase production using citrus waste by the bacterium B. megaterium VHM1, which is a cost-effective method. We have constructed a forcefully aerated packed bed bioreactor for enhanced production of α-galactosidase. The organism B. megaterium utilizes complex polysaccharides and produces extracellular α-galactosidase. B. megaterium is GRAS (generally regarded safe) status. The so-produced enzyme can be exploited in the processing of legumes in food process industries to remove the raffinose family oligosaccharides. The laboratory scale experiments may provide a basis for scale-up purposes of the processor on α-galactosidase production in SSF.
Conflict of Interests
There is no conflict of interest.
Author Contribution
AGP, NVK and GSK carried out the experiments, and AGP and VHM planned and wrote the Manuscript.
Funding
One of the authors, Aravind Goud G Patil, is thankful to the Council of Scientific and Industrial Research. New Delhi, for providing financial support in the form of a Senior Research Fellowship (SRF).
References
- Mulimani VH, Thippeswamy S, Ramalingam S. Enzymatic degradation of oligosaccharides in soybean flours. Food Chem. 1997; 59(2):279-282.
- Patil AG, Kote NV, Mulimani VH, Kadakol G. Enhanced production of α-galactosidase from novel strain Bacillus megaterium VHM1 in solid state fermentation by using citrus waste. 2007.
- Kumar SP, Surekha D, Joginder SD. Agro-industrial wastes and their utilization using solid state fermentation: A review. Biores and Bioproces. 2018; 5(1):13-15.
- Vary PS, Biedendieck R, Fuerch T, Meinhardt F, Rohde M. Bacillus megaterium from simple soil bacterium to industrial protein production host. Appl Microbiol Biotechnol. 2007;76(1):957-967.
- Claus D, Berkley RCW. Bergey's manual of systematic bacteriology. Baltimore: Williams and Williams. 1986:1104-1139.
- Liu CQ, Chen QH, Cheng QJ, Wang JL, He GQ. Effect of cultivating conditions on α-galactosidase production by a novel Aspergillus foetidus ZU-G1 strain in solid-state fermentation. J Zhejiang Univ Sci B. 2007;8(1):371-376.
- Mukherjee AK, Borah M, Rai SK. To study the influence of different components of fermentable substrates on induction of extracellular α-amylase synthesis by Bacillus subtilis DM-03 in solid-state fermentation and exploration of feasibility for inclusion of α-amylase in laundry detergent formulations. Biochem Eng J. 2009;43(2):149-156.
- Wang CL, Li DF, Lu WQ, Wang YH, Lai CH. Influence of cultivating conditions on the α‐galactosidase biosynthesis from a novel strain of Penicillium sp in solid‐state fermentation. Lett Appl Microbiol. 2004;39(4):369-375.
- Sodhi HK, Sharma K, Gupta JK, Soni SK. Production of a thermostable α-amylase from Bacillus sp. PS-7 by solid state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production. Process Biochem. 2005;40(2):525-534.
- Babu KR, Satyanarayana T. α-Amylase production by thermophilic Bacillus coagulans in solid state fermentation. Process Biochem. 1995; 30(4):305-309.
- Jakubowski A, Kwapisz E, Polak J, Galas E. The biosynthesis of Bacillus licheniformis α-amylase in solid state fermentation. In Progress in Biotechnology. 2000;172(1):35-239.
- Anisha GS, John RP, Prema P, Pandey A. Investigation on α-galactosidase production by Streptomyces griseoloalbus in a forcefully aerated packed-bed bioreactor operating in solid-state fermentation condition. Appl Biochem Biotechnol. 2010;160(1):421-427.
- Agrawal M, Pradeep S, Chandraraj K, Gummadi SN. Hydrolysis of starch by amylase from Bacillus sp. KCA102: a statistical approach. Process Biochem. 2005; 40(7):2499-2507.
- Kim JS, Kim JH, Ryu EK, Kim JK, Kim CK, Hwang IG. Versatile catabolic properties of Tn4371-encoded bph pathway in Comamonas testosteroni (Pseudomonas sp) NCIMB 10643. J Microbiol Biotechnol. 2004;14(2):302-311.
- Dey PM, Pridham JB. Biochemistry of α‐galactosidases. Adv Enzymol. 1972;36(1):91-130.
- Ramachandran S, Patel AK, Nampoothiri KM, Francis F. Coconut oil cake–a potential raw material for the production of α-amylase. Bioresource Technol. 2004; 93(2):169-174.
- Prakasham RS, Rao CS, Sarma PN. Green gram husks an inexpensive substrate for alkaline protease production by Bacillus sp in solid-state fermentation. Bioresour Technol. 2006;97(13):1449-1454.
- Das K, Doley R, Mukherjee AK. Purification and biochemical characterization of a thermostable, alkaliphilic, extracellular α‐amylase from Bacillus subtilis DM‐03, and a strain isolated from the traditional fermented food of India. J Biotechnol Appl Biochem. 2004; 40(3):291-298.
- Krishna C. Solid-state fermentation systems-an overview. Critic Rev Biotechnol. 2005;25(2):1-30.
- El-Gindy AA, Ali UF, Ibrahim ZM, Isaac GS. A cost-effective medium for enhanced production of extracellular α-galactosidase in solid substrate cultures of Aspergillus awamori and A. carbonarius. Aust J Bas Appl Sci.;2(4):880-889.
- Ashley VM, Mitchell DA, Howes T. Evaluating strategies for overcoming overheating problems during solid-state fermentation in packed bed bioreactors. Biochem Eng J. 1999; 3(2):141-150.
- Ahmed SA. Optimization of production and extraction parameters of Bacillus megaterium levansucrase using solid-state fermentation. J Appl Sci Res. 2008;4(10):1199-1204.
- Bocchini DA, Oliveira OM, Gomes E, Da Silva R. Use of sugarcane bagasse and grass hydrolysates as carbon sources for xylanase production by Bacillus circulans D1 in submerged fermentation. Process Biochem. 2005;40(12):3653-3659.
- Chellappan S, Jasmin C, Basheer SM, Elyas KK, Bhat SG, Chandrasekaran M. Production, purification and partial characterization of a novel protease from marine Engyodontium album BTMFS10 under solid state fermentation. Process Biochem. 2006;41(4):956-961.
- Jin F, Li Y, Zhang C, Yu H. Thermostable α-amylase and α-galactosidase production from the thermophilic and aerobic Bacillus sp JF strain. Process Biochem. 2001;36(6):559-564.
- LeBlanc JG, Silvestroni A, Connes C, Juillard V, de Giori GS. Reduction of non-digestible oligosaccharides in soymilk: application of engineered lactic acid bacteria that produce alpha-galactosidase. Genet Mol Res. 2004;3(3):432-440.
Citation: Kote NV, Mulimani HV, Kadakol G, Patil AGG (2023) Enhanced Production of ?-Galactosidase from Novel Strain Bacillusmegaterium VHM1 in Solid-State Fermentation by Using Citrus Waste. J Food Process Technol. 14:1074.
Copyright: © 2023 Kote NV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


