Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2020) Volume 11, Issue 2
The effect of citric acid on the physicochemical and microbiological parameters of processed burger meat
Elie Awad1,2, Dima Mnayer3* and Karine Joubrane12Food Safety Lebanese Commission, Presidency of the Council of Ministers, Lebanon
3Department of Sciences, Lebanese University, Lebanon
Received: 27-Feb-2020 Published: 20-Mar-2020, DOI: 10.35248/2157-7110.20.11.822
Abstract
Meat can undergo many changes and harbor a large number of pathogenic and spoilage microorganisms during primary and further processing. The objective of this research was to study the effect of citric acid on the physicochemical and microbiological parameters of burger meat.
This study was conducted in ten different processed meat factories in Lebanon during August 2018. The different samples of meat were marinated using Citric Acid (CA), with two concentrations of 1.5% (CA1.5) and 3% (CA3), untreated meat is used as a control (CA0). The burger minced meat samples were collected at four specific processing stages: grinding, spice addition, fine grinding, and molding, and then spray washed separately with citric acid. The samples were analyzed for their physicochemical properties such as pH, humidity, weight loss, meat protein. Microbiological analysis was also conducted on the meat samples (Total viable count, Escherichia coli, Staphylococcus aureus, Sulfite Reducing bacteria and Salmonella).
Results showed that 3% of citric acid concentration resulted in the greatest reduction in cooking loss and pH, with a greater increase in the moisture content of the burger meat and had no effect on the meat protein content. Moreover, the citric acid concentration at 3% resulted in the highest reduction of bacteria population on meat burgers. It was concluded that citric acid is highly effective in decontaminating meat burgers and in preserving its physicochemical properties and can be useful during the stages of processing especially at the spice addition stage.
Keywords
Citric acid; Meat burger; Microbiological analysis; Physicochemical properties
Introduction
For centuries, processed meat has been one of the most consumed foods in many countries around the world. Lebanon has a low rank in meat export as compared to the developed countries [1].
The richness of the meat in water and proteins of high biological value makes it an indispensable food for a balanced diet. Maintaining the sensory and textural properties of meat products is a challenge that requires more effort to protect the integrity of the product, its taste, flavor, and textural sensory attributes [2].
Traditionally, meat processing is a means of extending shelf-life (preserving) and producing a convenient item for use later and elsewhere. Processing is aimed at reducing the enzyme activity in the meat, retarding lipid oxidation, and preventing spoilage by microorganisms. In modern times, meat is processed not only as a means of preserving but also for producing consumeracceptable products compatible with modern lifestyles and philosophy of health-related quality of life [3].
Many bacterial communities present in poultry meat can include pathogenic species during primary and further processing. Post slaughter contaminations are due to unhygienic slaughterhouse floor, poor and almost completely unhygienic means of transportation, open meat cuts display and meat storage [4,5].
With respect to health and economic problems caused by bacteria, it is very important to reduce the initial microbial population on meat [6].
Various intervention strategies have been developed to reduce the level of bacteria on the surface of animals’ carcass such as washing and sanitizing with hot water, chlorinated water, foodgrade acids and salts [7].
Presently scientists focus is on reducing microbial load without having undesirable changes in meat sensory properties [8]. One of these interventional procedures is the use of organic acid; it is simple, cheap, fast and efficient in meat preservation technology [9]. An earlier study has proved that the dilute organic acid solutions have no undesirable effects on meat [10]. Organic acid solutions (1%-5%) such as lactic acid, acetic acid, citric acid, ascorbic acid, fumaric acid, and tartaric acid are the most frequently used chemical interventions for beef and lamb [11].
Citric acid is a 2-hydroxy 1,2,3-propane tricarboxylic acid (white powder) extracted from fruit juice. Several studies have demonstrated the effect of citric acid on the physicochemical and sensory properties of meat [12-14]. Moreover, citric acid is generally recognized as safe antimicrobial agents, and the dilute solutions of organic acids (1%-3%) are generally without effect on desirable sensory properties of meat [15].
Previous works focused on limited treatments during meat burger processing, therefore, this study aims at comparing the effect of two concentrations of citric acid (1.5% and 3%) on some important physicochemical and sensory properties such as pH, moisture, weight loss, and burger meat protein and on their antibacterial activities on some important bacteria species (Total viable count, Escherichia coli, Staphylococcus aureus, Anaerobic count (Sulfite Reducing bacteria) and Salmonella) in meat during the four stages of processing of the meat burger namely grinding, adding spices, fine grinding, and pressing.
Materials and Methods
Food matrix, chemicals
The food matrix used in this study is represented by frozen processed burger meat since it represents the basis of the most various dishes consumed in Lebanon. Seven hundred twenty samples collected from ten different factories in Lebanon were the subject of this work.
The citric acid used in our study is ProGarda flavor compound in powder under the name CITROX with two recommended dosages 1.5% and 3% prepared in sterile Distilled Water (DW). This type of organic acid is considered Generally Recognized As Safe (GRAS) and is commonly used in the food industry [16].
The Plate Count Agar medium, Tryptose Sulfite Cycloserine (TSC) Agar medium, and Muller-Kauffman tetrathionate broth were purchased from Sigma Aldrich (St. Louis, MO, USA).
RAPID ʹ Staph selective medium and RAPIDʹ E. coli 2 Agar medium were obtained from Bio-Rad (France), Potassium tellurite and Xylose-Lysine-Desoxycholate (XLD) selective agar were supplied by Hi-Media (Mumbai, India), egg yolk and peptone water were from Sigma Aldrich (Munich, Germany), API20E gallery was from Biomerieux (USA), Rappaport Vassiliadis broth was obtained from VWR (London, UK) and Trypticase Soy Agar (TSA) was supplied by Biomerieux (Marcyl’Étoile, France).
