Indexed In
- Open J Gate
- Genamics JournalSeek
- Smithers Rapra
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 9, Issue 1
Nitrogen Starvation of Assessment in the Production of Single Cell Oils and Biodiesel Quality in Heterotrophic Cultures of Cyanobacteria Phormidium autumnale
Erika Cristina Francisco1*, Eduardo Jacob-Lopes2, Karem Rodrigues Vieira2 and Telma Teixeira Franco32Food Science and Technology Department, Federal University of Santa Maria, Santa Maria, Brazil
3School of Chemical Engineering, University of Campinas, Campinas, Brazil
Received: 18-Jul-2019 Published: 08-Aug-2019, DOI: 10.35248/2090-4568.19.9.192
Abstract
The cyanobacteria have great potential for aquaculture industries, bioproducts, bioenergy and bioremediation, as in the reduction of ammonia, phosphorus and organic compounds. The manipulation of the medium and the modes of operation of the cultures are necessary to induce the production of biomass and bioproducts, such as lipids. The aim of the study was to evaluate the different nitrogen sources and their depletion in heterotrophic cultures of the cyanobacterium Phormidium autumnale aimed at obtaining high yields lipid. The heterotrophic cultures were carried out in bubble column bioreactor employing cassava starch as organic carbon source. In the first stage, were studied different nitrogen source under the C/N (carbon/nitrogen) ratios in the culture medium of 20, 40 and 60. In the second stage, selected to nitrogen source with the greatest potential for the production of lipids and held its depletion searching for the induction of a cellular stress. The fatty acids profile in the end of cultures was used in the estimation of biodiesel quality. In the first stage, the cultures employing sodium nitrate under CN ratio of 60 resulting in lipid content of 13.22% and lipid productivity of 7.62 mg/L.h. From the depletion of sodium nitrate, second stage, obtained a lipid productivity and lipid content of 10.43 mg/L.h and 25.07%, respectively. The fatty acid profile showed, in the presence of nitrogen, saturated fatty acid fraction and monounsaturated of 76.72% and 23.88%, respectively. The depletion of sodium nitrate induced a change in the lipid profile, directing the concentration of saturated fatty acids of 98.97%. The biodiesel properties obtained meet the standards set by standardization of the quality of biodiesel.
Keywords
Microalgae; Cyanobacteria; Heterotrophic; Cellular stress; Nitrogen; Lipids; Biofuel
Introduction
The cyanobacteria are widely known as producers of a variety of bio-compounds as pigments, are also sources of various products such as carotenoids, phycobiliproteins and bioactive compounds as anticancer, antibacterial, antifungal and immunosuppressive agents and lipids [1]. Because of potentially important compounds, many cyanobacterial strains are grown in the commercial sector. Most products are complex organic compounds with unique structures and cannot be synthesized in the laboratory. For this reason, cyanobacteria are known as "cell factories". Many researches focus on the study of the metabolism of cyanobacteria in order to produce high value-added bioproducts in large scale [2,3].
The intracellular lipids can be cited as an example of bioproducts widely studied in microalgal biotechnology. In general, the microalgal lipids can be employed in several areas, among them, production of biofuels. According to Francisco et al. [4], for sustainable production of bulk oil from microalgae becomes viable it is necessary employ organic substrates sources of low cost, as well as the development of induction technology to the accumulation of lipids in the cell.
Many factors are essential for the metabolism of cyanobacteria and direct routes to produce the desired final product. An example to be cited, is the production of biomass, which can be improved through certain feeding strategies, altering substrates and their concentrations, aiming at higher productivity rates [5].
Among such factors, the availability and source of nitrogen is one of the most important. Nitrogen is a constituent of structural and functional cellular proteins and under certain cultivation strategies, such as nitrogen limitation, cells may have high concentrations of lipids, such as in photosynthetic systems employing diatoms and chlorophytes [6]. However, one fact that needs to be highlighted is that unlike other microbial systems, microalgae have the capacity to grow in the absence of essential nutrients for their metabolism, such as phosphorus and nitrogen [7].
