Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2021) Volume 12, Issue 1
Positive Selection a simple approach to reduce high virus incidence and seed yam degeneration in Ghana.
Marfo EA1, Lamptey JNL1,2, Ennin SA1,2, Osei K1,2, Oppong A1, Quain MD1,2, Bosompem AN1, Danquah EO1, Frimpong F1 and Kwoseh C32CSIR College of Science and Technology, Ghana
3Department of Crops and Soil Sciences, Faculty of Agriculture, KNUST, Ghana
Received: 28-Jan-2021 Published: 04-Feb-2021, DOI: 10.35248/2157-7110.21.12.863
Abstract
This study was conducted to determine how seed yam selected from three sources namely: Positive Selection (PS), farmer practice and diseased can influence viral incidence and yields of three white yam varieties namely; “Pona”, “Laribako” and “Dente” in Ghana. Seed yams previously selected in 2015 from symptomless or mildly infected plants (PS), purchased seeds (farmer practice) and diseased seeds were established in field experiments in 2016 and 2017 at two locations; Ejura and Fumesua in a 3 × 3 factorial Randomized Complete Block Design (RCBD). The performances of the three seed sources were compared for their reaction to yam mosaic virus infection and tuber yield. Plants raised from PS performed significantly (p<0.05) better with least virus infection and disease severity scores irrespective of the variety. This study shows that PS is a good approach to reducing virus load as well as reducing seed yam degeneration while maintaining fairly good yields.Keywords
Degeneration; Farmer practice; Positive selection; Virus incidence; Virus severity
Introduction
Yam, Dioscorea spp. is an important staple food for more than 60 million people worldwide [1,2]. It belongs to the family Dioscoreaceae and genus Dioscorea. The genus Dioscorea has 600 species, however, only six are known to be economically and socially important in terms of food, cash and medicine [3]. In Sub- Saharan Africa specifically Ghana, it is regarded as vital crop for both domestic and export market [4].
The cropping of yam is done vegetatively using the edible underground tuber. These tubers may usually be damaged from nematodes, viruses, tuber rotting fungi and bacterial infections and these are major contributors to the poor tuber seed quality and low yields in yam. A loss in yield of up to 50% due to viruses has been reported in western Nigeria [5]. Cultivation of yam is being threatened by diseases caused by viruses in all areas where the crop is grown [6]. Various viruses belonging to different types of virus genera have been identified to cause viral diseases in cultivated and wild yams especially D. rotundata [7]. The viral infection produces symptoms such as mosaic, vein clearing, vein banding, chlorosis, mottle, stunting and leaf distortions in their host plants [8,7]. Among all the yam viruses Yam Mosaic Virus (YMV) is known to cause serious economic losses. They also reduce the internationally acceptable market standards as reported by Mantell et al. [9]. Those plants showing mosaic or mottle symptoms produce less vigorous plants and a decline in yield with internal brown spots which do not meet the required marketable standards. Furthermore, not only do yam viruses cause reduction in yield but also, they hamper the international exchange of planting materials within the Sub region.
In West Africa including Ghana, farmers practice what is known as ‘milking’ (harvesting the yam tubers early, without disturbing the shoot growth, and put the vine back into the soil. A cluster of seed yam sized tubers usually develop from the shoot, which is harvested as seed yams and are used for planting during the following planting season) [10,11]. This traditional method of producing seed yam often results in the production of poor quality, disease and pests infested planting material. Again in the traditional method of producing seeds, farmers do not pay attention to the health status of the plant being milked [7]. Thus in most cases diseased plants are milked resulting in the production of infected seeds for the next planting. This practice results in disease build up and yield losses in farmers’ fields.
