Indexed In
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 13, Issue 5
The Improvement of Ankle Reflex, Knee Reflex, Muscle Strength, and Sensory Perception after Fixed Dose Combination of Vitamin B1, B6, and B12 in Subjects with Peripheral Neuropathy (Secondary Analysis of the NENOIN Study)
Manfaluthy Hakim1, Roger D Gibb2, Poonam Nitin Sule3, Yan Lid4 and Rizaldy Taslim Pinzon5*2Department of Biostatistics, The Procter and Gamble Company, Mason, United States of America
3Department of Clinical Development, Procter and Gamble Health Limited, Mumbai, India
4Department of Medical and Technical Affairs, Procter and Gamble International Operation SA Singapore Branch, Singapore
5Department of Neurology, Duta Wacana Christian University, Wahidin, Indonesia
Received: 22-Sep-2023, Manuscript No. CPECR-23-23168; Editor assigned: 25-Sep-2023, Pre QC No. CPECR-23-23168 (PQ); Reviewed: 09-Oct-2023, QC No. CPECR-23-23168; Revised: 16-Oct-2023, Manuscript No. CPECR-23-23168 (R); Published: 23-Oct-2023, DOI: 10.35248/2161-1459.23.13.384
Abstract
Background: Most peripheral neuropathy (PN) treatments relieve pain but do not restore neuronal health and function. A fixed dose combination (FDC) of vitamin B1, B6 and B12 was previously reported to improve subjective symptom perception in patients with mild to moderate PN.
Methods: The Neurobion Non-interventional (NENOIN) study was a prospective, open-label, multi-center, single-arm observational study. 411 subjects with mild to moderate PN of various etiologies were prescribed once-daily dose of 100mg of vitamin B1, 100mg of B6 and 5mg of B12 (Neurobion® Forte tablets) for 90 days and evaluated with neurological examination comprising 51 assessments at baseline, Days 14, 30, 60, and 90.
Results: Statistically significant (p<0.001) reflex improvements were observed in 67.6%, 54.3%, 92.3%, and 75.0% patients with absent baseline reflex on their left ankle, right ankle, left knee, and right knee, respectively. The percentage of subjects with normal ankle dorsiflexion strength increased significantly (p<0.001) from 86% (baseline) to 97% (Day 90). Sensory assessments in the finger and toes showed significant (p<0.001) improvement. Significantly (p<0.001) greater improvement in Total Symptom Score was observed among subjects with comparatively more abnormal baseline sensory outcomes.
Conclusion: Treatment with an FDC of vitamin B1, B6, and B12 for 90 days progressively improved ankle and knee reflex, motor strength, and sensory perception status in the toes and fingers.
Keywords
Peripheral neuropathy; Motor neuropathy; Total Symptom Score; Fixed dose combination; Neurotropic vitamin B
Introduction
Peripheral neuropathy (PN) is a debilitating condition caused by damage to the peripheral nervous system. It is characterized by sensory symptoms such as tingling, numbness, pain, and muscle weakness predominantly in the hands and feet [1,2]. Treatment of PN typically focuses on mitigating neuropathic pain [3], a symptom of late PN in which the nerves have undergone significant damage [4]. Various pharmacological treatment options are available for PN; however, most treatments have moderate efficacy, may result in side effects and may present a potential risk of drug abuse [5,6]. Recommended first-line treatment options for neuropathic pain may also be contraindicated in patients using additional treatments, preventing the use of combination treatment in patients not sufficiently responding to monotherapy [7]. Further, while most treatments offer symptom relief often focusing on painful symptoms, few help to restore nerve function and integrity [3]. Thus, there is a need for alternative therapeutic approaches for PN.
Vitamin B deficiency is associated with PN and supplementation with vitamin B may lead to symptom improvement [8-13]. Specifically, the synergistic effect of vitamins B1, B6, and B12 is well studied and supports the physiological mechanisms required for peripheral nerve regeneration and function [14]. Vitamin B1 (thiamine) acts as an essential cofactor in carbohydrate metabolism and facilitates cellular energy production, helping to provide energy to nerve cells [15]. Vitamin B6 is an enzymatic cofactor that contributes to the metabolism of various cellular macromolecules including neurotransmitters [14,16,17]. In addition, vitamin B6 possesses potent anti-oxidative characteristics capable of effectively quenching reactive oxygen species, thus protecting cells from oxidative damage [14,17]. Vitamin B12 (cobalamin) plays important roles in neuron regeneration and survival, remyelination, and the maintenance of myelin sheaths [4,18]. Deficiency of vitamin B12 was found to be associated with lower sensory and motor function in a large cross-sectional study [19].