Several studies have been developed to evaluate the impact of nutritional stress on the increase of lipid content in microalgae cells, among them, nitrogen stress has been widely researched [8,9]. Different authors have shown that nitrogen deprivation can be considered as a "lipid trigger", resulting in an increase in lipid content in microalgae from 4.5% to 85.6% [10]. Nitrogen deprivation during microalgal metabolism leads to the induction of genes encoding enzymes involved in lipid metabolism. In contrast, the biosynthesis of proteins is reduced so that the cells are adapted to a lower availability of amino acids [11,12].
The economic factor is considered to be of extreme importance when we approach microalgal cultures in order to obtain bioproducts. In order to mitigate expenses with the operating systems used in the cultivation of large-scale microalgae, different cultivation systems can be used, such as autotrophic, mixotrophic and heterotrophic modes. Considering the metabolism of these organisms, although some species of cyanobacteria are obligatorily autotrophic and other heterotrophic facultative, many can be cultivated in total absence of luminosity, through equipment such as fermenters [13]. Large-scale heterotrophic cultivation is generally costadvantageous when compared to photosynthetic systems, yet the superior performance of biomass productivity is the driving force of these systems. Controlled growth under aseptic conditions in fermenters decreases the loss of nutrients and increases the quality of the product. The final cost of biomass productivity is the essential characteristic for the cropping systems and depends essentially on the conditions and medium of cultivation [14].
From these information, the objective was to study different sources of nitrogen and carbon/nitrogen (C/N) ratios, the strategy of nitrogen depletion in heterotrophic cultures of cyanobacteria Phormidium autumnale and evaluated the quality of biodiesel.
Materials and Methods
Microorganisms and culture media
Axenic cultures of Phormidium autumnale were originally isolated from the Cuatro Cienegas desert (26°59 ′ N, 102°03 ′ W-Mexico). Stock cultures were propagated and maintained in solidified agar-agar (20 g/L) containing synthetic BG11 medium [15] with the following composition (g.L-1): K2HPO4 (0.03 g.L-1), MgSO4 (0.075 g.L-1), CaCl2.2H2O (0.036 g.L-1), ammonium citrate and iron (0.0006 g.L-1), Na2EDTA (0.001 g.L-1), NaCl (0.00072 g.L-1), NaNO3 (0.015 g.L-1), citric acid (0.0006 g.L-1), Na2CO3 (1.5 g.L-1), trace metals [H3BO3 (0.0028 g.L-1), MnCl2.4H2O (0.0018 g.L-1), ZnSO4.7H2O (0.00022 g.L-1), Na2MoO4.2H2O (0.00039 g.L-1), CoSO4.6H2O (0.00004 g.L-1)]. The incubation conditions used were 25°C, a photon flux density of 15 μmolm-2s-1 and a photoperiod of 12 h. To obtain the inoculums in the liquid form, 1mL of sterile synthetic medium was transferred to slants, and the colonies were scraped and then homogenized with the aid of mixer tubes. The entire procedure was performed aseptically.
Bioreactor
Measurements were made in a bubble column bioreactor. The system was built of borosilicate glass and had an external diameter of 12.5 cm and height of 16 cm, resulting in a height/ diameter (h/D) ratio equal to 1.32 and a nominal working volume of 2.0 L. The dispersion system of the reactor consisted of a 2.5 cm diameter air diffuser located inside the bioreactor. The air flow was monitored by flow meter (KI-Key Instruments®, Trevose-PA, USA) and the inlet of air and outlet of gases were filtered through filtering units made up of polypropylene membrane with a pore diameter of 0.22 μm and total diameter of 50 mm (Millex FG®, Billerica-MA, USA). The bioreactor including filtering units was previously sterilized by autoclaving at 121°C for 40 minutes and then for 30 minutes containing the synthetic medium.
Obtaining kinetic data in an experimental bioreactor
Experiments were performed in a bioreactor operating under a batch regime, fed on 2.0 L of culture medium. The experimental conditions were as follows: initial concentration of inoculum of 100 mg/L, temperature of 26°C, pH adjusted to 7.6, aeration of 1 volume of air per volume of culture per minute and absence of light.
