Indexed In
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2023) Volume 12, Issue 3
Paradigms of a Prophylaxis and Early Treatment in New Variants of SARS-CoV-2
Liliana Elena Weimer1*, Cattari Giovanna2, Fanales-Belasio Emanuele3, Cuccuru Elena2 and Vidili Gianpaolo22Department of Internal Medicine, Day Hospital, University Hospital of Sassari, Sardinia, Italy
3Department of Infectious Diseases, DMI; Istituto Superiore di Sanita, Rome, Italy
Received: 23-May-2023, Manuscript No. CMO-23-21925; Editor assigned: 26-May-2023, Pre QC No. CMO-23-21925 (PQ); Reviewed: 09-Jun-2023, QC No. CMO-23-21925; Revised: 19-Jun-2023, Manuscript No. CMO-23-21925 (R); Published: 26-Jun-2023, DOI: 10.35248/2327-5073.23.12.343
Abstract
Despite the challenges of outpatient administration and associated costs, monoclonal antibodies were a mainstay of the COVID-19 armamentarium from November 2020, when bamlanivimab first received US food and drug administration Emergency Use Authorization (EUA), through November 2022, when the bebtelovimab EUA was revoked.
Ideal qualities of treatments include effectiveness in preventing hospitalization and death, safety and tolerability for patients, easy administration in the outpatient environment, and cost-effectiveness. Monoclonal Antibodies (mAbs) that neutralize SARS-CoV-2 fit the safety and efficacy profile in early randomized clinical trials.
Monoclonal antibodies targeting the anti-SARS-CoV-2 Spike (S) protein are prescribed in high income countries to prevent severe disease in at risk patients. Although studies report efficacy as between 50% to 85%, global access is currently largely inequitable.
Multivariate omicron (B.1.1.529) and sub variant (BA.2 followed by BA.4 and BA.5) dominance has challenged the treatment landscape for mild to moderate disease, introducing considerable uncertainty on the efficacy of monoclonal antibodies and leading to changes to initial recommendations for some of them. Contemporaneously, oral, direct acting antivirals with a reported efficacy ranging from 30% (molnupiravir) to 89% to 90% (nirmatrelvir/ritonavir) have recently received conditional or emergency approval in some countries and been recommended in international guidelines such as the world health organization guidelines. S-217622, also known as ensitrelvir, a 3CL protease inhibitor that has been shown to significantly reduce the infectious viral load, is currently in phase 3 trials and waiting for emergency approval in Japan and should be submitted soon in China. The main purpose of this opinion paper is to highlight the possible strategies to optimize and protect current and future therapeutic options to treat the most vulnerable patients.
Keywords
Combination therapy; Monoclonal antibodies; SARS-CoV-2; Early treatment
Introduction
Scientists around the word have fervently searched for safe and effective therapies for COVID-19 since the advent of the pandemic [1].
Monoclonal antibodies targeting the anti-SARS-CoV-2 Spike (S) protein are prescribed in high-income countries to prevent severe disease in at-risk patients. Although studies report efficacy as between 50% to 85%, global access is currently largely inequitable [2,3].
Multivariate omicron (B.1.1.529) and sub variant (BA.2 followed by BA.4 and BA.5) dominance has challenged the treatment landscape for mild to moderate disease, introducing considerable certainty on the efficacy of monoclonal antibodies and leading to changes to initial recommendations for some of them [4-6]. Contemporaneously, oral, direct acting antivirals with a reported efficacy ranging from 30% (molnupiravir) to 89% to 90% (nirmatrelvir/ritonavir) have recently received conditional or emergency approval in some countries and been recommended in international guidelines such as the World Health Organization guidelines [7,8]. S-217622, also known as ensitrelvir, a 3CL protease inhibitor that has been shown to significantly reduce the infectious viral load, is currently in phase 3 trials and waiting for emergency approval in Japan and should be submitted soon in China [9]. The main purpose of this opinion paper is to highlight the possible strategies to optimize and protect current and future therapeutic options to treat the most vulnerable patients [10].