The Neurobion Non-interventional (NENOIN) clinical study reported the efficacy and safety of the commercially available fixed dose combination (FDC) of vitamin B1 (100 mg), B6 (100 mg), and B12 (5 mg) (Neurobion® Forte) in mild to moderate PN of various etiologies [20-23]. The trial demonstrated that treatment with the FDC of B vitamins was effective in reducing subjective perception of symptoms when assessed using Total Symptom Scores (TSS) [24], Visual Analog Scores (VAS) and improved Quality of Life (QoL) scores [25]. The treatment was also shown to be well-tolerated. Subgroup analysis of the trial further showed consistent efficacy across different PN etiologies, baseline severity, age, and sex [22]. However, the evidence for vitamin B complex efficacy when assessed objectively is limited.
Here we present a post-hoc analysis of the neurological examination (NE) data from the NENOIN trial. This analysis aims to investigate the potential benefit of treatment with a FDC of vitamin B1, B6, and B12 with respect to neurological assessments in patients with mild to moderate PN and their nerve function.
Materials and Methods
Study design
The NENOIN study was a prospective, open-label, noninterventional (observational), single-arm study conducted at 8 centers across Indonesia (21). The study was performed in accordance with the Declaration of Helsinki (1964) and received approval from the Institutional Review Board and the National Agency of Drug and Food Control of Indonesia (BPOM; No. PN. 01.313.3.12.15.6802, approved: 31 December 2015). The trial is registered in the Indonesian Clinical Trial Registry (Registration No: INA-KPA0DYA). All subjects provided signed informed consent prior to their participation.
Study participants
Subjects were patients with PN of various etiologies, including diabetes mellitus, nutritional, alcoholic neuropathy, carpal tunnel syndrome, idiopathic, and other etiologies. Inclusion criteria were age ≥ 18 and ≤ 65 years and diagnosis of PN using either a Michigan Neuropathy Screening Instrument (MNSI) score of ≥ 7, a Patient Administered Questionnaire and Health care Professional Score of ≥ 2.5 or a Toronto Clinical Neuropathy Score (TCNS) of ≥ 6. Patients who visited any of the participating centers for PN or other conditions underwent an eligibility screening. Eligible patients were then invited to participate in the study.
Subjects were excluded if they: 1) had clinically significant cardiovascular, pulmonary, gastrointestinal, hematological, hepatic, renal, or endocrine diseases (except diabetes mellitus), 2) had a history/sign/symptoms suggestive of genetic neuropathy, or those who underwent any gastrointestinal surgery in the last 6 months, 3) had surgery planned during the study period, 4) had any clinically significant or unstable medical or psychiatric condition that in the opinion of the investigator would affect the subject’s ability to participate in the study, or 5) had participated in other clinical trials in the last month.
Further exclusion criteria were subjects with 1) intake of vitamin B complex products for more than 1 week consecutively, in the last 3 months before consent, 2) known hypersensitivity to any component of Neurobion® Forte tablet, or 3) severe neuropathy (TCNS ≥ 12 or VAS ≥ 7 for pain) who were on therapy with non-steroidal anti-inflammatory drugs (NSAIDs), gabapentin, pregabalin, or any other anti-inflammatory drugs. Subjects who were on any treatment which interferes with neuropathy like methotrexate or any other cytostatic drug treatment, were pregnant, planning to become pregnant, or were breastfeeding were also excluded from the study.
Intervention
Eligible subjects were given tablets containing a FDC of vitamin B1/thiamine mononitrate 100 mg, vitamin B6/pyridoxal hydrochloride 100 mg, and vitamin B12/cyanocobalamin 5 mg marketed as Neurobion® Forte in Indonesia. Neurobion® Forte is manufactured by PT Merck Tbk, Indonesia and Procter and Gamble the Marketing Authorization Holder (MAH). One tablet was taken once daily for 90 days. Following the baseline visit, subjects were followed up at Day 14 ± 3, Day 30 ± 3, Day 60 ± 3, and Day 90 ± 3. No other known additional therapies have been performed in the subjects.
Outcome measures
In this post-hoc analysis, NE comprising 51 assessments were conducted by a medical professional at baseline and at each follow-up visit. Tendon reflexes were assessed using an ordinal 4-point (absent, reduced, normal, increased) scale. Muscle strength and sensory perception were assessed as binary scales (normal/abnormal, present/absent). The current analysis focuses primarily on ankle reflexes, knee reflexes, ankle muscle strength, and neurological sensory tests (joint position, light touch, pain/temperature, and vibration) of the fingers and toes.
