Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Original Research Article - (2021) Volume 11, Issue 5
Three Bâs Local Bio-Material Wastes to Energy; Potential for Environmental Management and Sustainability
Kabok P Aguko*, Michael O Oloko and George K NgusaleSchool of Engineering and Technology, Jaramogi Oginga Odinga University of Science and Technology, 210-40601, Bondo, Kenya
School of Engineering and Technology, Jaramogi Oginga Odinga University of Science and Technology, 210-40601, Bondo, Kenya
Received: 08-Mar-2021 Published: 18-May-2021, DOI: 10.35248/2252-5211.21.11.411
Abstract
The Counties adjacent or neighboring Lake Victoria in Kenya constitute the Nile Basin and are not exempt from carbon uptake loss, increased non-renewable fuels use and environmental degradation risks such as global warming due to population growth. Energy demand for counties in the Nile Basin becomes a critical challenge in terms of wood fuel availability and the catchment services. This study ascertained the potential of bio-energy material based on site-specific (local) characteristic. Particularly, water hyacinth (Hy) in gulf, markets for sawdust, feacal matter (FM) in towns for Bio-ethanol, Biogas and Briquettes. 100 ml Bio-ethanol from hyacinth cooked three liters of tea in 25 minutes against an ordinary charcoal cooking stove (Jiko) for 45 minutes. FM and Hy (1:1) briquettes could substitute charcoal/firewood because of high calorific/heat values of proximate value analyses. That; a person produces averagely 123.6 grams of feacal per call or 0.12 Kg for about 0.02 m3 of methane. These 3B’s (Biogas, Bioethanol and Briquettes) energy options could drive waste management for entrepreneurships within Counties neighboring Lake Victoria for SDG’s strategies.
Keywords
Lake Victoria; Energy Demand; Bio-energy; Bio-ethernal; Biogas; Briquettes; Entrepreneurships
Introduction
Kisumu and Siaya Counties, the study area, are respectively located between latitudes 00° 20' to 00° 50' South; 0° 26’ to 0° 18’ north and longitudes 33° 20'E to 35° 20'E; 33° 58’ east to 34° 33’ west. Kisumu County is with a population of 968,909 (2009 Kenyan National Census) and at assumed growth rate of 2.7%, the current (2019) population is 1,155,574. Siaya County’s population was estimated at 885,762 (KNBS 2012 Population projections) and was expected to grow at a rate of 1.7 per cent per year and as of 2019 census the population was 993,183. These towns within the Lake Victoria Basin are not exempted from environmental degradation such as global warming due effects of population growth (basis of SDGS 1 to 17). Odada, et al., [1] summarizes that this includes; ‘perceptions and practices related to natural resources exploitation in the Lake Victoria, intertwined with livelihoods, culture and political expediency that makes it complex and threatens sustainable use of environmental resources’.
The demand for energy due to population increase (SDG 7) is a critical challenge within a locality (such as Kisumu and Siaya counties) in terms of availability and the world today. In Kenya, wood fuel accounts for 70% of the country’s energy consumption needs and traditionally is for heating (cooking) by 90% of the rural household and about 85% urban areas (SDG 15). The major source of energy (Transport, gas-cooking etcetera) is from non-renewable fossil fuel that is associated with global warming, environmental degradation, and human health problems (SDG 13) [2]. It is also cognizant that, the negative impacts of traditional fuel sources (firewood, charcoal, and kerosene etcetera) on the environment together with cost factors, led to the development of renewable energy sources for economic and environmental benefits (U.S Department of Energy-Energy Efficiency and Renewable Energy, 2011). Consequently, the growing energy demand encourages scientists’ worldwide to explore low cost, environmental friendly and sustainable alternative energy sources [3-6]; more so, use of locally available biomaterial to be encouraged.
The world energy council (WEC) 2000predicted, “Modern or improved biomass around the world will replace the traditional biomass in rural areas by 2020, and traditional biomass will decline to about 20% energy demand in developing countries [7]. This has not happened and is part of the SDG targets. In the developing world, the possible transformation of natural biomass to modernized renewable energy is through Briquetting, Biogas and Bio-ethanol (christened here as three B’s), apart from the solar and hydro sources.
The Three, B’s
Briquetting is the physical transformation of loose raw material mostly made of biomass waste into high-density fuel briquettes through compacting, for domestic and industrial use [8,9]. Raw materials include but not limited to: feacal matter, charcoal, coffee husk, maize stock, bagasse, sawdust [10-13]. However, FM as a raw material should be free of pathogens when heat treated properly and solely or combined with other biomass material for its relatively higher combustion characteristics and density. The factors for mixing FM and other biomaterial should consider availability, cost, burning characteristics, and their potential to ignite to burn to provide heat energy. Biogas is also an alternative renewable energy that can fulfill the demand for cooking where there exists no option other than firewood [14]. Bioethanol too as a clean, safe renewable energy resource, is a potential substitute to fossil fuels as is given emphasis [15-17]. Feacal matter, water hyacinth, sawdust, rice husks, paddy rice stalks, sugar cane stalks and bagasse do meet the criteria for Nile basin region (Kisumu and Siaya counties) for availability emphasized [18-21].
The lignocellulose water hyacinth fits bioethanol production and as is described [22-24,15]. Its origin is explained [25] and the unfavorable characteristics [26,27]. The favorable characteristics for briquetting are as discussed [28-32]. Just as is sawdust described by Kuti and Adegoke, [33] Switch grass described [34-36]; as are respectively also attractive sources for Briquettes and Bio- ethanol.