Sample preparation
The samples were treated at the Chamber of Commerce, Industry, and Agriculture of Tripoli and North Lebanon (CCIAT).
The samples were collected from four different stages of the burger flow chart; i) grinding, ii) adding spices, iii) fine grinding, iv) pressing.
A standardized sample is cut out from the meat, by using a sterile knife and forceps than placed in a sterile plastic cup. The sampling conducted in each factory was the following: in each stage, six samples remained intact, 6 samples were sprayed with a dosage of 1.5 % of Citrox® and 6 samples were sprayed with a dosage of 3% of Citrox® (Number of samples were taken based on in the Lebanese norms NL 722(2003)). Then, the samples were transferred quickly to the laboratory and stored in a refrigerant system at -20°C. Seventy-two samples were taken by one meat factory which makes a total of 720 samples analyzed from the ten meat industries.
For the analysis, the meat samples were cut into 25 g pieces, using scissors and a sterile forceps. The weighing is carried out using an analytical balance. A twenty-five-gram meat sample is used for the pH test, nine grams are weighed for the weight loss (cooking weight loss) and ten grams are weighed for the moisture and protein content tests.
The evaluation of the microbiological quality of burger meat required a whole process to look for the bacteria constituting the microflora of both original and pathogenic minced meat. The work methodology followed in this study is described by ISO 6887-2.
Physico-chemical properties
pH measurement: A 25 g meat sample was placed in a sterile Stomacher bag to which 225 ml of the buffer has been added for 1/10 dilution. Then the mixture was stirred for 30 seconds to allow a good distribution in the liquid homogeneously. The whole was incubated at room temperature for 15 minutes to complete the experiment. The sample was filtered through Wattman No. 4 paper. The pH of the filtrate was determined using a glass electrode pH meter (Eutech, China) after calibration.
Cooking weight loss: Nine grams of the meat was weighed (G1=weight 1), then the meat samples were prepared by placing them in immersed plastic bags in a water bath at 80°C until an internal temperature of 70°C (approximately 1 hour) was reached using a thermometer. The cooked meat was cooled to room temperature and weighed (G2=weight 2). The loss of cooking was calculated by the differences in weight before and after cooking.
Calculation formula: cooking weight loss=G1-G2
Moisture content: Moisture content was calculated using a moisture analyzer (OHAUS, Germany). The moisture content is deduced from the weight loss of the product during drying by measuring the mass variation of a sample while heating 10 g of the meat at a temperature of 103°C until the change in weight is stopped.
The protein content: Protein content was calculated using a protein analyzer (Indiamart, India). Using the analytical balance, ten grams of meat is weighed and placed in the protein analyzer after the addition of sulfuric acid (4 ml, 95% concentration) and the protein content for all samples was recorded.
Microbiological analysis
Processing of samples for analysis: Twenty-five grams of meat sample was aseptically cut into pieces using scissors and sterile forceps. The weighing was carried out using an analytical balance (Sartorius M-PROVE).
Each 25 g sample was individually placed in a sterile Stomacher bag to which 225 ml buffered peptone water was added for a 1/10 dilution. Then the mixture was stirred in the stomacher for 30 seconds to finally allow a good distribution of bacteria in the liquid in a homogeneous manner. The whole was incubated at room temperature for 15 minutes before proceeding to culture in the appropriate medium. The methods of enumeration used in this study were defined by ISO Technical Committee.
Enumeration of total viable count: The Total Viable Count (TVC) was counted according to ISO 4833: 2003. Colony count was performed on solid medium after inoculation with stock solutions and aerobic incubation at 30°C.
Seeding and incubation: Using sterile pipettes, 1 ml of the initial suspension was poured in sterile Petri dishes containing 15 ml of Plate Count Agar medium cooled to 45°C. The inoculum was thoroughly mixed with the culture medium by circular motions on a cool, horizontal surface. After solidification, the dishes were incubated at 30°C for 72 hours.
Expression of results: According to ISO 4833: 2003, each box selected must contain at most 300 colonies and at least 15 colonies. Counting was performed after incubation using a colony counter. The number of microorganisms per gram of product was calculated from the boxes selected per gram of product.
Enumeration of Staphylococcus aureus
The enumeration of Staphylococci was performed by the alternative method according to ISO 16140 under Attestation No: BRD 07/09 - 02/05, AOAC-RI approved N° 080602.
Seeding and incubation: One ml of the initial suspension was evenly distributed at the surface on three Petri dishes containing the RAPIDʹ Staph selective medium supplemented with potassium tellurite and egg yolk. The inoculum thus provided was delicately spread in a circular manner and the boxes were then incubated at 37°C for 24 hours.
Enumeration and confirmation: The principle of RAPID Staph medium was based on an optimized Baird-Parker formula to guarantee the detection and enumeration of S. aureus in 24 hours at 37°C. Typical Staphylococci produce gray to black colonies that are shiny and convex (1-2 mm) with a clear 2-5 mm halo due to proteolysis of the egg yolk, confirmed by the positive coagulase and latex agglutination test. It was possible to determine the number of S. aureus per gram of product.
Enumeration of E. coli
The enumeration of E. coli is performed according to ISO 16140 under Attestation No: BRD 07/1-07/93 and BRD 07/7-12/04, AOAC-RI N° 050601.