TSS was reported by subjects at baseline and at every postbaseline visit based on the frequency and intensity of stabbing pain, burning pain, paresthesia, and numbness reported by subjects (21). The TSS ranges from 0 (no symptoms) to a maximum of 14.64 points [all symptoms are severe and (almost) continuously present].
The NENOIN study was a real-world study in which the assessment of the endpoints was conducted by trained medical professionals at the respective study sites.
Statistical methods
The data for each of the 51 NE assessments were descriptively summarized in time-series plots and contingency tables to facilitate interpretation. For analysis purposes, ordinal-scaled measures (reflexes) were assigned numeric values from 1 to 4. For NE assessments found to have substantive changes over time, the statistical significance of changes versus baseline were assessed with the McNemar’s test (binary measures) and the paired-difference t-test (ordinal measures). Additionally, their association with the TSS data was explored with plots of the data and the Spearman correlation analysis. For the ordinal-scale reflex data, the association between the change from baseline to Day 90 and baseline level reflex was statistically assessed with contingency table analysis and the Cochrane-Mantel-Haenszel test for ordinal responses. All analyses were conducted with SAS 9.4 Enterprise Guide.
Results
Patient characteristics
The NENOIN study screened 414 subjects, out of which 411 met the eligibility criteria and were enrolled in the study [21]. Participant demographics of the NENOIN study have been previously described [21]. The mean age of the subjects was 50.9 ± 8.25 years (range: 22 to 65 years) with 72.3% (n=297) of them being female. The most common etiologies for peripheral neuropathy were idiopathy (n=112; 27.3%) and diabetes (n=104; 25.3%). Majority of the subjects had peripheral neuropathy due to a combination of etiologies (n=126).
The number of subjects with NE data at baseline, Day 14, Day 30, Day 60, and Day 90 was 411, 402, 399, 393 and 390, respectively. In subjects for whom NE assessments were conducted, 101,677 of the 101,745 (99.3%) assessments were successfully completed and recorded. Results from 30 of these 51 assessments at baseline and at Day 90 are available in the supplementary material (Supplementary Table 1). In 34 of the 51 NE assessments, the majority (≥ 95%) of subjects had favorable outcomes at most visits. Left biceps, right biceps, left triceps, and right triceps reflexes were favorable in approximately 80% of the subjects at baseline and showed no significant improvement over 90 days of treatment. Analysis results of the remaining 13 assessments: ankle tendon reflexes (left and right), knee reflexes (left and right), ankle dorsiflexion strength (left and right), and 7 sensory nerve detection tests are presented below.
Improvement in ankle and knee reflexes
Statistically significant (p<0.001) improvement in ankle and knee reflexes over the course of the 90-day treatment period was observed (Table 1). Specifically, among subjects with absent left (n=34) and right ankle (n=35) reflex at baseline, the majority demonstrated improvement in their ankle reflex at Day 90 [n=23 (67.6%) and n=19 (54.3%) of subjects, respectively]. At Day 90, none of the subjects with reduced left (n=192) and right (n=193) reflex at baseline had worsened reflex, and 47 (24.5%) and 48 (24.9%) subjects had improved left and right ankle reflex, respectively. Among subjects with normal left (n=164) and right ankle (n=161) reflex at baseline, only 11 (6.7%) and 7 (4.4%) respectively, had worse reflex after 90 days of treatment.