Sludge (from sewers, pit latrines and septic tanks) management on the other hand constitutes a key part of wastewater treatment process and involves substantial costs and resources [37,38]. In the developing countries like Kenya, the amount of faecal matter (a bio- matter) is increasing in direct proportion to population (concern of SDGS strategy 1 to 17) and is causing constraints in the maintenance and operation of treatment plants in urban areas.
The aim of this study was to ascertain the potential for utilization of local saw dust as a market waste material, water hyacinth (Hy) from Kenyan Lake Victoria gulf, feacal matter (FM) of towns or townships for production of Bio-ethanol, Biogas and Briquettes to meet collective needs in both cooking and energy for heating. Particularly to single out these renewable energy production potential options as the drivers to waste management, related entrepreneurships for sustainability and as the triple SDG strategy for Kisumu and Siaya Counties (a locality) in Kenya.
Methodology
Study Area
The Bio-ethanol/Biogas study productions were at Jaramogi Oginga Odinga University of Science and Technology (JOOUST) in Bondo sub-county, Siaya County and Kisumu City in Kisumu County (Kenya). The average annual temperature in Bondo and Kisumu are respectively 28.3̊C and above which ordinarily can support a fermentation process. The briquetting was undertaken in Nakuru county (Latitude 0° 18' 11.156" S Longitude 36° 4' 48.094" E) at the Nakuru Water Service and Sanitation Company (NAWASCO) treatment site in Kaloleni. The water hyacinth for Bio-ethanol and briquettes were from Lake Victoria, Usenge beach in Siaya County -Kenya.
The Bio-Materials Tests
Briquetting
Faecal matter and sawdust were respectively from pit latrines of low-income areas of Kivumbini/Kaloleni by exhauster truck and from local sawmills within Nakuru town. The potassium chlorate was from Nyanza Equipment laboratory, Kisumu, while laboratory analysis was at JOOUST, Government Chemist of Kisumu and the Maseno University (Kenya).
Raw Material Preparation
The water hyacinth (from Lake Victoria), faecal matter and sawdust were dried in the green house (Figure 1) for 7-14 days with periodic mixing to allow aeration, quick drying and was intended to reduce the moisture to about 12% [39,40]. Foremost, water hyacinth was milled to size equivalent to sawdust particles, and then was carbonized in open half drum. Faecal matter and sawdust were nonetheless carbonized in a drum kiln, an open half cut drum (Figures 2-4), and wereseparately milled to 0.5 mm by a hammer mill (Figure 5). The mixing ratios selected were as depicted in Table 1. The additive material was ground to size by a mortar and crucible. 20 liters of water to one liter of molasses was stirred as was mixed to form a binder solution.

Figure 1: From left: Water Hyacinth at Lake Victoria; Dry Water Hyacinth; Milled Water Hyacinth; Sawdust and Faecal Matter in the Greenhouse.

Figure 2: Drum Kiln.

Figure 3: Carbonization of Sawdust & Water Hyacinth.

Figure 4: Carbonized Sawdust &Water Hyacinth.

Figure 5: Hammer Mill and a Batch Mixture.
| Samples | Ratio |
|---|---|
| Faecal matter-sawdust potassium chlorate | 3:3:1 |
| Faecal matter –sawdust | 1:1 |
| Faecal-matter-water hyacinth | 1:1 |
| Faecal-matter-water hyacinth | 3:1 |
Table 1:Mixing Ratios.
Agglomeration: The non-pressure method and a dustpan respectively were used to grow the materials into spherical briquettes (Figure 6) and loading the drum with a 15 kg mixed ratio of the material. Dust was added at intervals followed by the binding agent (Molasses) sprinkled on the dust to allow compaction of the mixture to obtain 45 mm. The binder solution is desirable in a rotating drum to result into a mass such as a pellet or granule, variously cited as disc- pelletizing, rotary drum granulation, pin mixing and paddle mixing [11]. This increases or decreases the porosity or density of a material through nucleation, coalescence and consolidation. The final stage attrition; compacts the product and is characterized by surface wear due to friction. (Figure 7) shows the direct sunlight drying of briquettes subjected to tests after four days.

Figure 6: Agglomeration.

Figure 7: Drying Beds.
Physical characteristics and proximate analysis (density, moisture content, volatile matter, ash content and caloric value) were done for briquettes samples as per ASABE standards [41-45]. An Atomic Absorption Spectrophotometer-Shimadzu AA6300, Auto Sampler (ASC -6100) and a Graphite Furnace Automizer (GFA-EX 7i) for determining the ash component. Fixed carbon (FC) and heating value (Hv) were determined by the equations; FC=100-(% VM+% ASH and Hv=2.326 (147.6c+144v); where c = % fixed carbon, v = % volatile matter. In addition, it is that the calorific value of charcoal, is 25.7 kj/kg @ 1.7% MCwet and or 27.6-31.5 kj/kg @~5% MCwet.
Water Boiling Test
The water-boiling test (WBT) was according to version 4.2.3 and 4.1.2. Four replica WBT for each sample of briquettes were done and characteristics performance equations used in determining the parameters. The subsidiary equipment used included; digital thermometer, a small shovel for removing hot briquettes from the stove,tongs for handling briquettes, heat resistance gloves, and a wood fixture for holding the thermometer probe in water, timer, and standard pots. Altitude determined, was by a hand-held GPS and others determined were, Burning Rate, Specific Fuel consumption, Power Output and Burning Time (the average time taken for the briquette to bring the water to the boiling point; [46]).