Literature Review
Protecting emerging treatment options
Several crucial issues warrant urgent attention to optimize the use of these emerging treatment options (Figure 1). First, as proven to be transformational for HIV, rapid, affordable access to early antiviral treatment to slow the tide of new variants is critical to effective “test and treat” strategies to protect the most fragile patients and avoid a severe and/or persistent infection. After more than 2 years of pandemic, progress has been slow and public health attention has recently been attracted by the low-profile agreement during the) in Geneva in May 2022 [11]. Together with vaccination, early diagnosis and treatment have the ability to reduce disease worsening, to reduce transmission and to constrain variability in viral sequences.
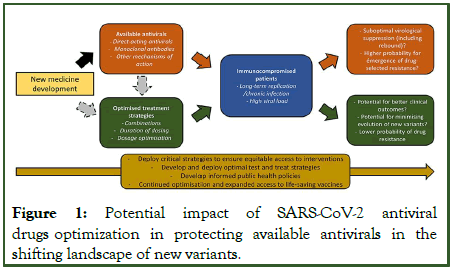
Figure 1: Potential impact of SARS-CoV-2 antiviral drugs optimization in protecting available antivirals in the shifting landscape of new variants.
Second, although the combined effect of omicron and increasing vaccine deployment in some regions has shifted the demand response from hospital to outpatient care, considerable uncertainty exists about who is now at risk for severe omicron disease [12]. While the risk/benefit ratio across at risk subpopulations has unquestionably changed in vaccinated populations, gains made can only be preserved if those at highest risk are rapidly diagnosed and receive treatment in less than one week.
Third, high levels of antiviral efficacy will be critically important, especially in immunocompromised patients who are grossly underrepresented in registration trials [13]. Causes of immunosuppression are diverse (including organ/stem cell transplants, cancer, immunosuppressive medications or uncontrolled HIV) and these patients represent a significant proportion of the population, e.g., 7 million adults in the USA, but also in low and middle income countries due to the high prevalence of uncontrolled HIV [14]. Overall, the mortality risk with omicron is still unclear, but protection of those who cannot be effectively vaccinated or protected by a prior SARS-CoV-2 infection remains imperative. Importantly, in regions where HIV is highly prevalent, there is a clear need and opportunity to reinforce HIV epidemic control by prompt diagnosis and sustained viral suppression with antiretroviral, key factors to also enable the control of SARS-CoV-2 spread in this group.
Although there are many other causes for variant emergence (host jump or adaptation, vaccine exposure, to name the most frequent), data confirm that immunocompromised patients with long-term SARS-CoV-2 replication are particularly susceptible to resistance and transmissible variant emergence. The emergence of resistance mutation thus impacting treatment efficacy is more likely if a patient has been exposed to specific antiviral drugs. In addition, it remains unclear if the small percent rebound occurrence (2%) observed with nirmatrelvir in the EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High Risk patients) trial, performed in the delta variant era, is underestimating a risk that would be particularly of concern in patients harboring an impaired immune system and in the omicron era. In one recent case series, one out of 7 patients who had a virologic rebound also had an immunosuppressing condition. Another recent case series revealed that all three patients with viral rebound were highly immunocompromised. This potentially raises concerns about the need of longer antiviral courses, especially in these patients.
Preclinical data have clearly demonstrated that virological efficacy is higher for combinations of existing antiviral drugs than single agents. To achieve the goal of changing the treatment guidelines in SARS-CoV-2 infected immunocompromised individuals, independent and academic clinical trials for drug combinations should be considered as an urgent, unmet research priority. Today, collaboration with industry to allow early access to antiviral drugs to be combined has been an objective still to be achieved. Certain potent monoclonal antibodies, such as bebtelovimab, cannot even be accessed for research or for routine care outside of the USA [15].