| Baseline left ankle reflex | Number of Subjects |
Day 90 left ankle reflex | ||
|---|---|---|---|---|
| Worsened | No change | Improved | ||
| Absent reflex | 34 | N/A* | 11 (32.4%) | 23 (67.6%) |
| Reduced reflex | 192 | 0 (0%) | 145 (75.5%) | 47 (24.5%) |
| Normal reflex | 164 | 11 (6.7%) | 153 (93.3%) | N/A* |
| Increased reflex† | 0 | 0 (0%) | 0 (0%) | 0 (0%) |
| Baseline right ankle reflex | Number of Subjects |
Day 90 right ankle reflex | ||
| Worsened | No change | Improved | ||
| Absent reflex | 35 | N/A* | 16 (45.7%) | 19 (54.3%) |
| Reduced reflex | 193 | 0 (0%) | 145 (75.1%) | 48 (24.9%) |
| Normal reflex | 161 | 7 (4.4%) | 154 (95.7%) | N/A* |
| Increased reflex† | 1 | 1 (100%) | 0 (0%) | 0 (0%) |
| Baseline left knee reflex | Number of Subjects |
Day 90 left knee reflex | ||
| Worsened | No change | Improved | ||
| Absent reflex | 15 | N/A* | 1 (6.7%) | 14 (92.3%) |
| Reduced reflex | 172 | 1 (0.6%) | 117 (68.0%) | 54 (31.4%) |
| Normal reflex | 202 | 11 (5.5%) | 191 (94.5%) | N/A* |
| Increased reflex† | 1 | 0 (0%) | 0 (0%) | 1 (100%) |
| Baseline right knee reflex | Number of Subjects |
Day 90 right knee reflex | ||
| Worsened | No Change | Improved | ||
| Absent reflex | 16 | N/A* | 4 (25.0%) | 12 (75.0%) |
| Reduced reflex | 168 | 0 (0%) | 114 (67.9%) | 54 (32.1%) |
| Normal reflex | 205 | 10 (4.9%) | 195 (95.1%) | N/A* |
| Increased reflex† | 1 | 0 (0%) | 0 (0%) | 1 (100%) |
Note: *N/A indicates that no improvement is possible; †For ‘Increased reflex’ at baseline, Day 90 ‘Normal’ reflex was considered ‘Improved’ and ‘Absent’ or ‘Reduced reflex’ was considered ‘Worsened’.
Table 1: Change in ankle and knee reflex status from baseline to Day 90 of treatment.
Among subjects with absent baseline left (n=15) and right knee reflex (n=16), 14 (92.3%) and 12 (75.0%) subjects demonstrated improvement at Day 90 respectively. Improvement was observed in 54 (31.4%) and 54 (32.1%) subjects with reduced left (n=172) and right knee (n=168) reflex at baseline, respectively. Only 11 (5.5%) and 10 (4.9%) subjects with normal left and right ankle reflex at baseline, respectively had worsened at Day 90.
Muscle strength
Figure 1 shows the percentage of subjects with normal ankle dorsiflexion strength at baseline and at follow-up visits through to Day 90. 86% of subjects had normal ankle dorsiflexion strength at baseline, but with daily treatment with the FDC of vitamin B1, B6, and B12, this percentage consistently increased with each successive visit to 97% at Day 90. This improvement is statistically significant for both legs (p<0.001).
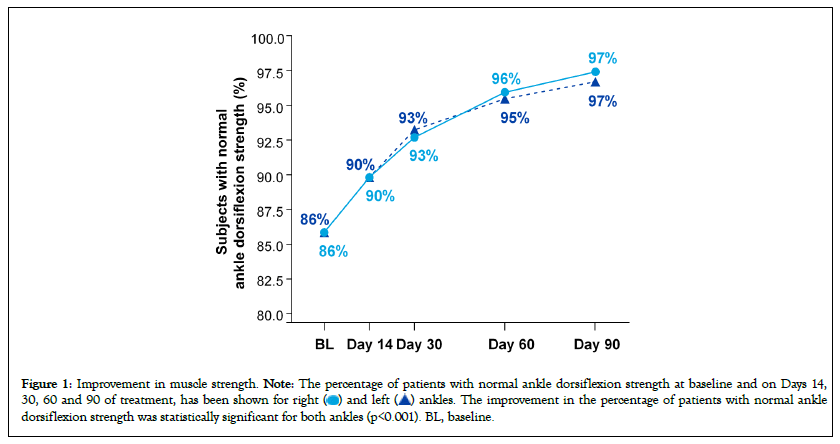
Figure 1: Improvement in muscle strength. Note: The percentage of patients with normal ankle dorsiflexion strength at baseline and on Days 14,
30, 60 and 90 of treatment, has been shown for right  ankles. The improvement in the percentage of patients with normal ankle
dorsiflexion strength was statistically significant for both ankles (p<0.001). BL, baseline.
ankles. The improvement in the percentage of patients with normal ankle
dorsiflexion strength was statistically significant for both ankles (p<0.001). BL, baseline.
Sensory perception
Figure 2 shows the percentage of subjects with positive results at baseline and follow-up visits for the following sensory perception assessments: joint position (right finger, Figure 2A), light touch (left and right toe, Figure 2B), pain/temperature (left and right toe, Figure 2C), and vibration (left and right toe, Figure 2D). Positive sensory perception at baseline ranged from 65% to 73% for all measures except for pain/temperature in the right toe which was notably higher at 92%. Consistent visit-to-visit improvement was observed in all cases, except pain/temperature in the right toe which had a higher starting point. Left and right toe scores improved comparably over the 90-day treatment period. The change from baseline to Day 90 was statistically significant (p<0.001) for all 7 sensory assessments.