Producing Bio-ethanol
Preparation: The procedure adopted was that; water hyacinth fetched from Usenge beach in Siaya County was cleaned, chopped, ground to pieces to increase the surface area for enzymes (yeasts) and placed in a container ready for fermentation. Hyacinth (Hy), distillers yeast (Dy), Sugar (Sg) and water (Wr) were mixed in a ratio of 1:0.05:0.05:1by weight (20:1:1:20 in Kgs) and put in a container to fit or at least 20% larger.
To remove oxygen, water was warmed then mixed with hyacinth in a container in equal ratio and was tightly closed and left for 24 hours for the aerobic process. Yeast was mixed, stirred and was left for more six hours. Sugar was added next, mixed and the container closed to preclude air (oxygen), and all was for six days to ferment. On the seventh day, the alcoholic content could smell ready for fractional distillation after sieving. The pH and temperature respectively measured in the process ranged from 3.0 to 5.0 and 27℃ and 35℃.
Extracting Bio-ethanol: The distillation apparatus were a Clamp and Triple-Stand, Beaker, Lie-big Condenser, Soxhlet extractor, Conical flask, Measuring cylinder, Rubber tubes, Burning plate and Running water (Figure 8). Foremost, a 250 mL of the ferment was poured into a conical flask, clamped and dipped into a beaker that had water on a burning plate ready for distillation. Next, a Lie- big condenser was (with water inlet and outlet rubber tubes) clamped and inserted into a Soxhlet extractor and to a conical flask. The burning plate was switched on, temperature adjusted and maintained at maximum of 360°C for it to heat water in the beaker. The mixture in the conical flask evaporated as heat increased and distillate collected in the soxhlet extractor. The volume was recorded next as it was transferred to a measuring cylinder, and the procedure was repeated several times for more bio-ethanol as time was recorded. It is worth noting that heating source changeable, as appropriate to solar for sustainability.

Figure 8: Bioethanol Extraction Apparatus.
Bioethanol Test: The alcohol type test from water hyacinth was conducted at the JOOUST laboratory. Foremost, a 20 mL of bioethanol solution was in a boiling tube, potassium dichromate solution added and the color recorded. Color of mixture as dilute sulphuric acid was added to the pre - warmed mixture, was noted. The effective cooking power was tested ordinarily by placing 250 mL in a bio-ethanol cooker, 2L of water poured into a cooking vessel, 500 mL of milk added and the mixture heated. Comparison was also for time taken to cook the same amount of tea in an ordinary jiko to that on Bio- ethanol cooker.
Producing Biogas
Slurry Addition and Gas Collection: Three digester types used were; Fixed Dome Bio-Digester (FDBD), Flexible Structure Bio- Digester (FSBD) and a Simple Ordinary Drum Bio-Digester (SODBD).
The FSBD (1 m3) at Kibuye in Kisumu City were fed with initial 600 liters of market wastes slurry while those at Nyamasaria (Kisumu City -2.4 m3), with an initial 1500 liters. Respectively this left a space of 400 (33%) and 900 liters (37.5%) for gas and every week 100 and 300 liters were added. In the case of the SODBD, as 75 liters of slurry poured through the inlet valve and closed, 25% space for the gas remained. The gas realization was to take between 20-30 days after digestion (estimated). Close monitoring during the period was undertaken and especially for leaks. Every after 14 days a balloon was used for collecting gas volume produced for a test (first and second). A simple burning test carried out for the gas by opening the gas valve, lighting a matchstick and pointing to the 25 mm gas outlet tube.
Results and Discussion
Briquetting:The Biomaterials and their bulk densities used in the study were; Faecal matter (FM - 490 kg/m3), water hyacinth (WH- 87 kg/m3) and sawdust (SD- 210 kg/m3). When these were briquetted by agglomeration to an average of 4cm diameter, the bulk densities changed with the Bio – material mixture (Figure 9) as; FM-WH (3:1–1036 kg/m3), FM-WH (1:1 -1024 kg/m3), FMSD & kclo3-999.41 kg/m3, and FM-SD (920.92 kg/m3). The raw materials significantly affected briquette densities for example particle size and moisture content, the smaller the particle size, the higher the density of the briquettes.
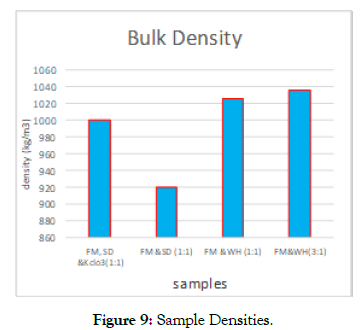
Figure 9: Sample Densities.
The proximate and combustion properties of the briquettes examined (Density, % volatile matter, % ash content, % fixed carbon and heating or calorific value) based on sample analysis of variance resulted in mean percentage volatile matter of 3.22 ± 3.11. Though FM-SD compared to FM -WH combinations had higher percentage of volatile matter as; FM-SD (5.68%), FM-SD & kclo3 (4.70%,) and FM -WH (1:1 & 3:1-2.64% and 0.08%).