The culture medium consisted of modified BG11 synthetic medium supplemented with 14 g/L cassava starch as a source of organic carbon and different sources of nitrogen (ammonium molybdate, nitrate sodium, nitrite sodium, urea, yeast extract).
The C/N (carbon/nitrogen) ratios of 20, 40 and 60 were tested, which were adjusted from different concentrations of nitrogen sources, as Table 1.
| Source of nitrogen (g/L) | C/N 20 | C/N 40 | C/N 60 |
|---|---|---|---|
| Ammonium molybdate | 2.18 | 1.06 | 0.75 |
| Nitrate sodium | 2.18 | 1.06 | 0.75 |
| Nitrite sodium | 1.75 | 0.85 | 0.6 |
| Urea | 0.75 | 0.35 | 0.25 |
| Yeast extract | 2.18 | 1.06 | 0.75 |
Table 1: Different carbon/nitrogen (C/N) ratio and sources of nitrogen concentrations.
Nitrogen depletion
After selection of the nitrogen source with the highest potential for production of lipids, held experiments in order to evaluate the accumulation of intracellular lipids from the depletion of nitrogen. The depletion was performed by separating the biomass from BG11 culture medium supplemented with cassava starch at a C/N ratio of 60 (condition selected as propitious among those tested), after 120 hours (exponential phase). Then, the biomass was added to a new bioreactor containing the medium in the absence of nitrogen source.
Sampling and analytical methods
Samples were collected aseptically in a laminar flow hood previously sterilized. The tips used for sample collection were previously sterilized by autoclaving at 121°C for 20 minutes. The cellular concentration, the dynamics of pH and the consumption of organic carbon were monitored every 24 hours during the growth phase of microorganism. The experiments were performed in duplicate and kinetic data refer to the average of four repetitions.
The dynamics of pH was determined by potentiometer (Mettler- Toledo, São Paulo-SP, Brazil). The cell concentration was gravimetrically evaluated by filtering a known volume of culture medium through a 0.45μm membrane filter (Millex FG®, Billerica-MA, USA), drying at 60°C for 24 h. The organic carbon concentration was expressed in terms of chemical oxygen demand (COD) analyzed according to the closed reflux, colorimetric method of Standard Methods for the Examination of Water and Wastewater [16].
The lipid fraction was extracted from the biomass by the Bligh and Dyer method [17]. The method of Hartman and Lago [18] was used to saponify and esterify the dried lipid extract to obtain the fatty acid methyl esters (FAMEs). The fatty acid composition was determined using a VARIAN 3400CX gas chromatograph (Varian, Palo Alto-CA, USA). The FAMEs were identified by comparison of the retention times with those of the standard (Supelco, Louis-MO, USA) and quantified by area normalization.
Kinetics parameters
Biomass data were used to the maximum specific growth rate (ln(X/X0)=μmax.t), where X is the final cell concentration (mg/L), X0 is the initial cell concentration (mg/L), μmax is the maximum specific growth rate (d-1) and t is time (d), and to calculate the biomass productivity (PX=μ.X). The different organic carbon sources were used to calculate the substrate consumption rate (rS=dS/dt), where S is the organic carbon concentration (mg/L) and t is the time (d), the conversion efficiency (CE=S0-S/S0), where S0 is the initial organic carbon concentration (mg/L) and the substrate yield coefficient (YX/ S=dX/dS). The lipid content of the biomass was used to estimate the lipid productivity (PL=PX.L), where PX is the biomass productivity (mg/L.d) and L is the lipid content of the biomass (%).
Analysis of biodiesel properties
The biodiesel properties, degree of unsaturation, saponification value, iodine value, cetane number, long-chain saturated factor, cold filter plugging point, cloud point, allylic position equivalents, bis-allylic position equivalents, oxidation stability, higher heating value, kinematic viscosity and density, were estimated using Biodiesel© Ver. 1.1 [19,20].