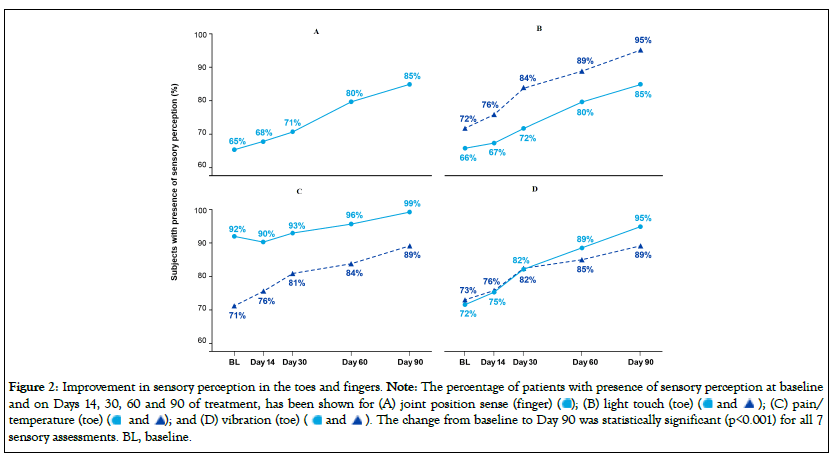
Figure 2: Improvement in sensory perception in the toes and fingers. Note: The percentage of patients with presence of sensory perception at baseline
and on Days 14, 30, 60 and 90 of treatment, has been shown for (A) joint position sense (finger)  (C) pain/
temperature (toe)
(C) pain/
temperature (toe)  The change from baseline to Day 90 was statistically significant (p<0.001) for all 7
sensory assessments. BL, baseline.
The change from baseline to Day 90 was statistically significant (p<0.001) for all 7
sensory assessments. BL, baseline.
Correlation analysis did not show significant correlation between the number of positive sensory results and TSS at baseline (data not shown). However, there was a statistically significant correlation between TSS change from baseline to Day 90 and the number of abnormal baseline sensory outcomes in the finger and toes at baseline (r=-0.2, p<0.001, Figure 3). At one extreme, subjects with 7 abnormal baseline sensory scores had an average TSS improvement of 4.7, whereas subjects with zero abnormal baseline sensory scores had TSS improvement of 2.9.
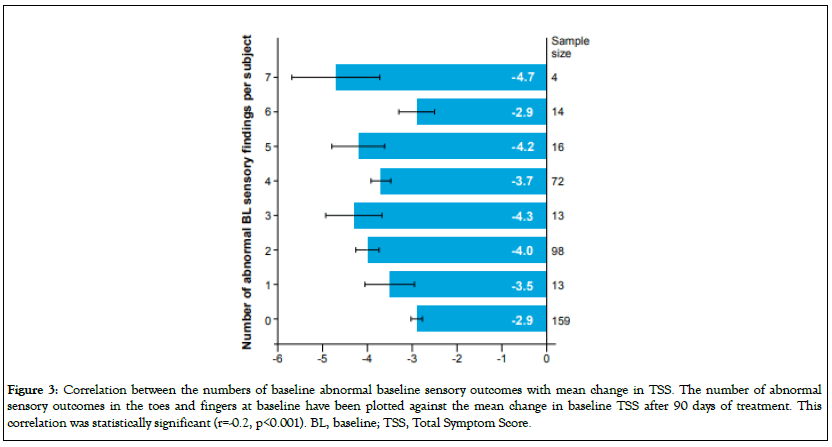
Figure 3: Correlation between the numbers of baseline abnormal baseline sensory outcomes with mean change in TSS. The number of abnormal sensory outcomes in the toes and fingers at baseline have been plotted against the mean change in baseline TSS after 90 days of treatment. This correlation was statistically significant (r=-0.2, p<0.001). BL, baseline; TSS, Total Symptom Score.
Discussion
Subjects in the NENOIN study were enrolled primarily due to their report of experiencing PN in the feet and hands. The NENOIN study previously reported significant improvements in the primary endpoint of TSS for PN symptoms as well as improvements in secondary endpoints in the VAS and in QoL measurements, in patients with PN, receiving a FDC of B vitamins [21]. The current analysis showing significant improvements in specific neurological assessments of the extremities, lends further evidence to the effectiveness of this treatment in PN.