Ash content: the average ash content obtained from the briquettes was 13.90 ± 0.72, though the ratio of FM-WH (1:1) had the highest ash content value of 18.96%. This was significantly higher than FM-SD (1:1). FM-SD & kclo3 (1:1) and FM-WH (3:1) shown in Figure 10. Additionally, the ash content was tested (Table 2) for presence of manganese, Lead and Nickel which could be harmful to the environment; with an Atomic absorption spectrophotometer (ASC-6100) and a graphite furnace automizer (GFA). According to food and agriculture (FAO) and World Health Organization (WHO) heavy metal regulation-Faolex, legal notice no.66/2003 the standard concentration of lead, nickel and manganese are respectively 0.061-0.461ppm, 0.150-1.031ppm and 401.8-583.7ppm. These metals tests were done because of their diverse effects in environment such as brain development and nervous system, risk of high blood pressure and kidney damage to humans. Exposure of pregnant women to high levels of lead leads to miscarriage, still, premature and low birth weight as well as minor malformations.
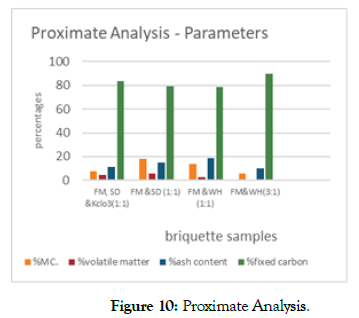
Figure 10: Proximate Analysis.
| Samples | Lead actual conc.ppm | Manganese (Mn) actual conc.ppm |
Nickel (Ni) actual conc.ppm |
|---|---|---|---|
| FM/SD/Kclo3 | 0.3526 | 36.1648 | 2.3712 |
| FM/SD | 0.1814 | 31.5590 | 1.2425 |
| FM/WH (1:1) | 0.4323 | 32.2784 | 2.6829 |
| FM/WH (3:1) | 0.5101 | 29.2941 | 0.9477 |
Table 2:Ash Content.
Heating and Calorific Values: The heating and calorific values from the FM–SD samples ranged from 122,422.31-127,445.85Kj/ Kg and for FM-WH ranged between 116,761.47-129,526.11Kj/Kg (Figure 11). The highest heating value was from potassium chlorate added material and the least was 1:1- ratio FM - WH. This shows that the heat value of sawdust added samples were higher to the water hyacinth samples.
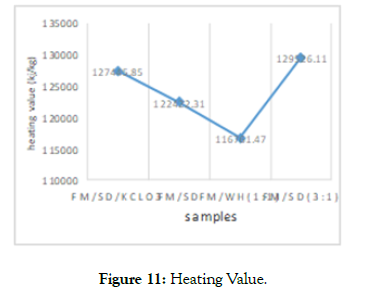
Figure 11: Heating Value.
Ignition time: A Kenya Ceramic Jiko (KCJ) cook stove was used in determining the time required for a flame to ignite a briquette sample; the ignition time in a controlled condition with no wind but a well-ventilated area. The commonly (locally) used method (papers) at homes was also applied and the ignition time recorded by a stopwatch. The briquette sample with equal ratio of FM -Wh ignited faster than the (3:1) ratio, this could be because faecal matter is denser than water hyacinth. Figure 12 below thus shows that ignition time decreased with the decrease in the ratio of faecal matter. Briquettes made from FM-SD/Kclo3 had a low ignition time of 299secs (Table 3) compared to the other samples, while the 3:1 FM –Wh samples had a high ignition time of 393sec (Table 3). This confirms that Potassium chlorate reacts vigorously and spontaneously to ignite when mixed with combustible materials.
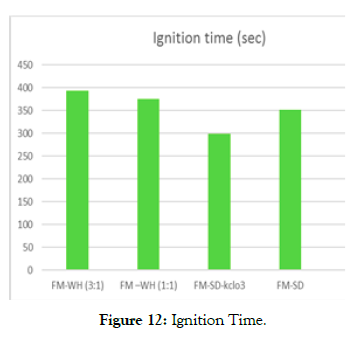
Figure 12: Ignition Time
| Samples | Lead actual conc.ppm | Manganese (Mn) actual conc.ppm |
Nickel (Ni) actual conc.ppm |
|---|---|---|---|
| FM/SD/Kclo3 | 0.3526 | 36.1648 | 2.3712 |
| FM/SD | 0.1814 | 31.5590 | 1.2425 |
| FM/WH (1:1) | 0.4323 | 32.2784 | 2.6829 |
| FM/WH (3:1) | 0.5101 | 29.2941 | 0.9477 |
Table 3: Briquette Sample Characteristics.
Burning time: Burning time of briquettes is the duration taken to bring one liter of water to boiling and is always affected by the material type (Figure 13). Likewise, the atmospheric pressure, altitude and amount of impurities affect the boiling point of water. It was thus determined as 940C at 1227m altitude, 0014’18” N and 34016’09” E during the month of February at Bondo in Siaya County.
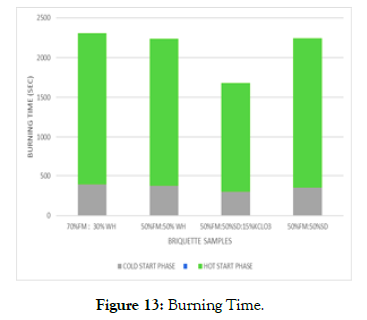
Figure 13: Burning Time.