It is therefore imperative that a source of clean planting material should be identified and used and tissue culture produced planting material can serve this purpose. The availability of cleaned planting materials from tissue culture or resistant varieties is a major constraint in Ghana. It has therefore become necessary to select apparently healthy looking plants from farmers’ field to be used as planting materials in the subsequent growing season. This method known as Positive Selection (PS) (also referred to as ‘Select the best’) means selecting the best-looking plants or apparently symptomless or mildly infected plants as source of seed for the next planting season [12]. Positive selection involves identification, tagging and monitoring healthy-looking plants during vegetative growth until the tubers are harvested and stored to be used as seed for the next cropping season [13]. It must be noted that before the positively selected seeds are planted, they are screened to reject tubers which have nematodes galls and cracks. Positive selection has been proven to be a promising complementary practice for smallholder potato farmers in Kenya, in addition to seed production and marketing by specialized seed growers [14]. Application of positive selection in potato is reported to have increased yields of smallholder potato farms without monetary investment and the important mechanism behind the effect of positive selection is the reduction of virus infection in plant population [15,16] reported that Positive selected seed (PSS) showed 12.6% latent Bacterial Wilt infection compared to 44.7% from Farmer Selected Seed (FSS) on potato. All samples from positive selection were free from Potato Leaf- Roll Virus (PLRV) and Potato Virus Y (PVY), and had lower infection incidence of Potato Virus S and Potato Virus X than Farmer selected seed. In Uganda, the incidence of Bacterial Wilt symptomatic potato plants in progeny crops did not exceed 3% in positive selection compared with 6.7% in Farmer selection seeds and incidence did not significantly differ from Basic Seed. It is supposed that when positive selection method is applied to produce seed yam it can result in the reduction of virus infected seed yams and disease build up in farmer fields.
Over the years, yam virus diagnostics have employed the use of Enzyme Linked Immunosorbent Assay (ELISA) and recently the use of molecular diagnostic tools. The different types of ELISA have been used to diagnose yam viruses including Triple Antibody Sandwich (TAS) ELISA, protein A sandwich (PAS) ELISA and double antibody sandwich (DAS) ELISA. In 2012, Eni et al. did a re-evaluation of the methods available for the detection of Yam Mosaic Virus (YMV) to see which of them was most efficient including Immune-Capture Reverse Transcription Polymerase Chain Reaction (IC-RT-PCR). The IC-RT-PCR was able to detect more samples infected with YMV than both PAS and TAS ELISAs which indicates the effectiveness of employing molecular detection. Currently, techniques such as reverse transcription-recombinase polymerase amplification (RT-RPA) [17,18] and the use of tissue culture and next-generation sequencing [19-22] are used in yam virus diagnosis after morphologically observing visible or no visible symptoms.
This work was thus carried out to ascertain whether Positive Selection (positive selection) can be applied to reduce the incidence of viral diseases on yam and thereby increase the productivity of the crop. The specific objectives were to determine how positive selection, farmer practice and diseased seed tubers could influence viral incidence and severity on three white yam varieties namely; “Pona”, “Laribako” and “Dente” in Ghana and also to determine the rate of seed yam degeneration among the different seed sources.
Methodology
Site of Experiment and Preparation of planting materials for 2016 trial
Multiplication sites were established at two locations, CSIR-Crops Research Institute at Fumesua Kumasi (latitude 60° 41’ N and 10° 28’ W longitude) and a Research out station at Ejura (located on 70° 23’ N, latitude and 10° 21’W longitude) during the major season in 2015. The purpose was to produce planting materials for two treatments namely; positive selection and field infected plants. A field size of (100 × 50) m (5000 m2) was ploughed, harrowed and ridged mechanically using tractor at both sites. Three white yam, Dioscorea rotundata varieties; locally known as Pona, Dente and Laribako were purchased from Ejura yam market. They were cut into minisett sizes of 70 g and treated with a cocktail of 70 g mancozeb and 75 ml of Karate (lambda cyhalothrin) in 10 L of water. The treated minisetts were dried under shade for 24 hours prior to planting. The fields were established with a spacing of 1.0 ×
0.5 m. Weeding was done as and when necessary to manage weeds. Yam vines were trailed unto 2 m bamboo sticks two weeks after sprouting for effective interception of sunlight for photosynthesis. The sprouted plants were tagged using blue and red ribbons, based on disease severity score on a scale of 1-5. A score of 1 represents no obvious symptoms, 2 represents symptoms on 1%-24% of leaves, 3 represents symptoms on 25%-50% of leaves, 4 represents symptoms on 51%-74% of leaves and 5 represents symptoms on 75%-100% of leaves (20). Yam plants with disease severity score of 1 and 2 (symptomless and mildly infected) were tagged with blue ribbons and those scoring 4 and 5 were tagged with red ribbons. The blue tagged plants were routinely inspected in order to ensure that they were not severely infected with passage of time. Any blue tagged plant that scored above 2.0 in the course of the season was rejected and the tag removed accordingly. The symptomless and mildly infected (blue tagged plants) of the three varieties represented the positive selection treatment whilst the severely infected (red tagged plants) plants represented the diseased treatment. Harvested seeds were treated with 79 g of a fungicide, mancozeb super (mancozeb and methaxyl mixture) and 50 ml of an insecticide Karate (Lambda- cyhalothrin ); mixed in 15 L of water against fungal and insects damage during storage. They were then air dried for four (4) hours after which they were arranged in a bamboo yam barn at CSIR- CRI, Fumesua. The seeds were stored and used for the main field experiment in the 2016 major season.