Seeding and incubation: Using sterile pipettes, 1 ml of the initial suspension was poured in sterile Petri dishes containing 15 ml of RAPID ʹ E. coli 2 Agar medium and poured into each Petri dish. The inoculum was thoroughly mixed with the culture medium by circular motions on a cool, horizontal surface. After solidification, the dishes were incubated at 44°C for 24 hours.
Expression of results: After incubation, coliforms (other than E. coli) form characteristic blue-green colonies and E. coli form characteristic pink to violet colonies. As E. coli is a species belonging to the coliform group, the enumeration of total coliform was achieved by adding the number of blue-green colonies and the number of pink-violet colonies. Only those plates that contain between 15-150 CFU should be retained.
Enumeration of Sulphite-Reducing Anaerobes
Sulphite-reducing Anaerobes (ASR) develop in Iron Sulphite Agar in 24 hours, giving typical black colonies according to ISO 15213: 2003. ASR spores are usually indicative of ancient contamination.
Seeding and incubation: Using sterile pipettes, 1 ml of the initial suspension was poured in sterile Petri dishes containing 15 ml of Tryptose Sulfite Cycloserine (TSC) Agar medium and poured into each Petri dish. The inoculum was thoroughly mixed with the culture medium by circular on a cool, horizontal surface.
After solidification, 10 ml of the same medium were poured into the Petri dishes as a coating. The dishes were incubated in an anaerobic jar at 37°C for 24 h.
Expression of results: Any black colony is enumerated and that surrounded by a black area was considered a sulfite-reducing bacteria. The total colonies per gram of the product to be analyzed were determined.
Enumeration of Salmonella
The method is described according to ISO 6579/A1 (02/2006) as follows:
Selective enrichment: 1 ml of the initial suspension was sterilely transferred into 10 ml of Muller-Kauffman tetrathionate broth (OXOID CM0148) using a sterile pipette. Similarly, 0.1 ml of the initial suspension was sterilely transferred into 10 ml Rappaport Vassiliadis broth (R.V/SCHARLAU 02-379). The tubes were then incubated at 37°C and 41.5°C respectively for 24 h.
Isolation and identification: From the cultures thus obtained, the isolation of Salmonella was done on Xylose-Lysine- Desoxycholate (XLD) selective agar following the method of quadrant striations. A step of incubating the dishes at 37°C for 24 hours followed the latter. Thus, the resulting suspect colonies were subcultured onto Trypticase Soy Agar (TSA) agar and incubated at 37°C for 24 h in order to obtain pure and colorless colonies for subsequent confirmation steps.
Serologic confirmation: This step constituted the first confirmation step and consisted of determining the characteristic O and H antigenic factors of Salmonellae. This was done using slide agglutination tests, using the specific polyvalent anti O and anti-H sera. If agglutination took place, the second confirmation step proceeded.
Biochemical confirmation: Colonies presumed to be Salmonella were verified using a biochemical system of identification. Using a sterile platinum loop, colonies grown on TSA agar were removed and dispersed in 5 ml of sterile physiological saline. Then, using a sterile dropper, the wells of the specific API20E gallery for the identification of Salmonella were loaded by this suspension, while respecting the indications on each well. The galleries were then incubated at 37°C for 24 hours. The next day the appropriate reagents were added to the wells and the reading of the results was done. The interpretation of the microbiological analysis results of the samples was based on the Lebanese Standards Institution-LIBNOR NL-504:2004 which is reproduced in Table 1.
| Bacterial characteristics | Standards (CFU/g) |
|---|---|
| Anaerobic count (Sulfite Reducing Bacteria) at 37°C | 30 |
| Escherichia coli at 44°C | 100 |
| Salmonella species at 37°C | Absence |
| Staphylococcus aureus at 37°C | 100 |
| Total viable count at 30°C | 5 × 105 |
Table 1: Meat Burger standard licensed by LIBNOR.
Statistical analysis
The Statistica 10 program was used for all analyses. Analysis of Variance (ANOVA test) was performed to assess whether there was a significant difference between different stages of data collection. The results were expressed as means ± standard deviation and considered significantly different at p<0.05.
Results and Discussion
Physicochemical properties
Effect of citric acid on cooking weight loss: Figure 1 represents the loss of cooking weight of the hamburger meat in the different stages: grinding (stage 1), adding spices (stage 2), fine grinding (stage 3) and pressing (stage 4) without organic acid (CA0) and with organic acids at different concentrations of 1.5% (CA 1.5) and 3% (CA3). The results showed significant values with respect to the processing of samples (CA0), CA1.5 and CA3 (p<0.05) and the four different stages (p<0.05), while the results are not significant compared to the samples per stage (p>0.05).
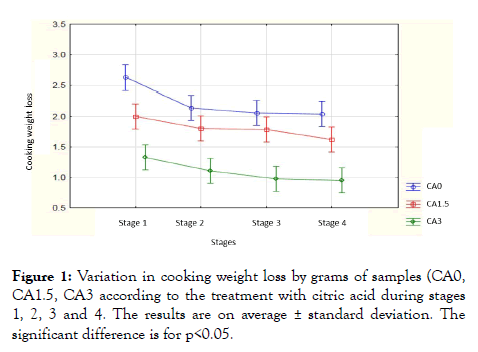
Figure 1: Variation in cooking weight loss by grams of samples (CA0, CA1.5, CA3 according to the treatment with citric acid during stages 1, 2, 3 and 4. The results are on average ± standard deviation. The significant difference is for p<0.05.