FM-SD-KCLO3 sample indicated the lowest burning time (1380.6sec), as faecal matter (3:1) for sample of water hyacinth showed the highest burning time (1891 sec). FM- SD samples indicated less burning time compared to FM-Wh, confirming that the determined high volatile matter content of FM- SD samples (4.10~5.68 %) compared to the low volatile matter content of FMWh of 0.08~2.64% played a role. Those samples with high ratio of FM-WH (3:1) thus higher density (1036 kg/m3) with reduced porosity hence low oxygen infiltration with decreased burning rate and increased burning time of the briquettes. This compares well with the work of Chaney et al. [47], Sotannde et al. [48] and by Loo, and Koppejan [49], that states that volatile matter content influences the thermal behavior of solid fuels.
Burning Rate
The FM-Wh briquettes burnt faster than FM-SD since water hyacinth is less dense than sawdust and feacal matter (490 kg/ m3). Briquettes with lower density burn faster and is consistent t because high porosity allows oxygen to diffuse into the briquettes [47]. The burning rate measures the quantity of briquettes burnt in a given time. Faecal matter -sawdust-kclo3 briquettes had the highest burning rate of 7.278 g/min while Faecal matter-water hyacinth (3:1) had the lowest burning rate of 5.471 g/min (Figure 14). The highest burning rate was obtained at high power cold start phase while the lowest at low power phase compared to low power that the heat value of sawdust added samples were higher to the water hyacinth samples.
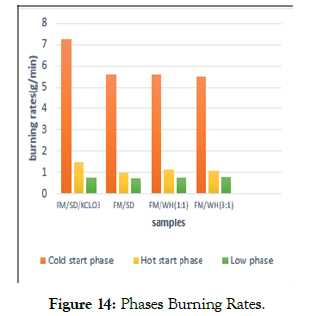
Figure 14: Phases Burning Rates.
Ignition time: A Kenya Ceramic Jiko (KCJ) cook stove was used in determining the time required for a flame to ignite a briquette sample; the ignition time in a controlled condition with no wind but a well-ventilated area. The commonly (locally) used method (papers) at homes was also applied and the ignition time recorded by a stopwatch. The briquette sample with equal ratio of FM -Wh ignited faster than the (3:1) ratio, this could be because faecal matter is denser than water hyacinth. Figure 12 below thus shows that ignition time decreased with the decrease in the ratio of faecal matter. Briquettes made from FM-SD/Kclo3 had a low ignition time of 299secs (Table 3) compared to the other samples, while the 3:1 FM –Wh samples had a high ignition time of 393sec (Table 3). This confirms that Potassium chlorate reacts vigorously and spontaneously to ignite when mixed with combustible materials.
Burning time: Burning time of briquettes is the duration taken to bring one liter of water to boiling and is always affected by the material type (Figure 13). Likewise, the atmospheric pressure, altitude and amount of impurities affect the boiling point of water. It was thus determined as 940C at 1227m altitude, 0014’18” N and 34016’09” E during the month of February at Bondo in Siaya County.
FM-SD-KCLO3 sample indicated the lowest burning time (1380.6 sec), as faecal matter (3:1) for sample of water hyacinth showed the highest burning time (1891 sec). FM- SD samples indicated less burning time compared to FM-Wh, confirming that the determined high volatile matter content of FM- SD samples (4.10~5.68 %) compared to the low volatile matter content of FMWh of 0.08~2.64% played a role. Those samples with high ratio of FM-WH (3:1) thus higher density (1036 kg/m3) with reduced porosity hence low oxygen infiltration with decreased burning rate and increased burning time of the briquettes. This compares well with the work of Chaney et al. [47], Sotannde et al. [48] and by Loo, and Koppejan [49], that states that volatile matter content influences the thermal behavior of solid fuels.
Burning Rate The FM-Wh briquettes burnt faster than FM-SD since water hyacinth is less dense than sawdust and feacal matter (490 kg/ m3). Briquettes with lower density burn faster and is consistent t because high porosity allows oxygen to diffuse into the briquettes [47]. The burning rate measures the quantity of briquettes burnt in a given time. Faecal matter -sawdust-kclo3 briquettes had the highest burning rate of 7.278 g/min while Faecal matter-water hyacinth (3:1) had the lowest burning rate of 5.471 g/min (Figure 14). The highest burning rate was obtained at high power cold start phase while the lowest at low power phase compared to low power hot start phase. The potassium chlorate briquettes burned faster.
Specific fuel consumption: The average specific fuel consumption of three replications of water boiling test of the sampled briquettes at selected mix ratios ranged between (399.558-449.797) g/l for high power cold start, (480.7187-541.162) g/l for high power hot start and (603.25-679.105) g/l for low power phases as shown in (Figure 15). On average and on a decreasing trend, faecal mattersawdust -potassium chlorate had a higher specific fuel consumption compared to FM-Wh and to FM-SD samples. This is attributed to higher ignitability and low density of water hyacinth blend compared to sawdust blend. The specific fuel consumption decreased with increase in the faecal matter with higher calorific value compared to the water hyacinth and sawdust. Fuel consumption was more in the cold start than in the hot start as some heat warmed the cook stove and the surrounding. Specific fuel consumption was also higher in low power phase against the high-power phase for all the samples because the water was at a temperature as close as possible to 3oC below the boiling point for a long time (45 minutes).
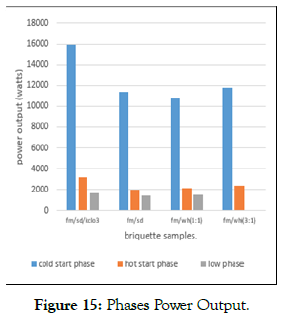
Figure 15: Phases Power Output.