Establishment of 2016 field trial Virus Detection
In 2016, fields at Fumesua and Ejura were ploughed and poultry manure (6 tons/ha) spread before harrowing. Ridges were made at a distance of one meter apart. All these preparations were done mechanically with a tractor. In all, there were nine treatments made up of three varieties of D. rotundata (Pona, Dente and Larabako) and three different seed sources (Positive Selection Seed (PS), Farmer Practice (FP) (purchased from the market) and seed from diseased plants). The experimental design was a 3 × 3 factorial in a Randomized Complete Block Design (RCBD) with three replications. There were six rows per plot or treatment for all the nine treatments at both locations. Rows measured 6 m long and 2 m apart. The spacing was 1.0 × 0.5 m with 12 plants per row. The seed tubers for the various treatments were cut into minisett sizes of 70 g and treated with a cocktail of 70 g mancozeb and 75 ml of Karate in 10 L of water as described earlier. Planting was done at Fumesua and Ejura in April and May, 2016 respectively. The experiment was repeated in 2017 to observe the rate of seed degeneration among the treatments. Data collected included number of sprouts after three weeks of planting, virus disease incidence, severity and tuber yield.
Virus Detection
Molecular based diagnosis for Yam Mosaic Virus (YMV) and Yam Mild Mosaic Virus (YMMV) (two known common viruses of yam in these locations) was conducted to confirm the health status of the positively selected plants. Young symptomatic and asymptomatic leaf samples were collected from the field for laboratory diagnosis using Polymerase Chain Reaction (PCR). Total nucleic acid was extracted using modified combined CTAB and Phenol- Chloroform based DNA extraction protocol (21). The pellets obtained were suspended in low TE (Tris EDTA) buffer and kept in -80℃ prior to testing. The Nanodrop 2000c spectrophotometer (Thermo Scientific) was used to quantify DNA and RNA. Reverse transcriptase (RT)-Polymerase Chain Reaction (PCR) was carried out using Protoscript® II RT-PCR kit (New England Biolabs Inc). The PCR was a one-step multiplex system the master mix was a
12.5 uL reactions containing 6.25 µl one taq one step reaction mix (2x), 0.5 uL one tag one step enzyme mix (25x), 1.0 µl each of primer mix-YMV (F+R) and YMMV (F+R), 1.75 µl PCR water and 2 µl of RNA template. The cycler (AB Applied Biosystem PCR thermal cycler), was set to the following conditions: 42℃ for 30 min for reverse transcription, 92℃ for 5 mins followed by 35 cycles of 94℃ for 40s, 55℃ for 40s, 72℃ for 5min and final extension of 72℃ for 5min. Amplification products were resolved on 1.5% agarose gel (Cleaver Scientific Ltd electrophoresis in 1x Tris-Boric acid-EDTA (TBE) buffer.
Results
Field establishment
Field experiment data analysed with Genstats statistical package (version 12) is as presented in Tables 2-7.