The results showed significant values of the different stages compared to the CA0 treatment (p=0.00031), and the different treatments compared to the first stage (p=3.34-7). The control samples showed an average difference cooking loss value of 2.76 ± 0.24 g in the first stage and decreased rapidly to an average value of 2.14 ± 0.24 g in the second stage and continued decreasing progressively to an average value from 2.05 ± 0.28 g to the third stage and 2.03 ± 0.31 g to the fourth stage. While with the two processed samples, the average results showed lower values, with a larger decrease in the averages of the results for the 3% citric acid concentration solution. The solution of 1.5% citric acid showed an average value of 2.0 ± 0.15 g in the first stage and decreased to an average value of 1.8 ± 0.17 g in the second stage and it continued to decrease to reach an average value of 1.62 ± 0.26 g in the fourth stage. The 3% solution of citric acid showed an average value of 1.33 ± 0.34 g in the first stage and it decreased to 1.11 ± 0.24 g in the second stage and it continued to decrease to a value average of 0.95 ± 0.16 g in the fourth stage.
Our results are in accordance with the literature reporting that weight loss decreases in meat samples marinated with citric acid [17,18]. The decrease in loss due to cooking acid treatments were explained by the swelling effect on muscle proteins that could hold more water [19]. The same authors showed also that the loss of cooking decreased at each stage, especially after the addition of the spices because of its acidic pH, and then decreased gradually in proportional relation to the pH. However, the influence of acids on the tissues depends on the type of fiber muscle in the meat as well as acidification [19]: a meat-rich in collagen has a less capacity to bind the water because most of the water present in the muscle is retained in the myofibrils [20]. Indeed, the study conducted by Burke and Monahan [21] on the effect of citrus juice on weight loss showed that marinated beef that contained a lot of collagen content did not show a statistically significant difference in meat weight loss.
Effect of citric acid on protein content: Table 2 shows the protein content of burger meat tested during the four stages of grinding (stage 1), adding spices (stage 2), fine grinding (stage 3) and pressing (stage 4) with the different treatments (CA 0, CA 1.5 and CA 3). The results did not show significant values compared to the treatment of the samples CA 0, CA 1.5 and CA 3 (p>0.05) and the four stages (p>0.05) and compared to the samples in stages (p>0.05). Control samples showed a mean value between 19.51 ± 1.82 % and 21.72 ± 1.95 % during the different stages. The 1.5% citric acid solution showed an average value between 20.17 ± 1.56 % and 21.50 ± 1.31 % at the different stages. The 3% citric acid solution showed a mean value between 19.32 ± 1.97 % and 21.11 ± 1.43 % at the different stages. The results obtained on the control and treated meat were comparable to those of the literature. Immersing the meats in a citric acid solution did not affect their protein levels [22].
| Samples | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
|---|---|---|---|---|
| CA0 | 19.51 ± 1.82a | 21.01 ± 1.51a | 21.45 ± 0.54a | 21.72 ± 1.95a |
| CA 1.5 | 21.32 ± 0.63a | 20.17 ± 1.56a | 20.50 ± 0.63a | 21.50 ± 1.31a |
| CA 3 | 21.11 ± 1.43a | 20.21 ± 1.65a | 19.50 ± 1.04a | 19.32 ± 1.97a |
The results are on average ± standard deviation. Values followed by the same letter are not significantly different (p>0.05)
Table 2: Variation in protein content (%) as a function of citric acid treatment during the different stages of processing.
Effect of citric acid on pH: Figure 2 shows the pH of the hamburger meat according to the stages of grinding (stage 1), adding spices (stage 2), fine grinding (stage 3) and pressing (stage 4) without organic acid and with organic acid at different concentrations (1.5% and 3%). The results showed significant values compared to the treatment of samples CA0; CA1.5 and CA3, (p<0.05) and the four stages (p <0.05), and compared to the samples in stages (p<0.05). Results showed significant values of the different stages compared to the CA0 treatment (p=4.36-10), and different treatments compared to the first stage (p=1.49-12). The control samples showed an average value of 6.76 ± 0.25 in the first stage and this value decreased to an average value of 5.95 ± 0.17 in the second stage and continued decreasing progressively to an average value of 5.73 ± 0.15 in the third stage and reached an average value of 5.5 ± 0.01 in the fourth stage. While the 1.5% solution of citric acid showed an average value of 5.18 ± 0.07 in the first stage and it gradually decreased to reach an average value of 4.61 ± 0.075 in the fourth stage. In addition, the 3% citric acid solution showed an average value of 4.7 ± 0.09 in the first stage and decreased to an average value of 4.05 ± 0.16 in the fourth stage.
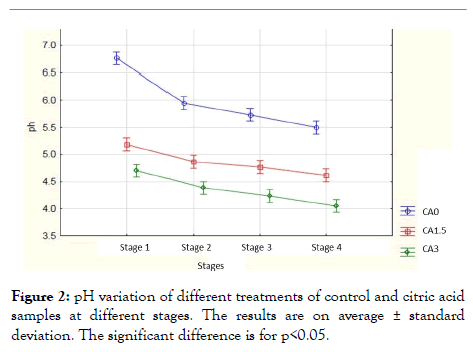
Figure 2: pH variation of different treatments of control and citric acid samples at different stages. The results are on average ± standard deviation. The significant difference is for p<0.05.
Our results showed that all marinated meats had the lowest pH. Moreover, the decrease of the pH in the second stage is due to the addition of the spices which have an acidic pH, and at each stage, the pH decreases progressively (at the second grinding stage and at the final stage). Indeed, the marinade in citric acid leads to a lower pH of the meat [23].