Less mass of faecal matter -water hyacinth (3:1) brought the water to the boiling because of its high calorific value and density. The findings agree with that of Chinyere et al. [50], Abdul Rasheed et al., [51] and Oyelaran et al.,[52] that the low specific fuel consumption of biomass briquettes respectively could be as a result of high heat content, high density and that low power phase was higher than the hot power phase for groundnut shell briquettes.
Power Output The average amount of energy released in burning FM-SD and FM-Wh in each phase of the three replications of water boiling tests at selected mix ratios respectively ranged between (10853.437-15919.162 watts, in high power cold start,), (2005.856-3175.530 watts, in high power hot start) and (1396.520-1623.754 watts, in low power phases) - (Figure15). The power output of the briquettes samples at the three phases considering the selected ratios depended on the type of feedstock used. Higher power output was with FM-SD and potassium chlorate briquettes than the other samples. Figure 16 below shows the combustion of the briquette samples in different Jiko stoves

Figure 16: Combustion of Briquette Samples.
Bio- Ethanol
Alcohol extracted from water hyacinth when tested; changed color from orange to yellow (Figure 17) and this indicated a primary alcohol type. Shown in Figure 19 is that day 1 - 2 was the maximum best time of bioethanol extraction from ferments of the water hyacinth. The amount extracted decreased drastically from day 1 to day 7, as most likely the quantity of enzymes acting on the substrate, also decreased. Thus, the alcohol obtained burnt with a bluish flame and 100 ml of bio-ethanol was able to cook 3L of tea in 25 minutes. In comparison, the ordinary “Jiko” cooked the same amount of tea but within a period of 45 minute. This indicated it had the heating power.
Figures 17-19 shows the orange colour of alcohol with addition of potassium dichromate; the mixture of Alcohol and Patassium dichromate warming; and yellow colour of alcohol when was warmed and dilute sulphuric acid added (Figure 20).

Figure 17: Orange Colour.

Figure 18: Warming.

Figure 19: Sulfuric Acid Added.
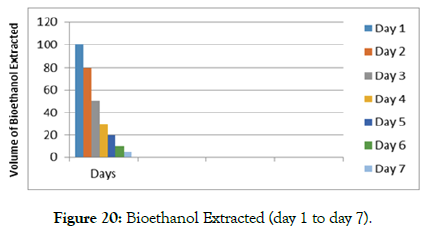
Figure 20: Bioethanol Extracted (day 1 to day 7).
BIOGAS The Obunga Bio-Tower (FDBD in Kisumu City) used by the Peri Urban community within the vicinity of the facility, continuously fed the toilets that were with an output as in Table 4.
Biogas
The estimated input of feacal matter to Obunga Bio-Tower was from cash income as in Table 4. In the first month of the three months (31, 30 and 31 days for the months) examined, the Bio-Tower generated an income of Kshs. 18,600, with an average number of 42 long calls per day, according to the Bio-tower caretaker's. The second and the third month were respectively; Kshs. 17,450 and Kshs. 25,420 at 39 and 47 long calls per day. Every person as then assumed, produced 123.6 grams of feacal per long call or 0.12 Kg.
The amount of gas produced therefore averaged to 0.02 m3.
| Month | Av. No Long calls. | Av. Mass Daily Feacal Matter (Kg) | Monthly Mass of Feacal Matter (Kg) | Av. Biogas Produced (m3) | Av. Biogas Produced (m3/Kg) |
|---|---|---|---|---|---|
| 1 | 1,302 | 0.12 | 156 | 3.1248 | 0.02 |
| 2 | 1,170 | 0.12 | 140 | 2.808 | 0.02 |
| 3 | 1,457 | 0.12 | 175 | 3.4968 | 0.02 |
Table 4: Obunga Feacal Matter Gas Production Values.
Conclusion and Recommendation
In comparing the performance characteristics of rapid igniting briquettes with different ratios of FM-Wh and FM-SD, the type of feedstock affected the briquettes as was observed. Thus briquettes with equal ratio of FM and Wh (1:1) is a suitable alternative source of energy to charcoal/firewood because of high calorific value/heat value, though with higher ash content which increased burning time and decreased the burning rate. FM-Wh briquettes of ratio 3:1 had high concentration of lead (0.5101 ppm) that could negatively affect the human health and safety. Potassium chlorate briquettes had high heat value, high specific fuel consumption and high power output. Emissions characteristics of the rapid igniting briquettes and water hyacinth blend though need further studies.
Water hyacinth is a potential substrate for briquetting/Biogas/ bioethanol production in substituting fossil fuel energy sources and an innovative way of managing the invasion of the weed in freshwater bodies (Lake Victoria). This consequently permits a “productive fight” against the plant invasion, with use of other wastes (feacal matter, market waste such as Sawdust) that are continually increasing in the townships surrounding the Lake Victoria, Kenyan side; and by large the east African countries. Hyacinth is also a strong evidence on the state of fertilizer use in the Kenyan Lake Victoria water shed and therefore a need for development of value addition and sustainable (SDG’s) economic exploitation strategies.