| Primer name | Sequence | Reference |
|---|---|---|
| YMV F | ATCCGGGATGTGGCAATGA | (22) |
| YMV R | TGGTCCTCCGCCACATCAAA | |
| YMMV F | GGCACACATGCAAATGAARGC | (22) |
| YMMV R | CACCAGTAGAGTGAACATAG |
Table 1: Primers and their sequences used in this study (Inqaba Biotechnical Industries (Pty) Ltd., Hatfield 0028, South Africa)
| Treatment | Mean no. of sprouts |
Mean viral disease Incidence (%) |
Mean viral disease severity (scale:1-5) |
Mean tuber yield (Mg/ha) |
||||
|---|---|---|---|---|---|---|---|---|
| Variety | 2016 2017 | 2016 2017 | 2016 2017 | 2016 2017 | ||||
| Dente | 10.00 | 3.22 | 84.32 | 90.67 | 3.35 | 4.27 | 6.96 | 1.76 |
| Larabako | 9.33 | 4.78 | 89.50 | 92.22 | 2.85 | 3.27 | 7.98 | 4.62 |
| Pona | 8.89 | 3.78 | 85.99 | 92.78 | 2.96 | 3.50 | 7.47 | 4.02 |
| LSD (5%) | NS | 1.00 | 4.51 | 1.79 | 0.19 | 0.59 | NS | 0.52 |
| CV | 8.5 | 11.3 | 2.2 | 0.9 | 2.8 | 7.1 | 6.1 | 6.6 |
| Source | ||||||||
| PS | 11.56 | 5.11 | 62.28 75.67 | 2.16 3.22 | 10.07 5.17 | |||
| FP | 8.44 4.00 | 4.00 100.0 | 3.01 3.72 | 7.92 3.55 | ||||
| Disease | 8.22 2.67 | 2.67 100.0 | 4.00 4.11 | 4.41 1.67 | ||||
| LSD (5%) | 0.92 0.83 | 0.83 7.16 | 0.32 0.25 | 0.54 0.53 | ||||
| CV | 8.5 11.3 | 11.3 3.4 | 2.8 7.1 | 6.1 6.6 | ||||
| Variety*Source | ||||||||
| Dente PS | 12.33 4.33 | 56.67 72.00 | 2.33 4.00 | 10.50 3.96 | ||||
| Dente FP | 8.33 3.33 | 96.29 96.29 | 3.56 4.33 | 6.83 0.93 | ||||
| Dente diseased. | 9.33 2.00 | 100.0 100.0 | 4.16 4.50 | 3.53 0.40 | ||||
| Larabako PS | 11.33 6.00 | 72.22 76.67 | 2.00 2.66 | 9.70 6.16 | ||||
| Larabako FP | 8.33 5.00 | 96.29 96.29 | 2.73 3.33 | 8.10 5.13 | ||||
| Larabako diseased | 8.67 3.33 | 100.0 100.0 | 3.80 3.83 | 6.13 2.56 | ||||
| Pona PS | 11.00 5.00 | 57.96 78.33 | 2.16 3.00 | 10.00 5.40 | ||||
| Pona FP | 8.67 3.67 | 100 100.0 | 2.73 3.50 | 8.83 4.60 | ||||
| Pona diseased | 7.00 2.67 | 100 100.0 | 4.00 4.00 | 3.57 2.06 | ||||
| LSD (5%) | NS NS | 7.13 3.41 | 0.47 NS | 1.11 0.83 | ||||
|
CV |
9.6 20.80 |
5.1 2.40 |
10.2 6.7 |
7.1 15.0 |
||||
Table 2: Performance of variety, seed source and their interaction at Ejura during 2016 and 2017 planting seasons.
| Treatment | Mean no. of sprouts |
Mean viral disease Incidence (%) |
Mean viral disease severity (scale:1-5) |
Mean tuber yield (Mg/ha) |
|---|---|---|---|---|
| Variety | 2016 2017 | 2016 2017 | 2016 2017 | 2016 2017 |
| Dente | 12.00 2.89 | 86.30 97.20 | 3.05 3.77 | 9.30 3.07 |
| Larabako | 8.33 1.78 | 94.30 97.80 | 3.21 3.77 | 8.49 3.87 |
| Pona | 10.44 2.33 | 84.80 100.0 | 2.77 2.83 | 9.62 3.16 |
| LSD (5%) | 1.59 0.61 | 6.85 NS | 0.44 NS | 0.32 0.47 |
| CV | 6.8 11.7 | 3.4 2.6 | 6.6 11.9 | 1.6 6.2 |
| Source | ||||
| PS | 12.11 3.00 | 74.90 95.00 | 2.37 2.38 | 12.47 5.57 |
| FP | 10.44 2.11 | 91.10 100.0 | 2.61 3.94 | 9.44 2.41 |
| Disease | 8.22 1.89 | 100.0 100.0 | 4.05 4.05 | 5.50 2.13 |
| LSD (5%) | 0.92 0.97 | 7.16 NS | 0.33 0.42 | 0.60 0.38 |