Early treatment optimization
Treatment optimization has been truly transformational for other viral diseases e.g., HIV/hepatitis C virus and was only achieved when antiviral drug combinations became the mainstay. With few drugs currently available, the opportunity must be seized prior to the emergence of resistance to drugs deployed widely as monotherapies. Combinations of polymerase inhibitors and polymerase/protease inhibitors have proven highly successful for other viruses and in animal models for SARS-CoV-2. Thus, as drugs that are appropriate to combine are available, there is no good reason not to study them clinically. In addition to the opportunities that combinations present for a more potent antiviral response (individual benefit), there can be no doubt that the rate at which resistance emerges will also be reduced (public health benefit). Higher potency will result in a lower variability in sequences through a lower degree of replication. In addition, the probability of the occurrence of multiple mutations to drive resistance to multiple antivirals simultaneously is much lower than for a single agent. This is particularly the case where concentrations achieved are close to the therapeutic efficacy threshold or in the case of low compliance.
It is incumbent upon the international research community and the pharmaceutical industry to pool knowledge and provide the critical information that the world health organization and country level authorities so urgently require, as well as early diagnosis and increased access to vaccines and antiviral therapy. The resistance risk for existing drugs has been woefully understudied throughout development, making it extremely challenging to rationalize during policy development. Looking beyond efficacy, drug combinations will unquestionably reduce the rate at which resistance and new variants impacting treatment options emerge and could be made available and accessible to those in need if timely efforts are made.
In conclusion, we call for combination therapies to be tested in adequately powered clinical trials in the target population of immunocompromised patients, both in wealthy and in low income countries where HIV-driven immunosuppression is prevalent. If higher efficacy is confirmed, the diversity of possible combinations will enable the tailoring of therapeutic options to individual patient needs (e.g., avoiding drug-drug interactions in transplant patients) as well as their specific regional context (e.g., oral-only combinations).
Does their use as a prophylactic or treatment potentially affect natural long-term immunity?
Considering the large doses used and the relative half-life of antibodies (~3 weeks for IgG molecules), there is a pertinent consideration whether the presence of circulating neutralizing mAbs could impact active immunity, whether through memory from infection or vaccination.
From the collective clinical data with MAb114, REGN-EB3 and palivizumab, the general benefits and risks associated with neutralizing mAbs are similar to those observed with traditional passive immunization against infectious agents. The agents themselves are relatively tolerable for patients, efficacious during the early onset of disease symptoms and in certain cases as a prophylactic, but with limited efficacy once infections are severe. The distinctions between these therapies are largely logistical; CPT is more rapidly implemented during an emerging pandemic when few therapeutic options are yet available, while neutralizing mAbs take time to discover and it takes time for regulatory approval for their use to be obtained as well as to scale up manufacturing capacity. The use and promise of passive immunization during the coronavirus outbreaks of the twenty-first century (that is, with SARS-CoV, Middle East respiratory syndrome related Coronavirus and SARS-CoV-2) have re-emphasized these past lessons while also highlighting additional insights, as we discuss next.
Fortunately today, the process to mass-produce recombinant mAbs has become scalable to meet demand and is costcompetitive with other treatments. Neutralizing mAbs overcome limitations intrinsic (for example, the risk of blood borne diseases, time to development of detectable high affinity antibodies and risk of low antibody titres, as well as variable epitope specificity. Furthermore, a high titre of neutralizing antibodies which current evidence indicates is necessary for the efficacy is inherent with neutralizing mAbs. As of April 2021, at least 20 neutralizing mAb therapies were being tested in late stage clinical trials or had already been approved for use in nine infectious diseases, including RSV infection and Ebola.
