Indexed In
- Open J Gate
- Genamics JournalSeek
- Access to Global Online Research in Agriculture (AGORA)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2022) Volume 11, Issue 1
The Effect of Iron Related Nanoparticles on Bio-Butanol and Bio-Hydrogen Production from Clostridial Fermentation: A Mini-Review
Sayed Tariq Pachakhan1, Hasamudin Sayedi2 and Mujtaba Barikzai1*2Department of Medical Laboratory Science and Biotechnology, Spinghar Institute of Higher Education, Kabul, Afghanistan
Received: 04-Sep-2022, Manuscript No. FMT-22-17926; Editor assigned: 07-Sep-2022, Pre QC No. FMT-22-17926 (PQ); Reviewed: 19-Sep-2022, QC No. FMT-22-17926; Revised: 20-Jan-2023, Manuscript No. FMT-22-17926 (R); Published: 27-Jan-2023, DOI: 10.35248/2167-7972.23.1.001
Abstract
The rapid depletion of non-renewable fossil fuels like coal, petroleum, and gas led the world to an energy crisis and environmental pollution. Hydrogen and biobutanol renewable energy sources are considered essential and the cleanest and high potential energy sources (122 kj/g). To produce renewable energy, nanoparticles were involved in inducing the production of hydrogen and bio-butanol with the assistance of microorganisms (Clostridial). Hydrogenase metal atoms present in their active site are NiFe, FeFe, and Fe hydrogenase. Hydrogen production is influenced by Iron (Fe) and Nickel (Ni) metals, which enhance the hydrogenase enzyme activity. Fe is a vital component of cytochromes in anaerobic microbes; hence the assumed addition of Fe3O4 can promote microbial cell growth and hydrogenase activity. The size and concentration of nanoparticles affect the production of hydrogen and bio-butanol. According to protein sequence analysis has been shown that amylase in Clostridium may contain a multiactive site for activity and has the ability for translation.
Keywords
Iron related nanoparticles; Bio-butanol; Bio-hydrogen production; Clostridial fermentation
INTRODUCTION
Worldwide utilizes fossil fuels, a significant source of energy, which harms the natural environment and human health. To overcome this kind of problem and obtain alternative energy to our world’s needs, a new source of energy. The production of biofuels reduces the usage of fossil fuels and, if possible, replaces them to overcome the limitation of their fixed natural resources. The major worry is within the next 150 years-200 years will face a lack of coal and petroleum energy. Little potential have been presented to produce biofuel in upcoming years, from one side to reduce environmental contamination. And additionally had pretty much biofuel energy, including bio methanol, bio- Hydrogen (H), bio-methane, and biodiesel. The rapid depletion of non-renewable fossil fuels like coal, petroleum, and gas led the world to an energy crisis and environmental pollution. By usage of new technology and chemical, materials want to obtain a new form of clean energy. Hydrogen and bio-butanol are renewable energy sources considered essential and the most pristine and high potential energy sources (122 Ki/g) [1]. Butanol is a crucial biological liquid fuel, which can be produced from renewable resources by the anaerobic Acetone Butanol Ethanol (ABE) fermentation process conducted by solvent genic Clostridium strain, such as Clostridium acetobutylicum and Clostridium beijerinckii. For renewable energy production, some chemical particles or nanoparticles were involved in inducing the production of hydrogen and bio-butanol with the assistance of microorganisms. The significant developments in nanotechnology have improved their potential application in amplifying the biological process. Hydrogen production by microorganisms catalyzes these reactions by hydrogenase (Hydrogenases are critical enzymes in the energy metabolism of many organisms. Especially in anoxic habitats where molecular Hydrogen (H2) is an important intermediate, these enzymes expel excess reducing power by reducing protons or oxidizing H2 as an energy and electron source). Hydrogenase metal atoms present in their active site are NiFe, FeFe, and Fe-hydrogenase. Hydrogen production is influenced by the presence of the Iron (Fe) and Nickel (Ni) metal, Ione, which enhance the hydrogenase enzyme activity. Nanoparticles, according to the size shown in their efficiency, Fe based Ni based nanoparticles, and ions, including Mg, Cu, Na, NH4, and K, have different responses to Hydrogen Yield (HY) and Hydrogen Evolution Rate (HER). Manipulation and changing the size and concentration of nanoparticles have enhanced the hydrogen yield for Fe based but not Ni based NPs, and Ione. For example, the smaller (less than 42 nm) NPs improved the hydrogen yield, whereas, for the hydrogen evolution rate, bigger size of NPs (40 nm-50 nm) seemed to increase the hydrogen Hydrogen Evolution Rate (HER) the reason was the stability of large size nanoparticles, for maintaining its frame for a long time. The study was ethically approved by the SU ethics committee (code: 1386-1413) [2].