| CV | NS 11.7 | 3.4 2.60 | 6.6 11.9 | 1.6 6.20 |
| Variety Source | ||||
| Dente PS | 14.00 3.00 | 68.30 91.70 | 2.33 2.16 | 13.47 5.36 |
| Dente FP | 11.67 3.00 | 96.29 100.0 | 3.56 4.66 | 9.13 1.83 |
| Dente diseased | 10.33 2.67 | 100 100.0 | 4.16 4.50 | 5.30 2.03 |
| Larabako PS | 11.33 3.33 | 91.30 93.3 | 2.00 2.66 | 10.97 6.23 |
| Larabako FP | 8.00 1.00 | 96.29 100.0 | 2.73 4.33 | 9.57 2.70 |
| Larabako diseased | 5.67 1.00 | 100 100.0 | 3.80 4.33 | 4.93 2.70 |
| Pona PS | 11.00 2.67 | 65.00 100.0 | 2.16 3.00 | 12.97 5.13 |
| Pona FP | 11.67 2.33 | 100 100.0 | 2.73 2.83 | 9.63 2.70 |
| Pona diseased | 8.67 2.00 | 100 100.0 | 4.00 3.30 | 6.27 1.66 |
| LSD (5%) | 1.8 NS | 11.16 NS | 0.47 0.97 | 0.87 0.63 |
| CV | ||||
| 8.8 40.80 | 7.9 2.40 | 10.9 12.0 | 6.40 15.00 | |
Table 3: Performance of variety, seed source and their Fumesua during 2016 and 2017 planting seasons.
| Seed source | 2016 Mean Yield (Mg/ha) |
% loss in Yield | 2017 Mean Yield (Mg/ha) |
% loss in Yield |
|---|---|---|---|---|
| PS | 10.07 | - | 5.17 | - |
| FP | 7.92 | 21 | 3.55 | 31 |
| Diseased | 4.41 | 56 | 1.67 | 68 |
Table 4: Effect of using Positive selection, Farmer practice and Diseased planting materials on the yield of seed yam production at Ejura.
| Seed source | 2016 MeanYield (Mg/ha) |
% loss in Yield | 2017 MeanYield (Mg/ha) |
%loss in Yield |
|---|---|---|---|---|
| PS | 12.47 | - | 5.57 | - |
| FP | 9.44 | 24 | 2.41 | 57 |
| Diseased | 5.50 | 56 | 2.13 | 62 |
Table 5: Effect of using Positive selection, Farmer practice and Diseased planting materials on the yield of seed yam production at Fumesua.
| 2016 Cropping season 2017 Cropping Season | ||||||||
|---|---|---|---|---|---|---|---|---|
| Yarn | Yield positive selection | Yield farmer practice | Yield increase | Yield positive selection | Yield farmer practice | Yield increase | ||
| Variety | (Mg/ha) | (Mg/ha) | Mg/ha | % | (Mg/ha) | (Mg/ha) | Mg/ha | % |
| Dente | 10.5 | 6.8 | 3.7 | 53 | 4 | 0.9 | 3 | 325 |
| Laribako | 9.7 | 8.1 | 1.6 | 19 | 6.2 | 5.1 | 1 | 20 |
| Pona | 10 | 8.8 | 1.2 | 13 | 5.4 | 4.6 | 0.8 | 17 |
Table 6: Yield from positive selection compared with farmer practice for the three local varieties from Ejura for the 2016 and 2017 cropping seasons.
| 2016 Cropping season 2017 Cropping Season | ||||||||
|---|---|---|---|---|---|---|---|---|
| Yam variety | Yield | Yield | Yield increase | Yield | Yield | Yield increase | ||
| positive selection | farmer practice | positive selection | farmer practice | |||||
| (Mg/ha) | (Mg/ha) | (Mg/ha) % | (Mg/ha) | (Mg/ha) | (Mg/ha) % | |||
| Dente | 13.5 | 9.1 | 4.3 | 46 | 5.4 | 1.8 | 3.5 | 193 |
| Laribako | 11 | 9.6 | 1.4 | 14 | 6.2 | 2.7 | 3.5 | 130 |
| Pona | 13 | 9.6 | 3.3 | 35 | 5.1 | 2.7 | 2.4 | 90 |
Table 7: Yield from Positive selection compared with Farmer practice for three local varieties from Fumesua for the 2016 and 2017 cropping seasons.