Results and Discussion
Table 2 shows the kinetic parameters for different nitrogen sources. The yeast extract in C/N ratio of 40 resulted in the higher substrate consumption rate (108.65 mg/Lh) and maximum specific growth rate (0.068 h-1). The substrate yield coefficient, maximum cell concentration and biomass productivity were obtained with ammonium molybdate in C/N ratio of 20, being 2.41 mgcell/mgCOD, 9120 mg/L and 62.57 mg/Lh, respectively. For parameters that represent the lipid fraction, the lipid content (20:58%) and lipid productivity (7.62 mg/Lh), representative values obtained using sodium nitrate to a C/N ratio of 60. Some studies have reported the urea in the range of 0.8-1.7 g/L, as nitrogen source with the highest potential in obtaining lipids in microalgae cultivation under certain culture conditions, such as photosynthetic systems [21,22].
| Source of Nitrogen | C/N ratio | rS (mg/L.h) | YX/S (mgcell/mgCOD) | µmax (h-1) | Xmax (mg/L) | Lipid (%) | PX (mg/L.h) | PL (mg/L.h) |
|---|---|---|---|---|---|---|---|---|
| Yest extract | 20 | 108.22 | 0.37 | 0.019 | 4960 | 4.29 | 40.5 | 1.74 |
| 40 | 108.65 | 0.35 | 0.068 | 4660 | 15.86 | 47.5 | 7.53 | |
| 60 | 107.57 | 0.35 | 0.035 | 4740 | 4.37 | 38.66 | 1.69 | |
| Ammonium molybdate | 20 | 25.95 | 2.41 | 0.045 | 9120 | 1.32 | 62.57 | 0.82 |
| 40 | 80.63 | 0.66 | 0.027 | 5220 | 0.93 | 35.55 | 0.33 | |
| 60 | 48.37 | 0.51 | 0.023 | 3080 | 2.37 | 20.55 | 0.49 | |
| Sodium nitrate | 20 | 31.6 | 0.84 | 0.038 | 3980 | 7.95 | 26.66 | 2.12 |
| 40 | 33.14 | 0.65 | 0.016 | 3200 | 13.22 | 21.53 | 2.84 | |
| 60 | 30.82 | 1.2 | 0.029 | 6320 | 20.58 | 37.02 | 7.62 | |
| Sodium nitrite | 20 | 49.39 | 0.22 | 0.017 | 880 | 9.71 | 10.83 | 1.05 |
| 40 | 55.85 | 0.43 | 0.038 | 2400 | 14.66 | 23.85 | 3.49 | |
| 60 | 42.73 | 0.24 | 0.039 | 860 | 7.13 | 10.55 | 0.75 | |
| Urea | 20 | 50.37 | 0.92 | 0.024 | 5680 | 12.44 | 38.75 | 4.82 |
| 40 | 45.42 | 0.98 | 0.016 | 6520 | 5.75 | 44.58 | 2.56 | |
| 60 | 60.82 | 0.96 | 0.029 | 7140 | 6.03 | 48.88 | 2.95 | |
| rS: substrate consumption rate, YX/S: substrate yield coefficient, µmax: maximum specific growth rate, Xmax: maximum cell concentration, Px: productively in biomass, PL: lipid productivity. | ||||||||
Table 2: Kinetic parameters for different nitrogen source and C/N ratio.
From the lipid yield under different culture conditions, shown in Figure 1, it appears that the best source of nitrogen and C/N ratio for heterotrophic cultivation of cyanobacteria Phormidium autumnale employing cassava starch as organic carbon source, is sodium nitrate in C/N ratio of 60.
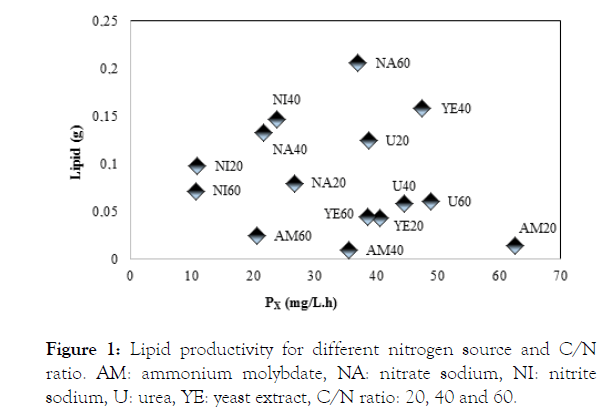
Figure 1: Lipid productivity for different nitrogen source and C/N ratio. AM: ammonium molybdate, NA: nitrate sodium, NI: nitrite sodium, U: urea, YE: yeast extract, C/N ratio: 20, 40 and 60.