Literature Review
Impact of Fe based ions and NP addition
Impact of the concentration of Fe-ion/Fe NPs size effect on HY and HER in bio-hydrogen production. Statistically analyzed via ANN-RSM, the result showed that size and concentration strongly affected production (Table 1) [3].
| NPs | Opt (mgL-1) | Substrate | Sc (gL-1) | Size (nm) | HY (mmolg-1) | HER (mmol L-1h-1) |
|---|---|---|---|---|---|---|
| Fe (NPs) | 400 | Grass | 10.7 | 50 | 2.9 | 54 |
| Fe (NPs) | 25 | Starch | 5 | 35 | 3 | - |
| Fe (NPs) | 300 | Malate | 3 | 16 | 20 | 04 |
| Fe (NPs) | 50 | Xylose | 30 | 75 | 13.3 | 2 |
| Fe (NPs) | 200 | MS | 10 | 50 | 0.9 | 24 |
| Fe (NPs) | 200 | Sucrose | 7.5 | 50 | 15.9 | 10.1 |
| Fe (NPs) | 175 | Glucose | 7.5 | 59 | 12.9 | 5.69 |
| Fe (NPs) | 50 | Starch | 6 | 35 | 5 | - |
| Fe (NPs) | 250 | Malate | 4 | 12 | 24.2 | 0.8 |
| Fe2O3 (NPs) | 50 | Glucose | 5 | 50 | 1.92 | 2.5 |
| Fe2O3 (NPs) | 50 | CDW | 15.3 | 33 | 16.75 | 102.5 |
| Fe2O3 (NPs) | 200 | DW | 56 | 23 | 7.85 | 624 |
| FeO3 (NPs) | 50 | Waste water | 110 | 6.5 | 1.9 | 49.4 |
| Fe2O3 (NPs) | 200 | MEG | 4 | 100 | 8.4 | 0.6 |
| Fe2O3 (NPs) | 300 | CAS | 10 | 20 | 3.875 | 1.92 |
| Fe2O3 (NPs) | 200 | Glucose | 10 | 20 | 9.2 | 3.1 |
| Fe2O3 (NPs) | 60 | Glucose | 6 | 60 | 1.92 | 2.5 |
| Fe3O4 (NPs) | 10 | Glucose | 2.5 | 100 | 10.1 | 0.23 |
| Fe3O4 (A-C-NPs) | 250 | Glucose | 5 | 30 | 11.656 | 3.2 |
| GT-INP (Fe2O4 and FeO (OH)) (NPs) | 1000 | CO | 1.008 | 70 | 1.58 | 0.0662 |
| Magnetite (NPs) | 200 | SJ | 3 | 50 | 6.7 | 0.23 |
| Hematite (NPs) | 200 | Sucrose | 12.5 | 55 | 10.4 | 6 |
Table 1: Comparison of bio-H2 production with the addition of Fe-based nanoparticles.