In 2016, at Ejura, there was significant difference among the varieties, seed sources and their interaction in parameters such as virus disease symptoms incidence, severity and yield except for sprouting where there was no significant difference among the varieties and the interaction between the source of planting material and variety (Table 2, 2016). Positively selected planting materials interacted positively with source of planting material and variety (Table 2, 2016). With regards to tuber yield, among the varieties, Larabako recorded the highest yield with the lowest severity score. Plants from positive selection had the highest yield with the lowest incidence and severity scores as compared to plants from farmer practice as well as diseased tubers (Table 2). Positive selection Dente had the highest yield under the interaction table (Table 2). There was positive interaction between seed source and variety. Positively selected Dente seed recorded the highest yield of 10.50 Mg/ha which was not different from positive selection Pona seed and Larabako seed but out yielded farmer practice Dente and diseased Dente by 34.95% and 66.38% respectively At Fumesua, among the varieties, Pona recorded the highest yield with the least virus incidence and severity scores whiles positive selection materials performed significantly best among the other seed sources (Table 2, 2016). With interaction between seed source and variety, similar to the situation at Ejura, the interaction between seed source and variety was significant where positively selected Dente out yielded farmer practice and diseased by 32.22% and 60.65% respectively. Not only that positively selected Dente recorded the lowest virus incidence and severity scores, it also had the highest sprouting percentage (Table 2, 2016).
Generally, in 2017, there was high incidence and severity of virus at both locations which resulted in lower sprouting percentage and ultimately lower yields since yield is a function of plant population
[23] At Ejura, Larabako recorded the highest yield among the varieties with the lowest virus severity score and positive selection materials performed best among the different seed sources (Table 2, 2017). The interaction of positive selection Larabako resulted in highest yield at Ejura. The superiority of positive selection over farmer practice and diseased planting materials was further revealed. At Fumesua, Larabako was the highest yielding variety whilst positively selected Larabako out yielded the other seed sources (Table 2, 2017). Yields at Fumesua were generally higher than Ejura.
The use of positive selection as a technique to select planting materials for subsequent planting was able to save 21% and 31% yield losses in the year 2016 and 2017 respectively at Ejura whiles at Fumesua, positive selection saved 24% and 57% yield losses in 2016 and 2017 respectively as compared to Farmer Practice. This has been represented in Tables 4 and 5.
Among the three varieties, Dente had the highest yield increase for both locations and the two seasons as a result of Positive selection in 2016 followed by Larabako with Pona having the least yield increase but Larabako recorded the highest yield in 2017 followed by Pona and Dente respectively. Comparing yields from the two seasons, there was yield reduction from 2016 to 2017 (Table 6).
Virus detection
The PCR product resolved on agarose gel electrophoresis had the images as shown in Figures 1 and 2. In all, 44 leaf samples were tested for YMV and YMMV. Out of the 44 samples, 14 had amplifications for both viruses at the expected band sizes of 586 bp and 249 bp for YMV and YMMV respectively. None of the samples showed single infection by one virus. Out of the 44 samples, seven (7) were positive selection plants and out of the seven, five (5) samples did not amplify for any of the viruses but two (2) had mixed infections of YMV and YMMV.

Figure 1: Multiplex RT-PCR products in 1.5% agarose gel. Lane 1: ladder, lane 2: space, lane 3: YMV positive sample and lane 4: YMMV positive sample.
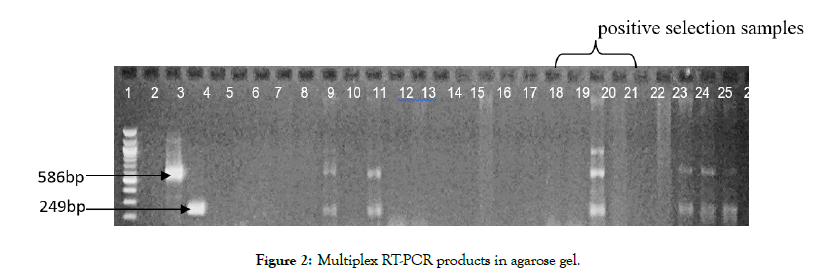
Figure 2: Multiplex RT-PCR products in agarose gel.