Effect of citric acid on humidity: Figure 3 shows the moisture of the hamburger meat according to stages 1, 2, 3, and 4 without organic acid and with organic acid at different concentrations (1.5% and 3%). The results showed significant values compared to the treatment of samples CA0, CA1.5 and CA3 (p<0.05) and the 4 stages (p<0.05), while the results were not significant compared to the samples by stages (p>0.05). Results showed significant values of the different stages compared to the CA0 treatment (p=0.0001), and the different treatments compared to the first stage (p=6.24-06). Control samples showed an average value of 47.16 ± 4.53% in the first stage and increased at each stage to reach an average value of 53.13 ± 1.02% in the fourth stage.
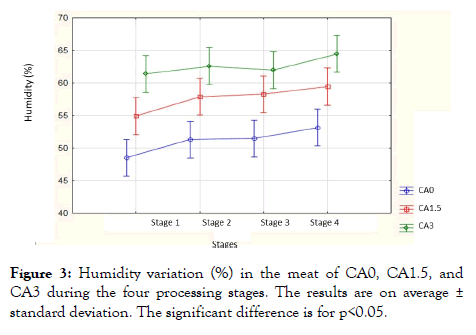
Figure 3: Humidity variation (%) in the meat of CA0, CA1.5, and CA3 during the four processing stages. The results are on average ± standard deviation. The significant difference is for p<0.05.
While the 1.5% solution of citric acid showed an average value of 54.9 ± 3.58% in the first stage and it gradually increased to reach an average value of 59.43 ± 1.89% in the fourth stage. The 3% solution of citric acid showed an average value of 62.55 ± 1.64% in the first stage and it increased to an average value of 63.00 ± 1.44% in the second stage and it remained constant in the third stage; afterward, it increased to reach an average value of 64.47 ± 5.23% at the fourth stage.
Results showed that marinated meat absorbed moisture, while intact meat showed a loss of fluid. Moisture is inversely proportional to pH. In addition to stage 2, moisture increased due to spices in samples that lower pH. Decreased pH could lead to increased moisture absorption in marinated meat [8]. In fact, the loss of liquid during the storage of meat is due to the denaturation of meat proteins [8]. Rao et al., [19] have shown that at pH values near the isoelectric point of the meat proteins, it is favorable to the muscle fiber to create extra water space added to the meat. Sofos [8] describes the mechanism of inflammation of the muscle under acidic conditions as follows: a reduction of the pH below the isoelectric point of the muscle proteins leads to the protonation of COO- negatively charged on groups of protein molecules and the breakdown of electrostatic bonds with certain NH+ groups on adjacent protein chains. The increase in net positive charge is thought to cause repulsion between groups of similarly charged proteins creating space for excess water.
Microbiological analysis
The microbiological tests described previously have been applied to all the samples taken at each stage of the process. All the samples examined were conformed to Lebanese Standards Institution- LIBNOR NL - 504:2004 - concerning the following parameters: Total Viable Count, Anaerobic Count (Sulfite Reducing Bacteria), Salmonella, Escherichia coli, and Staphylococcus aureus.
Contamination degree during processing: Although all the samples tested were confirmed according to the Lebanese standards, but contaminations were also recorded. Therefore, the degree of contamination was evaluated at different stages of meat processing according to citric acid treatment. Table 3 showed the percentages of contamination at each stage of the meat burger processing of CA, CA1.5, and CA3.
| Samples | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
|---|---|---|---|---|
| CA0 | 30% | 11% | 19% | 40% |
| CA1.5 | 25% | 14% | 19% | 42% |
| CA3 | 22% | 18% | 22% | 38% |
Table 3: Percentages of contamination during the four stages of processing for control and treated samples.
By referring to Table 3, close results were observed during each stage. The lowest contamination degree was graded during the second stage (adding spices) with values respectively of 11%, 14%, and 18%. The percentage of contamination increased during stage 3 (fine grinding) with values of 19% and 22%. During stage 1 (grinding) the percentages were higher than stages 2 and 3 with values of 30%, 25%, and 22%. Finally, we noted that stage 4 (molding) had the highest contamination degree with values respectively of 40%, 42%, and 38%.
During our visits and our observations in the food industries, the molding of the burger was accomplished in a processing area within another floor level and that was connected to it by the usage of an elevator, so the meat was transferred covered in the plastic container through the elevator where the area upstairs was missing any adequate controlled temperature. However, based on FAO [24] the room temperature during the four stages must be below +10°C to avoid the risk of meat spoilage, which can explain why the final stage showed the highest contamination degree. In addition to the previous, the molding machine lacked any disinfection, hygiene, and cleanliness before forming the meat burger. Mackey and Roberts [25] showed the importance of controlling temperature and time on pathogen growth in food industries.
Effect of citric acid on Total viable count: Figure 4 represents the number of Total Viable Count (TVC) in the meat burger based to the treatment of samples at both concentrations (1.5% and 3%) and controlled samples by comparing them at those different four stages of the process.
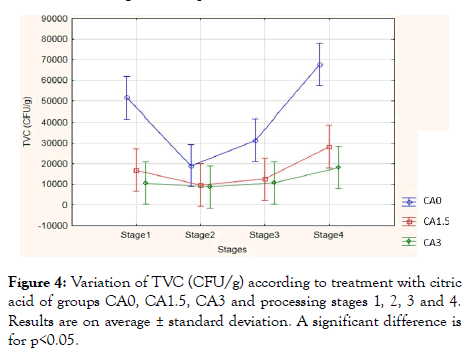
Figure 4: Variation of TVC (CFU/g) according to treatment with citric acid of groups CA0, CA1.5, CA3 and processing stages 1, 2, 3 and 4. Results are on average ± standard deviation. A significant difference is for p<0.05.