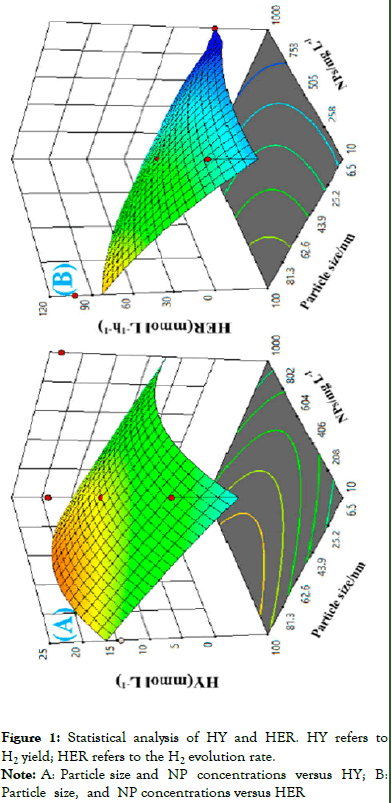
The effect of NPs size and concentration together has an impact on hydrogen yield, and hydrogen evolution was explored in the Figure 1.
Figure 1: Statistical analysis of HY and HER. HY refers to H2 yield; HER refers to the H2 evolution rate.
Note: A: Particle size and NP concentrations versus HY; B: Particle size, and NP concentrations versus HER
The above Figure 1 indicates the highest value in the range of NP size (81 nn-100 nn) and NP concentration (406 mg/-604 mg/l). For HER NPs size and NPs concentration tend to show the highest of HER is located size range of (81 nm-100 nm) its indicates that optimum size and concentration effects the production of hydrogen) [4].
Influence of nanoparticles on bio-hydrogen production
Nanoparticle (NPs) has been increasing significantly for application, protein immobilization, biosensors, and biofuel production. NPS can influence the microbial metabolic activity for the production of bio-hydrogen. Various NPs, positive effects such as Silver (Ag), Fe, Ni, Copper (Cu), Gold (Au), and Palladium (Pd), these NPs stimulate BHP (Bio-Hydrogen Production) by their surface [5]. The large size of NPS enables a solid ability to adsorb and transfer the electron. Different substrates are used to produce bio-hydrogen and bio-butanol, such as glucose, sucrose, lignocellulose, and rice mill wastewater, catalyzing by microorganism action of effective microorganism help the production rate and quality of hydrogen. This study developed an amylolytic Clostridium sp. strain BOH3 to produce butanol and hydrogen from food waste without enzymatic pretreatment. Strain BOH3, which possesses genes encoding amylases, can produce 14.1 g/L butanol and 16.2 mmol hydrogen’s from 180 g/L food waste. According to protein sequence analysis has been shown that amylase in Clostridium sp. strain BOH3 may contain a multi-active site for activity, and has high ability of translation than Clostridium beijerinckii NCIMB 8052. As a solvent, butanol is an important chemical to produce drugs, antibiotics, and vitamins, which aroused considerable industrial interest. In general, sugars stored in food waste are in the form of macromolecules (such as starch), which are usually hydrolyzed to monosaccharides prior to microbial fermentation. To improve the hydrolysis efficiency of food waste, we have to do various pretreatment methods such as physical, thermochemical, and enzymatic hydrolysis. Among these methods, supplementing commercial enzymes such as amylase and xylanase obtained the ideal effect to improve the hydrolysis efficiency of starch in food waste. Although efficiently enzymatic pretreatment can enhance butanol production from food waste, the economic value of this process is decreased as commercial enzymes cause high costs. Therefore, to address the two bottlenecks above, the main aims of this study are developing one step butanol and hydrogen production from food waste with amylase self-production using an amylolytic Clostridium sp. strain BOH3; enhancing butanol production and yield by promoting amylase activities. Hydrogenase is an enzyme for the production of hydrogen. Nickel (Ni) is present in the act e core site of hydrogenase, increasing H production, and at an optimum level of Ni increases bio-hydrogen productivity inside microorganisms [6]. Cobalt metallic nanoparticles also demonstrate their enhancement role in bio-hydrogen production. NPS some will decrease the production level as well. Ni and CO based metal nanoparticles reduce the toxicity for microorganisms to properly induce the production with all mentioned particles, Nickel Ferrite Nanoparticles (NiFe2O4 NPs) were arranged to progress hydrogen production via dark fermentation. The output of any element concentration and temperature is a significant issue for every nanoparticle has an optimum to produce the highest product. For example, between (50 mg/l-200 mg/l) promote hydrogen generation, excess NiFe2O4 NPs (over 400 mg/l) lowered hydrogen productivity, but at the concentration of 100 mg/l (37°C) and 200 mg/l (55°C) has shown 38.6% and 28.3% increase than control according to metabolic mentoring, NiFe2O4 NPs enhanced the butyrate pathway corresponding to the increasing abundance of Clostridium barium in mesophilic fermentation. The reason for nanoparticle usage is that the activity of hydrogen producing microbes may be