Discussion
The results from the two-year trial at both locations showed that positive selection planting material performed best under all four
parameters namely, sprouting, virus incidence and severity and tuber yield. The current results conform to the observations made [13,14] that positive selection is able to reduce virus infection thereby improving yield of the crop. From the results obtained in the 2017 trial, it was observed that the seed was losing its quality with time (seed degeneration), in terms of disease infection and tuber yields, irrespective of the source of the planting material positive selection materials performed better than farmer practice and diseased planting materials. This indicates the deleterious effect of recycling seed yam year after a year without recourse to the health of the seed. The fact that positive selection is capable of reducing yield losses by maintaining appreciable yields even when there is loss of seed quality with time makes it a very promising technique to be adopted by farmers. Also Positive selection again if adopted in Ghana by farmers would contribute to the reduction of virus load in yam growing areas which could impact positively on tuber yields of yam as was similarly observed on potato [15].
The presence of multiple or mixed infection of YMV and YMMV in the experimental plots in the two different agroecological zones conforms to similar observations are made [24]. The results of this trial in terms of the effect on virus infection on yields of especially plants raised from farmer practice and diseased tubers confirms the importance of YMV on white yams in Ghana. Its effect may become more devastating when it combines with other viruses like YMMV as observed in this study. Co-infection of different species of viruses often results in synergistic interactions that usually result in more severe leaf symptoms leading to greater yield losses [25]. Moreover, virus-virus interaction of yam viruses in mixed infections enhances the probability of genomic recombination which may result in more virulent strains of existing viruses or entirely new virus species [26].
The amplification of the two samples collected from plants raised from positive selection (which appeared symptomless) possibly gives an indication of latent infection by the culprit virus (es). Such plants exhibit high level of tolerance and often appear symptomless or mildly infected. If farmers could access tolerant planting materials as a result of positive selection, acceptable yields could be realized thus improving the income of the resource poor farmer. Samples collected from a few symptomatic plants did not amplify during the molecular diagnosis, and this could be due to lower virus concentration in those samples probably at the time for sample collection [27,28].
Conclusion
With farmers’ current practice of recycling seeds from one season to another, this study clearly shows that positive selection is a good approach to reducing virus load in farmers’ fields as well as reducing seed yam degeneration while maintaining fairly good yields. Thus, it will serve as an immediate approach to reducing the high disease prevalence in areas where the crop is grown while lasting or permanent approaches like breeding for resistant varieties and the use tissue culture techniques to produce clean planting materials become available for farmers to adopt.
Acknowledgement
We are grateful to the Community action to improve farmer saved seeds (CAY-Seed project) project at CSIR-CRI and all technical staff at the pathology section of the Plant health division of CRI, Fumesua.
Conflict of Interest Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- IITA. Healthy yam seed production Key fact. Research of Nourish Africa. 2013;1-6.
- Mignouna DB, Abdoulaye T, Alene A, Akinola AA, Maroya N. Baseline protocols: the case of Yam Improvement for Income and Food Security in West Africa (YIIFSWA) project; YIIFSWA working paper series, No. 4 2014.
- Aighewi BA, Maroya NG, Asiedu R. Seed yam production from minisetts: A training manual. 2014.
- Armah, M. Investment opportunity Ghana. Commercial Yam seed production. Millennium Development Authority. 2010;1-22.
- Emehute JK.U, Ikotun T, Nwauzor EC, Nwokocha HN. Crop Protection of Yams. Food Yams: Adv in Research 1998.
- Asiedu R, Ng SYC, Bai K.V, Ekanayake IJ, Wanyera NMW. Genetic improvement. Food yams: Advances in research. 1998;63-104.
- Kenyon L, Shoyinka SA, Hughes JDA, Odu BO. An overview of viruses infecting Dioscorea yams in sub-Saharan Africa. In Proceedings of a conference on Plant Virology in Sub Saharan Africa. 2001;432-439.
- Séka K, Diallo A, Kouassi K, Aké S. Screening ten yam (Dioscorea spp.) varieties for resistance to Yam Mosaic Virus and Cucumber Mosaic virus in Côte d'Ivoire. Afri J Plant Sci Biotech. 2009;3(1):36-43.
- Mantell SH, Haque SQ, Whitehall AR. A Rapid Propagation System for Yam. Caribbean agricultural research and development institute 1979.
- Asante BO, Otoo E, Acheampong P, Osei-Adu J, Nsiah-Frimpong B. Willingness to adopt the vine multiplication technique in seed yam production in the forest savanna transition agro-ecological zone, Ghana. J Dev Agri Eco. 3(16);710-719
- Aidoo R, Nimoh F, Bakang JEA, Ohene-Yankyera K, Fialor SC, Abaidoo RC. Economics of small-scale seed yam production in Ghana: implications for commercialization. J Sustain Dev Afr. 2011;13(7):65-78.