Combination or single Biomaterials ratios are thus usable for briquetting, biogas and bio-ethanol production as case of water hyacinth, feacal matter and Sawdust. Potential Bio-materials in a region (for example sugar cane bagasse, market wastes, rice husks or stalks, saw dust and even switch grass and their economics should thus be researched (singly or in combination) for renewable energy. Large production quantities (briquettes, bioethanol and Biogas) also need bigger plants researched on, designed and constructed. The aspect of modelling the corresponding awareness creation (includes designs, policy reviews, enforcement, management and monitoring), also need to be determined to help in achieving the SDG’s.
REFERENCES
- Odada EO, Ochola WO, Olago DO. Understanding future ecosystem changes in Lake Victoria basin using participatory local scenarios. Afr J Ecol. 2009;47:147-53.
- Patil JH, Lourdu MA, Raj A, Gavimath CC. Impact of dilution on biomethanation of fresh water hyacinth. Int J Chem Sci Appl. 2011;2(1):86-90.
- Cheng J, Lin R, Song W, Xia A, Zhou J, Cen K. Enhancement of fermentative hydrogen production from hydrolyzed water hyacinth with activated carbon detoxification and bacteria domestication. Int J hydrogen energy. 2015;40(6):2545-51.
- Cheng YS, Chen KY, Chou TH. Concurrent calcium peroxide pretreatment and wet storage of water hyacinth for fermentable sugar production. Bioresource technology. 2015;176:267-72.
- Lin L, Yang L, Xu F, Michel FC, Li Y. Comparison of solid-state anaerobic digestion to composting of yard trimmings with effluent from liquid anaerobic digestion: effect of total solids content and feedstock to effluent ratio. In2014 Montreal, Quebec Canada July 13–July 16, 2014 2014 (p. 1). American Society of Agricultural and Biological Engineers.
- Merino-Pérez O, Martínez-Palou R, Labidi J, Luque R. Microwave-assisted pretreatment of lignocellulosic biomass to produce biofuels and value-added products. In Production of Biofuels and Chemicals with Microwave 2015 (pp. 197-224). Springer, Dordrecht.
- WEC. Energy assessment. Energy and the challenge of sustainability; WEC. 2000.
- El-Saeidy EA. Technological fundamentals of briquetting cotton stalks as a bio-fuel [dissertation]. Humboldt: Humboldt University. 2004.
- Shekhar D. Popularization of biomass briquettes: A means for sustainable rural development. Asian Journal of Management Research. 2011;2(1):457-73.
- Kabok PA, Nyaanga DM, Mbugua JM, Eppinga R. Effect of shapes, binders and densities of faecal matter–Sawdust briquettes on ignition and burning times. J Petroleum & Environmental Biotechnology. 2018;9:370.
- Mbuba J, Nyaanga DM, Kabok P, Eppinga R. Effect of mix ratios and binders on physical and physical combustion characteristics of faecal matter-sawdust briquettes. Int J Emerging Technol Adv Eng. 2017;7(4):94-9.
- Muspratt AM, Nakato T, Niwagaba C, Dione H, Kang J, Stupin L, et al. Fuel potential of faecal sludge: calorific value results from Uganda, Ghana and Senegal. Journal of Water, Sanitation and Hygiene for Development. 2014;4(2):223-30.
- Jenkins B, Baxter LL, Miles Jr TR, Miles TR. Combustion properties of biomass. Fuel processing technology. 1998;54(1-3):17-46.
- Bryan BA, King D, Wang E. Biofuels agriculture: landscape-scale trade-offs between fuel, economics, carbon, energy, food, and fiber. Gcb Bioenergy. 2010;2(6):330-45.
- Rezania S, Ponraj M, Din MF, Songip AR, Sairan FM, Chelliapan S. The diverse applications of water hyacinth with main focus on sustainable energy and production for new era: An overview. Renewable and Sustainable Energy Reviews. 2015;41:943-54.
- Zhao J, Xia L. Ethanol production from corn Stover hemicellulosic hydrolysate using immobilized recombinant yeast cells. Biochem Eng J. 2010;49(1):28-32.
- Das S, Bhattacharya A, Haldar S, Ganguly A, Gu S, Ting YP, et al. Optimization of enzymatic saccharification of water hyacinth biomass for bio-ethanol: comparison between artificial neural network and response surface methodology. Sustainable Materials and Technologies. 2015 Apr 1;3:17-28.
- Ojeifo M, Ekokotu PA, Olele NF, Ekelemu JK. A review of the utilisation of water hyacinth: alternative and sustainable control measures for a noxious weed.
- Ntiba MJ, Kudoja WM, Mukasa CT. Management issues in the Lake Victoria watershed. Lakes & Reservoirs: Research & Management. 2001;6(3):211-6.
- Hu Z, Ma X, Li L. Optimal conditions for the catalytic and non-catalytic pyrolysis of water hyacinth. Energy Conversion and Management. 2015;94:337-44.
- Craft C, Megonigal P, Broome S, Stevenson J, Freese R, Cornell J, et al. The pace of ecosystem development of constructed Spartinaalterniflora marshes. Ecological applications. 2003;13(5):1417-32.
- Dien BS, Cotta MA, Jeffries TW. Bacteria engineered for fuel ethanol production: current status. Applied microbiology and biotechnology. 2003;63(3):258-66.
- Ganguly A, Chatterjee PK, Dey A. Studies on ethanol production from water hyacinth—A review. Renewable and Sustainable Energy Reviews. 2012;16(1):966-72.m
- Bergier I, Salis SM, Miranda CH, Ortega E, Luengo CA. Biofuel production from water hyacinth in the Pantanal wetland. Ecohydrology & Hydrobiology. 2012;12(1):77-84.