inhibited in several ways, including accumulation of Volatile Fatty Acid (VFA), over organic load, and lower microbial activity. To overcome this limitation, use the nanoparticle, showing nanoparticles’ importance. Some metal nanoparticles (e.g., FeO3 and TiO2) accumulate inside the microorganism cells and interact with intercellular comportment via chemical, physical, or biological mechanisms [7]. Nanoparticles could be single metal and two single metals. For example, the spinal ferrite NPs, such as Nickel Ferrite (NiFe2O4), Copper Ferrite (CuFe2O4), and Manganese Ferrite (MnFe2O4), nanomaterials is used in magnetic drug delivery, electronic device, and information storage. Iron, which affects hydrogen and butanol production, is considerable to understand; iron is an important factor in producing the two mentioned products, Iron corporation with pH. Butanol production could be enhanced with suitable iron content. The hydrogenase content iron cluster is a unique type of Fe-s center called the hydrogen cluster. According to many research reports, hydrogen evolution or butanol production are individuals, But few reported simultaneous hydrogen and butanol production. Hydrogen and butanol production appears to be a competitive aspect of metabolic electron transferring and balance between NADH and NAD [8].
Due to the unique physical and chemical properties of different nanoparticles, numerous interests have been attention to use these nanoparticles in dark hydrogen fermentation to enhance and obtain low cost bioactivity of hydrogen producing microbes with a high yield rate of hydrogen production [9].
Ferric Oxide Nanoparticles (FO NPs) are used for dark hydrogen fermentation using Enterobacter aerogenes. Ferric oxide enhances electron transfer. Also, the hematite and nickel oxide were explored, which involves improving the ferredoxin oxidoreductase activity by electron transferring rate, thereby enhancing hydrogenase activity [10].
Discussion
Effects of FO NPs on bio-hydrogen fermentation and production from glucose and pre-treated cassava starch
Various concentrations from (0 mg/l-400 mg/l) FO NPs affect hydrogen yield and glucose substrate production rate. Gradually increased the hydrogen yield from 164.5 ml/g to 171 ml/g, 183 ml/g and 192 ml/g with different concentrations of FO NPs (0 mg/l-200 mg/l). The concentration of 200 mg/l FO NPs resulted in the highest hydrogen production as a camper to no addition of nanoparticles of FO NPs, which enhanced the hydrogenase activity and electron transfer efficiency in E. erogenous cells. By adding the FO NPs to fermentation, the electron transfer process was likely enhanced by FO NPs due to their excellent conductive properties. The purpose of using Clostridium is to efficient bio hydrogen and biobutanol producers among all other microorganisms.
Enterobacter strains are considered to be more promising for industrial scale for hydrogen production, the reason was the rapid growth rate of this microorganism, and its ability to utilize a wide range of substrates and have strong adaptability to dissolved, oxygen, hydrogen pressure and pH [11].
Conclusion
Nanoparticles are helpful for the production of hydrogen and bio-butanol. In hydrogen and bio-butanol production, the nanoparticles significantly impact the output. Nanoparticles with different concentrations with different sizes implement in the production. These nanoparticles have been used to produce hydrogen, and bio-butanol increased the action of hydrogenase as a cofactor. Hydrogen and bio-butanol renewable energy sources are considered essential and the cleanest and high potential energy sources (122 kj/g). NPS can influence microbial metabolic activity for the production of bio-hydrogen. Amylolytic Clostridium sp. strain BOH3 to produce butanol and hydrogen from food waste without enzymatic pretreatment. Strain BOH3, which possesses genes encoding amylases, can produce 14.1 g/L butanol and 16.2 mmol hydrogen’s from 180 g/L food waste. Iron, which affects hydrogen and butanol production, is considerable and an essential factor in producing the two mentioned products an iron corporation with pH. In general, sugars stored in food waste are in the form of macromolecules (such as starch), which are usually hydrolyzed to monosaccharides prior to microbial fermentation. Efficiently enzymatic pretreatment can enhance butanol production from food waste.