Since PN is a progressive disease which starts at the extremities, NE findings at baseline were favorable in ≥ 95% subjects for most assessments except those in the knees, feet and hands. Further, since the NENOIN trial recruited patients with mild to moderate PN, normal neurological findings at non-peripheral regions of the body were expected. Therefore, this analysis focused on screening for PN symptoms at the extremities. The proportion of subjects with favorable biceps and triceps reflexes at baseline were lower at 80% but did not improve at Day 90. However, this is expected given the subjects' age range. As such, analysis was focused on the other 13 of the 51 assessments where a considerably high percentage of subjects showed abnormal NE findings: Ankle dorsiflexion strength (left and right), ankle reflexes (left and right), knee reflexes (left and right), joint position (right finger), light touch (left and right toe), pain/ temperature (left and right toe), and vibration (left and right toe).
The current analysis shows that 90 days of treatment with a FDC of vitamin B1, B6, and B12 provided statistically significant improvement in the left and right ankles and left and right knees reflexes. This result is significant since a considerable proportion of patients with PN experience loss of ankle or knee reflex [26] which may contribute to loss of balance and increase their risk of falling [27]. Among patients with absent reflex on their left ankle, right ankle, left knee, and right knee at baseline, 67.6%, 54.3%, 92.3%, and 75% experienced improvement respectively. The proportion of subjects with normal ankle dorsiflexion strength also increased significantly from 86% to 97%. The treatment also resulted in statistically (p<0.001) significant improvements to joint position sense in the fingers and sensory nerve perception of touch, vibration and temperature in the toes and fingers. These results support the finding that treatment with the FDC of B vitamins improves proprioception in patients with PN, which has also been associated with ankle reflex [28,29].
Further analysis demonstrated a positive correlation between TSS improvement and the number of negative toe and finger sensory findings at baseline. This suggests that patients with worse sensory impairments in the feet and hands at baseline, may experience a larger, more favorable improvement in their symptom severity after treatment with the FDC of B vitamins. This is consistent with previously reported findings that patients with moderate PN are more likely to improve in TSS with this treatment than patients with mild PN [22].
The beneficial effects observed in this study align with previous clinical studies which demonstrated the efficacy of B vitamins in treating PN [9,12,30]. These beneficial effects of therapy can be attributed to different and synergistic modes of action of B vitamins that work simultaneously for nerve regeneration, nerve activation and myelinization [4,31]. However, there is a lack of studies investigating the benefits of vitamin B using a more objective measure. A randomized controlled trial of 24 diabetic patients investigated the benefit of B vitamin combination utilizing objective measures [13]. In agreement with our study, the trial also showed B vitamin combination to significantly improve nerve conduction velocity with a trend for improvement on vibration perception threshold [13]. To the best of our knowledge, ours is the first study that investigates the benefits of vitamin B on both neural reflex and motor strength in a large number of patients having varied etiologies of PN, demonstrating a potential improvement in nerve function.
The results reported in this analysis add stronger evidence of the efficacy of the FDC of B vitamins, supplementing the previous findings of the NENOIN study [20-23]. Firstly, the data reported here are based on medical professionals assessing clinical signs of nerve reflex and muscle strength using established NE methods. This analysis using objective measures adds further objectivity to the previously reported TSS data, which was based on patient perception of symptoms (burning pain, stabbing pain, numbness, tingling/prickling) severity and frequency. Secondly, the NE data was collected prospectively, whereas the TSS data involved patients reporting on their recollection of symptoms over the past 24 hours. Recall errors associated with patient-reported pain assessment is a well-documented phenomenon [32].
The limitation of this study is the single-arm and open-label design which introduces the risk of measurement bias. Despite this, significant improvements were observed over the follow-up period using NE by medical professionals, suggesting real-world benefits of a vitamin B combination on neurological functions, in patients with PN. While this is an uncontrolled trial where subjects were prescribed the treatment without further intervention, compliance was confirmed during the trial by subject inquiry at each follow-up visit, to minimize errors in endpoint assessment due to non-compliance.
Conclusion
In conclusion, a FDC of vitamin B1, B6, and B12 improved ankle reflex, knee reflex, and motor strength (ankle dorsiflexion) after 90 days of treatment, which might be based on improved nerve function. The treatment also led to a significant improvement in sensory test results in the toes and finger. The benefit of treatment in TSS was also greater in patients with more abnormal sensory findings in the toes and finger at baseline.