Results showed a significant value compared to the treatment of the CA0, CA1.5 and CA3 (p<0.001), compared to the four stages (p<0.001) and the stage treatment effects (p<0.001). Control samples showed an average value of 5 × 104 CFU/g at the first stage and it decreased in the second and third stages to a value of 2 × 104 and 3 × 104 CFU/g respectively. However, the TVC average value increased in the fourth stage to 6.8 × 104 CFU/g whereas, with both treated samples the TVC showed lower values. The 1.5% citric acid wash solution showed an average value of 1.8 × 104 CFU/g at the first stage and it decreased to 1 × 104 CFU/g at the second and third stages; however, it increased to 3 × 104 CFU/g at the final stage. For the 3% citric acid wash solution, the results were the most significant with 1 × 104 CFU/g, (p<0.00001) during the first three stages while it increased to 1.8 × 104 CFU/g in the fourth stage. During our meat sampling in different industries, we noted that some food safety aspects were not taken into consideration: lack of personal hygiene (absence of masks, entering with untreated street shoes), which may be a risk of cross-contamination. According to FAO [26], “ a high level of personal hygiene is required and therefore essential that staff involved in processing must be in good health. Staff members should never enter the food processing area in street clothes and shoes but wear clean protective clothing, and also carefully attend to personal hygiene”. Moreover, after each stage, the meat was kept in a big plastic container that was placed on the floor. According to FAO [26], containers for meat, fat, or semi-or fully processed meat products must not be placed directly on the floor but on hygienic stands, pallets to avoid the risk of contamination.
The contamination degree that was noted in our study during the first and third stages could be explained by the poor hygiene of the grinding machine. It is essential that the facilities, machinery, and tools are properly cleaned before and immediately after the process [26]. In some industries, other types of meat (makanek and sujuk) were processed in the same grinding machines without cleaning and disinfecting between each product processing which may lead to a risk of crosscontamination. The ICMSF [27] noted that during mincing, microorganisms present on the surface of the meat are distributed throughout the minced meat. Mincing itself may also increase the temperature of the meat. The extent of this increase depends on the process. The mincer itself may constitute a significant source of cross-contamination if not effectively cleaned before use and between batches [27].
During stage 2 where the spices were added to the meat burger, the average of TVC was lower than the first stage, Indeed, studies explained that many compounds isolated from spices have shown antimicrobial activity against some of the most common microorganisms that affect the food quality and shelf life [28,29]. This inhibitory effect affected also stage 3 and this is due to the positive interaction of spices with the citric acid in inhibiting microbial growth.
The highest contamination in the final stage was mainly attributed to the exposition of meat to inadequate temperature and the lack of hygiene of the molding machine.
Based on these results, we noted that the highest level of contamination degree was graded during the final stage and that both concentrations of citric acid decreased the average of the total viable count whereas the 3% citric acid concentration showed the best results.
Citric acid effect on the bacterial load during the four stages of processing: Figure 5 represents the number of E. coli (CFU/g) in the meat burger based on the citric acid treatments of samples at the different four stages of the process. Results showed a significant value compared to the CA0, CA1.5 and CA3 samples (p<0.001), while no significant value was detected compared to the four stages (p>0.05) and compared to the stages treatment effect (p>0.05). E. coli average value in control samples was 50 CFU/g at the first stage, while during the second stage it decreased to 35 CFU/g, then it increased in the third and fourth stages to score an average value of 62 CFU/g. However, in both citric acid wash solutions, E. coli was eliminated with an average value of zero CFU/g during all the 4 stages of processing.
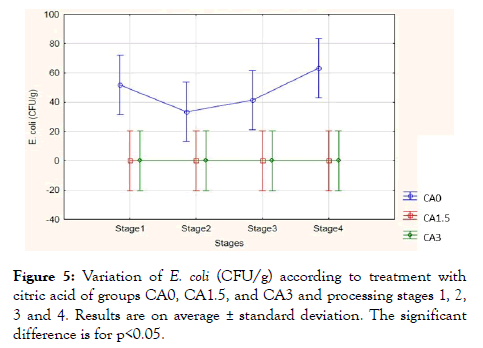
Figure 5: Variation of E. coli (CFU/g) according to treatment with citric acid of groups CA0, CA1.5, and CA3 and processing stages 1, 2, 3 and 4. Results are on average ± standard deviation. The significant difference is for p<0.05.
Regarding ASR, Figure 6 represents the number of ASR in the meat burger based on the treatment of samples at both concentrations (1.5% and 3%) and controlled samples by comparing them at the different four stages of the process.
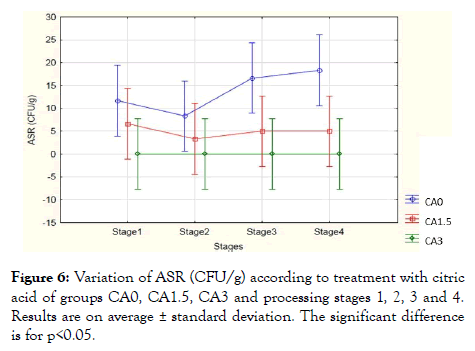
Figure 6: Variation of ASR (CFU/g) according to treatment with citric acid of groups CA0, CA1.5, CA3 and processing stages 1, 2, 3 and 4. Results are on average ± standard deviation. The significant difference is for p<0.05.