Association with several SARS-CoV-2 neutralizing monoclonal antibodies therapies with adverse outcomes of COVID-19
Elsewhere in JAMA network open, Ambrose, et al. evaluated the association of several SARS-CoV-2 neutralizing mAb therapies with adverse outcomes of COVID-19 in subpopulations at high risk of poor outcomes and across multiple variant epochs [16]. A population of 167183 patients met study inclusion criteria, of whom 25 241 (15.1%) received mAb treatment. All patients were non-hospitalized, had a EUA-defined risk factor for progression to severe disease, and received no other outpatient therapy for COVID-19. From November 2020 through January 2022, mAb treatment was associated with reductions in the odds of hospitalization of almost 50% and the odds of emergency department visits by 24% compared with no mAb treatment. The odds of 30 days all-cause death were reduced by 86% (OR, 0.14; 95% CI, 0.10-0.20). After adjusting for confounders, the Number Needed to Treat (NNT) to prevent the composite outcome of hospitalization or death at 30 days was 42. This association was observed against a backdrop of remarkable safety, with only 0.2% of patients experiencing any kind of adverse event.
The association of mAb therapy with improved outcomes was not uniform across all SARS-CoV-2 variants or across all patients. Patients who were unvaccinated or immunocompromised benefited the most from mAb therapy. The NNT to prevent 1 hospitalization at 14 days was 35 in the unvaccinated group and 17 in the immunocompromised group compared with 60 in the fully vaccinated group. In addition, the authors found that the mAb treatment effect size increased incrementally among patients with greater probability of poor outcomes (i.e., those with multiple or more severe comorbidities). It is unclear whether any patient in the study received tixagevimab-cilgavimab for prevention of COVID-19; however, this long acting mAb combination was granted EUA in early December 2021 and was not widely distributed until February 2022. Therefore, it is unlikely that its use substantially overlapped with the study period. Regardless, the authors’ findings are consistent with most other studies of COVID-19 therapies wherein patients who were seronegative at baseline were more likely to progress to severe disease and benefit from treatment. For immunocompromised individuals, the safety and efficacy of mAbs are especially notable because many of these patients have drug interactions or contraindications to other recommended outpatient COVID-19 therapies.
Unfortunately, at the time of publication, there are no mAb therapies available for the treatment or prevention of COVID-19. All EUAs were revoked or paused due to the emergence of substantial in vitro drug resistance among currently circulating SARS-CoV-2 variants. The question of whether in vitro potency directly correlates with clinical efficacy remains unanswered. In the absence of clinical data, regulatory bodies had to make decisions to offer or withdraw therapies relying on laboratory data alone. For example, the EUAs for both bamlanivimab-etesevimab and casirivimab-imdevimab were revoked on January 26, 2022, due to inability to neutralize omicron variants. Intriguingly, Ambrose, et al. found that casirivimab-imdevimab was associated with decreases in 14 days hospitalization (OR, 0.05; 95% CI, 0.01-0.42) in a small sample of 115 patients infected with sequence-confirmed omicron BA.1 despite the significantly reduced in vitro neutralizing ability of this mAb against this variant. Only 7.6% of patients received sotrovimab (which was expected to retain in vitro neutralization against early omicron variants) despite approximately 25% of the patients being diagnosed in the omicron era. When the omicron-era analysis was limited to patients who received sotrovimab, the treatment was associated with significant reductions in the odds of death within 30 days (bamlanivimabetesevimab and casirivimab-imdevimab were not).
What should clinicians and researchers do with these results, which describe 14 months of safe and effective therapy that is no longer available? Monoclonal antibodies provide important lessons that inform our future research and practice. First is the salient reminder to evaluate both the relative and absolute treatment effects when allocating scarce health care resources and/or determining the economic value of any given treatment. For instance, while the relative odds of 14 days hospitalization were exactly 49% lower in both unvaccinated and fully vaccinated groups, the NNT was notably smaller and more impactful in the unvaccinated group (NNTs of 35 vs. 60, respectively).
The second lesson is that the magnitude of a treatment’s effectiveness may change over time if the disease evolves. As Ambrose, et al. astutely comments, if severe disease and death decrease substantially between initial and later cases, treatments will have reduced effectiveness in preventing the same outcomes. For example, sotrovimab was associated with significant decreases in the odds of death within 30 days, but its NNT had increased to 666 by the omicron era.