Further studies on whether the benefits will be consistent across different etiologies of PN may be beneficial.
Funding
The conduct of the NENOIN trial was funded by Merck Limited CH Division. This post-hoc analysis was funded by P&G Health. Merck Limited CH Division was involved in developing and overseeing the conduct, primary analysis, interpretation and reporting of the NENOIN trial. P&G Health was involved in the post-hoc analysis and interpretation of data, and the decision to submit this article for publication.
Conflict of Interest
MH and RP have no conflict of interest to declare. PS, LY, and RG are employed by and own stocks in Procter and Gamble.
Acknowledgment
The authors acknowledge the Licensors of the questionnaires used in the study viz. a) Vera Bril and Bruce Perkins, the developers of the TCNS questionnaire b) SF-8TM Health Survey Questionnaire (a trademark of Quality Metric Incorporated (now OPTUM)). The authors wish to acknowledge the investigators of the NENOIN trial: Nani Kurniani, Dodik Tugasworo, Mudjiani Basuki, Hasnawi Haddani, Pagan Pambudi, Aida Fithrie and Audry Devisanty Wuysang for their role in conceptualizing the trial and generating the data used in this manuscript. Medical writing support was provided by Yen May Ong from McCann Health Singapore and Antony Hardjojo from Jaya Medical Writing Pte Ltd.
References
- Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31:5–20.
[Crossref] [Google Scholar] [PubMed]
- Azhary H, Farooq MU, Bhanushali M, Majid A, Kassab MY. . Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887–892. .
- Srinivasan A, Paranjothi S, Bhatthacharyya K, Maji D, Hazra D, Talwakar P, et al. Consensus recommendations for the management of peripheral neuropathy in India. J Indian Med Assoc. 2018;116:45–55.
- Baltrusch S. The Role of neurotropic B vitamins in nerve regeneration. Biomed Res Int. 2021;2021. .
[Crossref] [Google Scholar] [PubMed]
- Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:2058738419838383.
[Crossref] [Google Scholar] [PubMed]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173.
[Crossref] [Google Scholar] [PubMed]
- Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019;20(Suppl 1):S2–12.
[Crossref] [Google Scholar] [PubMed]
- Stein J, Geisel J, Obeid R. Association between neuropathy and B-vitamins: A systematic review and meta-analysis. Eur J Neurol. 2021;28:2054–2064.
[Crossref] [Google Scholar] [PubMed]
- Rizvi A, Ahmad A, Rizvi Z. Efficacy of combination of vitamin B1, B6 and B12 in management of diabetic peripheral neuropathy. Pak J Med Health Sci. 2013;7:801–804.
- Tjokroprawiro A. Review article and clinical experience: emerging multiple properties of high dose thiamine and B6-B12 vitamins. Therapeutic possibilities for diabetic vascular complications. Folia Medica Indonesiana. 2009;45:165–173.
- Woelk H, Lehrl S, Bitsch R, Köpcke W. Benfotiamine in treatment of alcoholic polyneuropathy: an 8-week randomized controlled study (BAP I Study). Alcohol Alcohol. 1998;33:631–638.
[Crossref] [Google Scholar] [PubMed]
- Abbas ZG, Swai AB. Evaluation of the efficacy of thiamine and pyridoxine in the treatment of symptomatic diabetic peripheral neuropathy. East Afr Med J. 1997;74:803–808.
[Google Scholar] [PubMed]
- Stracke H, Lindemann A, Federlin K. A benfotiamine-vitamin B combination in treatment of diabetic polyneuropathy. Exp Clin Endocrinol Diabetes. 1996;104:311–316.
[Crossref] [Google Scholar] [PubMed]
- Geller M, Oliveira L, Nigri R, Mezitis S, Ribeiro M, Fonseca A, et al. B Vitamins for Neuropathy and Neuropathic Pain. Vitam Miner. 2017;06.
[Crossref]
- Sriram K, Manzanares W, Joseph K. Thiamine in nutrition therapy. Nutr Clin Pract. 2012;27:41–50.
[Crossref] [Google Scholar] [PubMed]
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences–an overview. Nutr Hosp. 2007;22:7–24.
[PubMed]
- Mooney S, Leuendorf JE, Hendrickson C, Hellmann H. Vitamin B6: a long known compound of surprising complexity. Molecules. 2009;14:329–351.
[Crossref] [Google Scholar] [PubMed]
- Wu F, Xu K, Liu L, Zhang K, Xia L, Zhang M, et al. Vitamin B(12) Enhances Nerve Repair and Improves Functional Recovery After Traumatic Brain Injury by Inhibiting ER Stress-Induced Neuron Injury. Front Pharmacol. 2019;10:406.