- Barrett SCH. Waterweed invasions. Sci Am. 1989;260: 90-97.
- Perna C, Burrows D. Improved dissolved oxygen status following removal of exotic weed mats in important fish habitat lagoons of the tropical Burdekin River floodplain, Australia. Marine Pollution Bulletin. 2005;51(1-4):138-48.
- Malik A. Environmental challenge vis a vis opportunity: the case of water hyacinth. Environment international. 2007;33(1):122-38.
- Gopal B. Water Hyacinth. Elsevier Science Publishers, Amsterdam. 1987:471.
- Masto RE, Kumar S, Rout TK, Sarkar P, George J, Ram LC. Biochar from water hyacinth (Eichorniacrassipes) and its impact on soil biological activity. Catena. 2013;111:64-71.
- Buller LS, Ortega E, Bergier I, Mesa-Pérez JM, Salis SM, Luengo CA. Sustainability assessment of water hyacinth fast pyrolysis in the Upper Paraguay River basin, Brazil. Science of the Total Environment. 2015;532:281-91.
- Jiu BB, Li BX, Yu QJ. Effects of Pb on pyrolysis behavior of water hyacinth. Journal of analytical and applied pyrolysis. 2015;112:270-5.
- Okia DO, Ndiema CK, Ahmed MS. Physical and chemical properties of water hyacinth based composite briquettes. world. 2016;8:19.
- Kuti OA, Adegoke CO. Comparative performance of composite sawdust briquette with kerosene fuel under domestic cooking conditions. AU JT. 2008;12(1):57-61.
- Nurmi J, Hillebrand K. The characteristics of whole-tree fuel stocks from silvicultural cleanings and thinnings. Biomass and Bioenergy. 2007;31(6):381-92.
- Valentine J, Clifton-Brown J, Hastings A, Robson P, Allison G, Smith P. Food vs. fuel: the use of land for lignocellulosic next generation’ energy crops that minimize competition with primary food production. Gcb Bioenergy. 2012 Jan;4(1):1-9.
- Bayrakci AG, Koçar G. Second-generation bioethanol production from water hyacinth and duckweed in Izmir: A case study. Renewable and Sustainable Energy Reviews. 2014;30:306-16.
- NAWASCCO. From Pit to Product: Biomass-fuels and Bio-fertilisers from Faecal Matter and Urine in Nakuru, Kenya.VIA Water Progress Report. Nakuru Water and Sanitation Services Company. 2016.
- SNV.Sanitation Value Chain; Unlocking Opportunities in Sanitation; Scientific and Porpular publications for Nakuru (Kenya) County Sanitation Programme. 2018.
- Elaigwu V, Adetogun AC, Tembe ET. Combustion properties of briquettes produced from Groundnut shell and rice husk. 2010:4- 20.
- Imeh ST, Tembe ET. Combustion properties of Briquettes produced from sawdust of khayasenegalensis and Gmelinaarborea. 2011:14-18.
- Bamgboye AI, Bolufawi SJ. Physical Characteristics of Briquettes from Guinea corn (sorghum bi-color) Residue. Agricultural Engineering International: CIGR Journal. 2009.
- Davies RM, Abolude DS. Ignition and burning rate of water hyacinthbriquettes. Journal of Scientific Research and Reports. 2013:111-20.
- Antwi-Boasiako C, Acheampong BB. Strength properties and calorific values of sawdust-briquettes as wood-residue energy generation source from tropical hardwoods of different densities. Biomass and Bioenergy. 2016;85:144-52.
- ASTM E872-82. Standard test method for volatile matter in the analysis of particulate wood fuels. Annual Book of ASTM Standard, American Society for Testing and Materials. 2013.
- ASTM International. ASTM Standard E711-87, Standard test method for gross calorific value of refuse-derived fuel by the bomb calorimeter. 2004.
- Rotich PK. Carbonization and briquetting of sawdust for use in domestic cookers (Doctoral dissertation, University of Nairobi). 1996.
- Chaney JO. Combustion characteristics of biomass briquettes (Doctoral dissertation, University of Nottingham).
- Sotannde OA, Oluyege AO, Abah GB. Physical and combustion properties of briquettes from sawdust of Azadirachtaindica. J Forestry research. 2010;21(1):63-7.
- Van Loo S, Koppejan J, editors. The handbook of biomass combustion and co-firing. London: Earthscan; 2008.
- Chinyere DC, Asoegwu SN, Nwandikom GI. An Evaluation of Briquettes from Sawdust and Corn Starch Binder. Int J Sci Technol. 2014;2(7):149.
- Abdulrasheed A, Aroke UO, Ibrahim M. Compression pressure effect on mechanical & combustion properties of sawdust briquette using Styrofoam adhesive as binder. Am J Eng Res. 2015;4(8):205-11.
- Oyelaran OA, Bolaji BO, Waheed MA, Adekunle MF. An Experimental Study of Combustion Characteristics of Groundnut Shells and Waste Paper Admixture Briquettes. KKU Energy Journey. 2015; 42(4): 283-286.
Citation: Aguko KP, Oloko MO, Ngusale GK (2021) Three B’s Local Bio-Material Wastes to Energy; Potential for Environmental Management and Sustainability. Int J Waste Resour 11: 411.
Copyright: © 2021 Aguko KP, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.