Future Perspective
To discover those nanoparticles are friendly to the microorganism. And prevent and modify nanoparticles that inhabit the microorganism's metabolic activity.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, on reasonable request to the corresponding author.
Conflict of Interest Statement
All authors declared no potential personal or financial conflicts of interest.
Ethical Approval Statement
This study was ethically approved by the medical bioethics committee of the SIHE ethics committee (code: 1386-1413). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
STP was involved in the study’s conception, design, statistical analysis, and interpretation of the data. AMB and HS were involved in data collection, data cleaning, statistical analysis, and manuscript drafting. AMB supervised the study. All authors approved the final manuscript for submission.
Funding
N/A
References
- Lin R, Cheng J, Ding L, Song W, Liu M, Zhou J, et al. Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using Enterobacter aerogenes. Bioresour Technol. 2016;207:213-219. [Crossref] [Google Scholar] [PubMed]
- Patel SK, Lee JK, Kalia VC. Nanoparticles in biological hydrogen production: An overview. Indian J Microbiol. 2018;58(1):8-18. [Crossref] [Google Scholar] [PubMed]
- Liu Y, Liu J, He H, Yang S, Wang Y, Hu J, et al. A review of enhancement of biohydrogen productions by chemical addition using a supervised machine learning method. Energies. 2021;14(18):5916. [Crossref] [Google Scholar]
- Rambabu K, Bharath G, Thanigaivelan A, Das DB, Show PL, Banat F. Augmented biohydrogen production from rice mill wastewater through nano metal oxides assisted dark fermentation. Bioresour Technol. 2021;319:124-243. [Crossref] [Google Scholar] [PubMed]
- Wu H, Wang C, Chen P, He AY, Xing FX, Kong XP, et al. Effects of pH and ferrous iron on the coproduction of butanol and hydrogen by Clostridium beijerinckii IB4. Int J Hydrog Energy. 2017;42(10):6547-6555. [Crossref] [Google Scholar]
- Yang G, Wang J. Improving mechanisms of biohydrogen production from grass using zero valent iron nanoparticles. Bioresour Technol. 2018;266:413-420. [Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhao W, Yang J, Li Z, Zhang J, Zang L. Comparison of mesophilic and thermophilic dark fermentation with nickel ferrite nanoparticles supplementation for biohydrogen production. Bioresour Technol. 2021;329:124853. [Crossref] [Google Scholar] [PubMed]
- Srivastava N, Srivastava M, Mishra PK, Kausar MA, Saeed M, Gupta VK, et al. Advances in nanomaterials induced biohydrogen production using waste biomass. Bioresour Technol. 2020;307:123094. [Crossref] [Google Scholar] [PubMed]
- Alexandri M, Lopez-Gomez JP, Olszewska-Widdrat A, Venus J. Valorising agro industrial wastes within the circular bioeconomy concept: The case of defatted rice bran with emphasis on bioconversion strategies. Fermentation. 2020;6(2):42. [Crossref] [Google Scholar]
- Choudri BS, Baawain M. Bioenergy from biofuel residues and wastes. Water Environ Res. 2016;88(10):1446-1466. [Crossref] [Google Scholar]
- Sar T, Harirchi S, Ramezani M, Bulkan G, Akbas MY, Pandey A, et al. Potential utilization of dairy industries by-products and wastes through microbial processes: A critical review. Sci Total Environ. 2021:152253. [Crossref] [Google Scholar] [PubMed]
Citation: Pachakhan ST, Sayedi H, Barikzai AM (2023) The Effect of Iron-Related Nanoparticles on Bio-Butanol and Bio-Hydrogen Production from Clostridial Fermentation: A Mini-Review Ferment Technol. 12:001
Copyright: © 2023 Pachakhan ST, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.