The results showed a significant value compared to the treatment of the CA0, CA1.5 and CA3 samples (p<0.001), while no significant value was detected compared to the four stages (p>0.05) and compared to the stages treatment effect (p>0.05). The ASR average value for control samples was between (9 and 14) CFU/g during the four stages, however, the 1.5% citric acid wash solution showed a lower average value of 5 CFU/g during all the stages and for the 3% citric acid wash solution, ASR was eliminated to 0 CFU/g for all the stages of processing. However, Salmonella and S. aureus both were not detected during all the stages of processing initially in the controlled samples and in the treated one. In our experiment, the addition of citric acid wash solution at both concentrations (1.5% and 3%) showed significant antibacterial activity against the total viable count, E. coli and Anaerobes Sulfite reducing the population in meat burger samples. Antimicrobial activities of organic acids such as citric acid, lactic acid, acetic acid, propionic acid, and ascorbic acid have been evaluated by several researchers [30,31]. Elsewhere, studies have evaluated (0.1 to 24)% concentrations of organic acids for their efficacies on red meat; bacterial reductions were directly proportional to higher acid concentrations, acids combinations, and if the acids were applied to adipose tissues [32]. The 1.0 to 5.0% concentrations of organic acids were typically used in reducing microbial load on meat surface [33].
The results of our experiment revealed that citric acid (3%) was more effective than 1.5% in reducing the total viable count. According to Vasseur et al. [34], citric acid had the highest inhibitory effect because of its ability to diffuse through the cell membrane.
Our study further revealed that both citric acid concentrations reduced E. coli population to 0 CFU/g, so we noted that lower concentrations of the citric acid wash solution were almost as effective as higher concentrations. In a study conducted by Cutter and Siragusa [35], the mean log reductions of E. coli O157: H7 on beef showed 0.72, 0.77 and 0.84 log 10 CFU per ml, when exposed to citric acid at 1.0, 3.0 and 5.0% concentrations respectively. Our results revealed also that citric acid at a concentration of 3% was more effective in reducing Anaerobic Sulfite Reducing population in meat burger samples than at 1.5%. Graham and Lund [36] also observed that the growth inhibition of Clostridium botulinum (one of the ASR) was attributable to the chelation of metal ions by citric acid, apart from its effect on pH.
In our study, both Salmonella and Staphylococcus aureus were not detected in controlled samples and in treated samples with citric acid (1.5% and 3%). However, Vasseur et al. [34] showed that citric acid at concentrations of 1% to 3% reduced Salmonella serotypes when sprayed on beef and poultry carcasses by causing intracellular acidification. Tabak et al. [37] reported that citric acid (0.03%) significantly reduced the growth of S. aureus. Other studies reported that citric acid (2%) was more effective against Gram-positive bacteria (Bacillus cereus, and S. aureus) than Gram-negative bacteria (Salmonella enteritidis, and E. coli) [38].
Conclusion
This study has highlighted the effect of citric acid on the physicochemical and microbiological qualities of processed meat. The experiment was conducted in ten different meat processing industries across Lebanon in August 2018. Results showed that the addition of the citric acid solution at different concentrations (1.5% and 3%) allowed a decrease in pH, loss of cooking and improved moisture without affecting the protein content. The effect of citric acid increased with its concentration. As a result, immersing meats in citric acid solutions did not affect the nutritional quality of the meat and improved the organoleptic and technological qualities by improving the juiciness and tenderness of the meat. Moreover, the treatment of the meat with citric acid led to the lowering of their pH which may be at the origin of the decrease in the proliferation rate of the germs. In that context, the addition of spices was in synergism with citric acid effect. The citric acid concentration 3% had the highest reduction of bacteria population on meat burger compared to the 1.5% concentration. The most contaminated stages during processing were grinding and molding where specific attention should be taken into consideration such as staff hygiene, machine cleanliness and adapting adequate temperature/time parameters to avoid bacterial growth during high-risk contamination stages.
Consequently, our results allowed us to recommend the industries to use a spray with a dilution of 3% citric acid on the burger meat especially during the stage of spice addition for better organoleptic, technological, microbiological quality while maintaining the nutritional quality of the meat.
REFERENCES
- Lebanese Customs (2018). www.customs.gov.lb/customs/tariffs/national/tariff1.asp
- Kassem MG, Emara MM. Quality and acceptability of value-added beef burger. World J Dairy Food Sci. 2010;5:14-20.
- Hussain G, Rahman A, Hussain T, Uddin S, Ali T. Citric and lactic acid effects on the growth inhibition of E. coli and S. typhymurium on beef during storage. Sarhad J Agric. 2015;31:183-190.
- Ribeiro JS, Santos MJ, Silva LK, Pereira LC, Santos IA, Lannes SCS, et al. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019;148:181-188.
- Ud-Din S, Rahman A, Uddin S, Khan K. Level of pathogenic Escherichia coli on animal’s body coat and in meat under slaughter house environment. J Biol Agric Healthc. 2015;5:150-155.
- Hosseini SE. The effect of meat marinating with lactic and citric acid on some physicochemical and electrophoretic pattern of beef burger. Iranian J Vet Med. 2015;9:103-108.
- Dubal ZB, Paturkar AM, Waskar VS, Zende RJ, Latha C, Rawool DB, et al. Effect of food grade organic acids on inoculated S. aureus, L. monocytogenes, E. coli and S. Typhimurium in sheep/goat meat stored at refrigeration temperature. Meat Sci. 2004;66:817-821.
- Sofos J, editor. Improving the safety of fresh meat. Elsevier; 2005.
- Corry JE, James C, James SJ, Hinton M. Salmonella, Campylobacter and Escherichia coli O157: H7 decontamination techniques for the future. Int J Food Microbiol. 1995;28:187-196.