- Gildemacher P, Demo P, Kinyae P, Wakahiu M, Nyongesa M, Zschocke T. Select the best. Positive selection to improve farm saved seed potatoes. Trainers manual. Lima, Peru: International Potato Center 2007.
- Gildemacher RP, Schulte-Geldermann E, Borus D, Demo P, Kinyae P, Mundia P, et al. Dissecting a successful research-led innovation process: the case of positive seed potato selection in Kenya. Int J Tech Man Sust Dev. 2012;11(1):67-92
- Gildemacher, P., E. Schulte-Geldermann, D. Borus, P. Demo, P. Kinyae, P. Mundia, and P. Struik. 2011. Seed potato quality improvement through positive selection by smallholder farmers in Kenya. Potato Research 54: 253–266.
- Schulte-Geldermann E, Gildemacher PR, Struik PC. Improving seed health and seed performance by positive selection in three Kenyan potato varieties. Am J Potato Res. 2012;89(6):429-437.
- Kakuhenzire R, Lemaga B, Tibanyendera D, Borus D, Kashaija I, Namugga P, Schulte-Geldermann, E. Positive Selection: A Simple Technique for Improving Seed Potato Quality and Potato Productivity among Smallholder Farmers. Acta Hortic. 2013:1007:225-233).
- Silva G, Bömer M, Nkere C, Kumar PL, Seal SE. Rapid and specific detection of Yam mosaic virus by reverse-transcription recombinase polymerase amplification. J Virol Methods. 2015:222;138-144.
- Silva G, Oyekanmi J, Nkere CK, Bömer M, Kumar PL, Seal SE. Rapid detection of potyviruses from crude plant extracts. Anal Biochem. 2018;546:17-22.
- Bomer M, Rathnayake A I, Visendi P, Sewe SO, Sicat JPA, Silva G, et al. Tissue culture and next-generation sequencing: A combined approach for detecting yam (Dioscorea spp.) viruses. Physiol Mol Plant P. 2019:105;54-66.
- Eni AO, Kumar PL, Asiedu R, Alabi OJ, Naidu RA, Hughes JA, et al. First report of cucumber mosaic virus in yams (Dioscorea spp.) in Ghana, Togo, and Republic of Benin in West Africa. Plant Dis. 2008;92(5):833-833
- Egnin M, Mora A, Prakash CS. Factors enhancing Agrobacterium tumefaciens-mediated gene transfer in Peanut (Arachis hypogaea L.). In Vitro Cellular & Developmental Biology-Plant. 1998;34(4):310-318.
- Mumford RA, Seal SE. Rapid single-tube immunocapture RT-PCR for the detection of two yam potyviruses. J Viro Met. 1997;69(1-2):73-79.
- Eni AO, Hughes JDA, Rey MEC. Survey of the incidence and distribution of five viruses infecting yams in the major yam‐producing zones in Benin. Ann appl biol. 2008a;153(2):223-232.
- Akbar A, Pasha GR, Aslam M. Yield-density rapports: a non-parametric regression approach. Int Res J Financ Econ. 2010;43(1).
- Anjos JR, Jarlfors U, Ghabrial SA. Soybean mosaic potyvirus enhances the titer of two comoviruses in dually infected soybean plants. Physio & Biochem.1992;82(10):1022-27.
- Eni AO, Hughes JDA, Asiedu R, Rey MEC. Incidence and diversity of mixed viruses lower in yam tubers and tuber sprouts compared with field leaf samples: Implications for virus free planting material control strategy. Afr J Agric Res. 2013;8(23):3060-67
- Matthew R. PlantVirology. 3rd Edition. Academic press. Inc.San Diego, California. 1991;835.
- Oppong A, Lamptey JNL, Ofori FA, Anno-Nyako FO, Offei, SK., Dzomeku BM. Serological detection of Dioscorea alata potyvirus on white yams (Dioscorea rotundata) in Ghana. J Plant Sci. 2007:2(6);630-34.
Citation: Marfo EA, Lamptey JNL, Ennin SA, et al (2021) Positive Selection’ A Simple Approach to Reduce High Virus Incidence and Seed Yam Degeneration in Ghana. J Food Process Te.chnol 12: 863.
Copyright: © 2021 Marfo EA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.