[Crossref] [Google Scholar] [PubMed]
- Leishear K, Boudreau RM, Studenski SA, Ferrucci L, Rosano C, de Rekeneire N, et al. Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. J Am Geriatr Soc. 2012;60:1057–1063.
[Crossref] [Google Scholar] [PubMed]
- Hakim M, Kurniani N, Pinzon R, Tugasworo D, Basuki M, Haddani H, et al. Improvement of Quality of Life in Patients with Peripheral Neuropathy Treated with a Fixed Dose Combination of High-Dose Vitamin B1, B6 and B12: Results from a 12-week Prospective Non-interventional Study in Indonesia. J Clin Trials. 2018;08.
- Hakim M, Kurniani N, Pinzon RT, Tugasworo D, Basuki M, Haddani H, et al. Management of peripheral neuropathy symptoms with a fixed dose combination of high-dose vitamin B1, B6 and B12: A 12-week prospective non-interventional study in Indonesia. Asian J Med Sci. 2018;9:32–40.
- Hakim M, Kurniani N, Pinzon RT, Tugasworo D, Basuki M, Haddani H, et al. Vitamin B1, B6, and B12 Combination in the Management of Peripheral Neuropathy Symptoms in Indonesia: A Sub Group Analysis of NENOIN Study. Jurnal Kedokteran Indonesia. 2018;4: 43-49.
- Hakim M, Kurniani N, Tugasworo D, Basuki M, Haddani H, Pambudi P, et al. Analisis Korelasi Skor Gejala Total, Nyeri, dan Kualitas Hidup setelah Pengobatan Vitamin B1, B6, dan B12 Dosis Tinggi pada Neuropati Perifer. Neurona. 2018;35:86–92.
- Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, et al. Treatment With α-Lipoic Acid Improves Symptomatic Diabetic Polyneuropathy: The SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370.
[Crossref] [Google Scholar] [PubMed]
- Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164.
[Crossref] [Google Scholar] [PubMed]
- Sundkvist G, Lilja B, Nilsson H, J.- Å Nilsson, Rosén I. Peripheral nerve dysfunction is reflected by loss of ankle reflexes but not by autonomic neuropathy in diabetic patients. Muscle Nerve. 1997;20:740–743.
[Crossref] [Google Scholar] [PubMed]
- Richardson JK, Hurvitz EA. Peripheral neuropathy: A true risk factor for falls. J Gerontol A Biol Sci Med Sci. 1995;50:M211–M215.
[Crossref] [Google Scholar] [PubMed]
- Khudados E, Cody FWJ, O’Boyle DJ. Proprioceptive regulation of voluntary ankle movements, demonstrated using muscle vibration, is impaired by Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:504.
[Crossref] [Google Scholar] [PubMed]
- Xue X, Ma T, Li Q, Song Y, Hua Y. Chronic ankle instability is associated with proprioception deficits: A systematic review and meta-analysis. J Sport Health Sci. 2021;10:182–191.
[Crossref] [Google Scholar] [PubMed]
- Maladkar M, Tekchandani C, Dave U. Post-Marketing Surveillance of Fixed Dose Combination of Methylcobalamin, Alpha Lipoic Acid, Folic Acid, Biotin, Benfotiamine & Vitamin B6-Nutripathy for the Management of Peripheral Neuropathy. J Diabetes Mellit. 2014;4:124–132.
- Nedeljković P, Zmijanjac D, Drašković-Pavlović B, Vasiljevska M, Vučević D, Božić B, et al. Vitamin B complex treatment improves motor nerve regeneration and recovery of muscle function in a rodent model of peripheral nerve injury. Arch Biol Sci. 2017;69:361–368.
- Stull D, Leidy N, Parasuraman B, Chassany O. Optimal recall periods for patient-reported outcomes: Challenges and potential solutions. Curr Med Res Opin. 2009;25:929–942.
[Crossref] [Google Scholar] [PubMed]
Citation: Hakim M, Gibb RD, Sule PN, Lid Y, Pinzon RL (2023) The Improvement of Ankle Reflex, Knee Reflex, Muscle Strength, and Sensory Perception after Fixed Dose Combination of Vitamin B1, B6, and B12 in Subjects with Peripheral Neuropathy (Secondary Analysis of the NENOIN Study). J Clin Exp Pharmacol. 13:384.
Copyright: © 2023 Hakim M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