- Greer GG, Dilts BD. Lactic acid inhibition of the growth of spoilage bacteria and cold tolerant pathogens on pork. Int J Food Microbiol. 1995;25:141-51.
- Eniolorunda OO, Apata ES, Ogunlesi OE, Okubanjo AO. Quality Evaluation of Beef Preserved With Food Grade Organic Acids at Room Temperature. J Food Res. 2014;3:120.
- Hassan R, El-Kadi S, Sand M. Effect of some organic acids on some fungal growth and their toxins production. Int J Adv Biol. 2015;2:1-1.
- Kim TK, Hwang KE, Lee MA, Paik HD, Kim YB, Choi YS. Quality characteristics of pork loin cured with green nitrite source and some organic acids. Meat Sci. 2019;152:141-145.
- Liao H, Wu XP, Zhang KY, Ding XM, Bai SP, Wang JP, et al. The effect of citric acid acidification of drinking water on growth performance, cecal pH, and cecal microflora of meat duck. Livest Sci. 2018;209:54-59.
- Kotula KL, Thelappurate R. Microbiological and sensory attributes of retail cuts of beef treated with acetic and lactic acid solutions. Journal Food Prot. 1994;57:665-670.
- FSIS U. Safe and suitable ingredients used in the production of meat and poultry products. Accessed on Feb. 2006;24:2016.
- Ke S, Huang Y, Decker EA, Hultin HO. Impact of citric acid on the tenderness, microstructure and oxidative stability of beef muscle. Meat Sci. 2009;82:113-118.
- Önenç A, Serdaroğlu M, Abdraimov K. Effect of various additives to marinating baths on some properties of cattle meat. Eur Food Res Technol. 2004;218:114-117.
- Rao MV, Gault NF, Kennedy S. Variations in water-holding capacity due to changes in the fibre diameter, sarcomere length and connective tissue morphology of some beef muscles under acidic conditions below the ultimate pH. Meat Sci. 1989;26:19-37.
- Honikel KO, Hamm R. Measurement of water-holding capacity and juiciness. InQuality attributes and their measurement in meat, poultry and fish products Springer, Boston, MA, 1994;125-161.
- Burke RM, Monahan FJ. The tenderisation of shin beef using a citrus juice marinade. Meat Sci. 2003;63:161-168.
- Bonilha EF, Branco RH, Bonilha SF, Araújo FL, Cyrillo JN, Magnani E. Body chemical composition, tissue deposition rates and gain composition of young Nellore cattle selected for postweaning weight. R. Bras. Zootec. 2014;43:175-182.
- Stackhouse RJ, Apple JK, Yancey JW, Keys CA, Johnson TM. Citric Acid Enhancement at Solution pH Values Between 3.5 and 5.0 Does Not Alter the Fresh or Cooked Color of Dark-Cutting Beef. Anim Sci Arkansas Anim Sci. 2015:65.
- FAO. Composition of meat. Animal Production and Health. 2015.
- Mackey BM, Roberts TA, Mansfield J, Farkas G. Growth of Salmonella on chilled meat. Epidemiol Infec. 1980;85:115-124.
- Food and agriculture organization of the united nations, Bangkok: FAO 2010.
- International Commission on Microbiological Specifications for Foods. Microbial ecology of food commodities. Blackie Acad Profess. 1998.
- Kassem GM, Atta-Alla OA, Ali FH. Improving the quality of beef burger by adding thyme essential oil and jojoba oil. Archives of Zootechnics. 2011;60:787-795.
- Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199-1218.
- Castillo A, Lucia LM, Mercado I, Acuff GR. In-plant evaluation of a lactic acid treatment for reduction of bacteria on chilled beef carcasses. J Food Prot. 2001;64:738-740.
- Raftari M, Jalilian FA, Abdulamir AS, Son R, Sekawi Z, Fatimah AB. Effect of organic acids on Escherichia coli O157: H7 and Staphylococcus aureus contaminated meat. Open Microbiol J. 2009;3:121.
- Dickson JS, Anderson ME. Microbiological decontamination of food animal carcasses by washing and sanitizing systems: a review. J Food Prot. 1992;55:133-140.
- James SJ, James C. Thawing and tempering. Meat refrigeration. Woodhead Publishing Cambridge. 2002:159-187.
- Vasseur C, Baverel L, Hebraud M, Labadie J. Effect of osmotic, alkaline, acid or thermal stresses on the growth and inhibition of Listeria monocytogenes. J Appl Microbiol. 1999;86:469-476.
- Cutter CN, Siragusa GR. Efficacy of organic acids against Escherichia coli O157: H7 attached to beef carcass tissue using a pilot scale model carcass washer. J Food Prot. 1994;57:97-103.
- Graham AF, Lund BM. The effect of citric acid on growth of proteolytic strains of Clostridium botulinum. J Appl Bacteriol. 1986;61:39-49.
- Tabak M, Armon R, Rosenblat G, Stermer E, Neeman I. Diverse effects of ascorbic acid and palmitoyl ascorbate on Helicobacter pylori survival and growth. FEMS Microbiol Lett. 2003;224:247-253.
- Rio EL, Muriente R, Prieto M, Calleja CA, Capita R. Effectiveness of trisodium phosphate, acidified sodium chlorite, citric acid, and peroxyacids against pathogenic bacteria on poultry during refrigerated storage. J Food Prot. 2007;70:2063-2071.
Citation: Awad E, Mnayer D, Joubrane K (2020) The Effect of Citric Acid on the Physicochemical and Microbiological Parameters of Processed Burger Meat. J Food Process Technol 11:822. doi: 10.35248/2157-7110.20.11.822
Copyright: ©2020 Awad E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