Third, effective treatments are only effective if they can be readily administered to patients. Early in the pandemic, the outpatient infrastructure of US health care systems was not prepared or equipped to operationalize the rapid administration of intravenous infusions to highly contagious patients after diagnosis. Establishing processes to deliver mAb treatment was challenging, but the reward was great. Future investment in these therapies is even more important now that the infrastructure is in place to deliver them.
Fourth, mAb therapies highlighted the importance of rapid diagnostic and/or point of care testing. Patients with symptoms needed quick access to SARS-CoV-2 testing with rapid turnaround times. Because real-time variant sequencing was not available in the clinical setting, clinicians had to make challenging decisions about whether to continue providing mAbs for treatment based on forecasting per geographic region. With point of care precision testing, more treatments could have been administered for longer periods, which is particularly important during times of scarce resources.
While ethical allocation of scarce resources is challenging on many levels, it does bring into focus the fifth important lesson of mAb therapy: Using risk stratification strategies to optimize patient outcomes. These data from Ambrose, et al. further confirm that not all risk of COVID-19 progression is equal. Understanding this risk, ideally to the point of knowing patient specific baseline immunity, would facilitate precision medicine and would be the gold standard for deploying optimal, equitable, and value based care.
Ambrose and colleagues found that mAb therapy allowed us to consistently keep patients out of the hospital and alive. Acknowledging that mAb development and implementation seems like a constant race against the clock, scientists and manufacturers will need incentives to produce safe and effective therapies that are at risk of becoming obsolete. Authorizations for use of these therapies should focus on the patients most likely to benefit. Systematic efforts should continue to focus on both clinical and implementation science to capture clinical practice results as expeditiously as possible, which will allow us to effectively adapt to an ever changing landscape.
Effectiveness of monoclonal antibodies against COVID variants
The FDA has provisionally approved the following for the treatment and/or prevention of COVID-19.
Monoclonal antibodies that target the SARS-CoV-2 spike protein
• Casirivimab plus imdevimab (ronapreve).
• Regdanvimab (regkirona).
• Sotrovimab (xevudy).
• Tixagevimab and cilgavimab (evusheld).
Immune modulating monoclonal antibodies
• Tocilizumab (actemra).
Non monoclonal antibody antiviral agents used in the treatment of COVID-19
• Nirmatrelvir-ritonavir (paxlovid).
• Molnupiravir (lagevrio).
• Remdesivir (veklury).
Discussion
Monoclonal antibodies targeting the SARS-CoV-2 spike protein had shown clinical benefits against COVID-19 caused by variants predominant during the earlier stages of the pandemic.
These antibodies are designed to neutralise the virus by binding to the spike protein on its surface. However, emerging data show that anti-spike protein monoclonal antibodies demonstrate a significant decrease in their in vitro neutralising activities against many newer circulating SARS-CoV-2 variants, particularly Omicron and its sub variants. While there are few published clinical trials on the effectiveness of these monoclonal antibodies against clinical disease caused by these newer variants, it is expected that these mAbs will not provide clinical benefit in those people infected with the newer variants.
The activity of the monoclonal antibody tocilizumab is not reduced against variants as this antibody does not target the virus but acts as a modulator of the immune response. Non monoclonal antibody antiviral treatments such as nirmatrelvir/ritonavir (paxlovid), molnupiravir (lagevrio) and remdesivir (veklury), which have different mechanisms of action, are likely to retain their activity against the emerging strains.
In the word, the situation continues to evolve, with the epidemiology of circulating variants changing regularly. The characteristics of the circulating SARS-CoV-2 viruses should be considered when prescribing monoclonal antibodies for prevention or treatment of COVID-19. Healthcare professionals will need to consider alternative treatments as appropriate.
At this stage, the regulatory status of the products remain unchanged in the word. The FDA and its counterparts will continue to monitor the efficacy and safety of all COVID-19 medicines.
Potential updates to the Product Information for individual monoclonal antibodies will be published (if required) as they become available.
Conclusion
In the last clinical studies of outpatients with COVID-19, early treatment with different monoclonal antibodies used in accordance with prevailing authorizations and guidelines for specific SARS-CoV-2 variants was consistently associated with lower risk for hospitalization or death over nearly 2 years. The rapid evolution of new SARS-CoV-2 variants warrants timely, continuous evaluation of both mAb and non-mAb treatment approaches. Future investment in these therapies is even more important now that the infrastructure is in place to deliver them.
References
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med. 2021;385(23):e81.
[Crossref] [Google Scholar] [PubMed]
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237.
[Crossref] [Google Scholar] [PubMed]
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of Bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial. JAMA. 2021;325(7):632-644.
[Crossref] [Google Scholar] [PubMed]
- Abdelnabi R, Foo CS, Kaptein SJ, Zhang X, Do TN, Langendries L, et al. The combined treatment of molnupiravir and favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine. 2021;72:103595.
[Crossref] [Google Scholar] [PubMed]
- Gupta A, Gonzalez-Rojas Y, Juarez E, Casal MC, Moya J, Falci DR, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: A randomized clinical trial. JAMA. 2022;327:1236-1246.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Yisimayi A, Jian F, Song W, Xiao T, Wang L, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by omicron infection. Nature. 2022;608:593-602.
[Crossref] [Google Scholar] [PubMed]
- Yamasoba D, Kosugi Y, Kimura I, Fujita S, Uriu K, Ito J, et al. Neutralisation sensitivity of SARS-CoV-2 omicron sub variants to therapeutic monoclonal antibodies. Lancet Infect Dis. 2022;22(7):942-943.
[Crossref] [Google Scholar] [PubMed]
- United States Food Drug Administration. FDA updates sotrovimab emergency use authorization. FDA. 2022.
- World Health Organization. Therapeutics and COVID-19: Living guideline. 2022.
- Hasan Q, Elfakki E, Fahmy K, Mere O, Ghoniem A, Langar H, et al. Inequities in the deployment of COVID-19 vaccine in the WHO Eastern Mediterranean Region, 2020-2021. BMJ Glob Health. 2022;7(Suppl 4):e008139.
[Crossref] [Google Scholar] [PubMed]
- Skarbinski J, Wood MS, Chervo TC, Schapiro JM, Elkin EP, Valice E, et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread omicron (B. 1.1. 529) and delta (B. 1.617. 2) variant circulation in Northern California: A retrospective cohort study. Lancet Reg Health Am. 2022;12:100297.
[Crossref] [Google Scholar] [PubMed]
- John NA, John JE. Implications of COVID-19 infections in sickle cell disease. Pan Afr Med J. 2020;36:81.
[Crossref] [Google Scholar] [PubMed]
- Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316(23):2547-2548.
[Crossref] [Google Scholar] [PubMed]
- Langreth R, Muller M, Griffin R. Pfizer's grip on paxlovid thwarts research on COVID treatment. Bloomberg. 2022.
- Pecetta S, Finco O, Seubert A. Quantum leap of Monoclonal Antibody (mAb) discovery and development in the COVID-19 era. Semin Immunol. 2020;50:101427.
[Crossref] [Google Scholar] [PubMed]
- Ambrose N, Amin A, Anderson B, Barrera-Oro J, Bertagnolli M, Campion F, et al. Neutralizing monoclonal antibody use and COVID-19 infection outcomes. JAMA Netw Open. 2023;6(4):e239694.
[Crossref] [Google Scholar] [PubMed]
Citation: Weimer LE, Cattari G, Fanales-Belasio E, Cuccuru E, Vidili G (2023) Paradigms of a Prophylaxis and Early Treatment in New Variants of SARS-CoV-2. Clin Microbiol. 12:343.
Copyright: © 2023 Weimer LE, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