Figure 2 shows the cell growth curve, cassava starch consumption and production of lipids for cultivation employing sodium nitrate to a C/N ratio of 60, bet condition for lipid productivity. It can be observed that cyanobacteria do not present a period of adaptation, demonstrating the feasibility of using the microorganism in heterotrophic cultures using starch as a source of exogenous organic carbon. It is still observed that the biomass enters in the stationary phase after 156 hours and lipid production in 216 hours, and there was a 11.85% increase in lipid content after depletion of sodium nitrate.
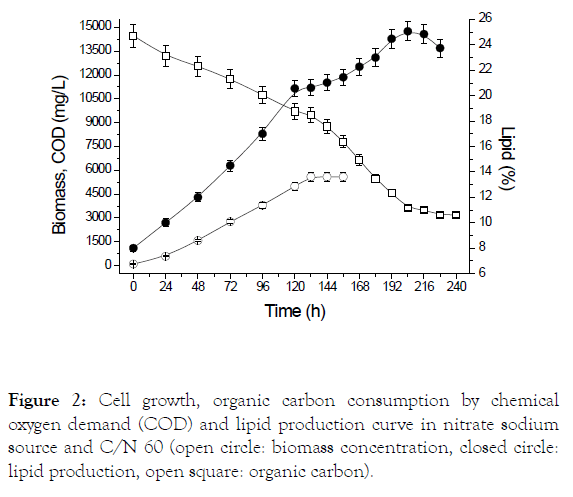
Figure 2: Cell growth, organic carbon consumption by chemical oxygen demand (COD) and lipid production curve in nitrate sodium source and C/N 60 (open circle: biomass concentration, closed circle: lipid production, open square: organic carbon).
For the nitrogen starvation strategy, the kinetics parameters were calculated in the end of the two phases, in the presence of From the analysis of them, there is an increased in the substrate consumption rate (46.66 mg/L.h), the maximum cell concentration (5590 mg/L), lipid content (24.07%) and lipid productivity (10.47 mg/L.h) performed after depletion of nitrogen from the medium. Hsieh and Wu [23] report that the induction of lipid accumulation can be performed from different conditions, such as the depletion of nitrogen, high concentrations of salts and stress induced by extreme temperatures and light intensities.
| Kinetic parameters | |||||||
|---|---|---|---|---|---|---|---|
| Phase | rS (mg/L.h) | YX/S (mgcell/mgCOD) | µmax (h-1) | Xmáx (mg/L) | Lipid (%) | PX (mg/L.h) | PL (mg/L.h) |
| A | 33.14 | 0.65 | 0.016 | 3200 | 13.22 | 21.53 | 2.84 |
| B | 46.66 | 0.46 | 0.0146 | 5590 | 25.07 | 41.59 | 10.43 |
| Phase A: nitrogen phase, phase B: nitrogen depletion phase. | |||||||
Table 3: Kinetic parameters for sodium nitrate depletion and biodiesel properties at C/N ratio of 60.
The profile of the fatty acids is shown in Table 4. A total of seven fatty acids were identified in the phases. In the phase A, the majority was acids caproic (65.9%) and oleic (23.88%) and in phase B, acids caproic (51.93%) and caprylic (43.62%).
| Fatty acid | 120 h | 144 h | 168 h | 192 h | 216 h | 240 h |
|---|---|---|---|---|---|---|
| Phase A | Phase B | |||||
| C6:0 (%) | 65.9 | 58.4 | 54.99 | 52.24 | 52.08 | 51.93 |
| C8:0 (%) | 5.43 | 37.49 | 40.35 | 42.39 | 43.41 | 43.62 |
| C10:0 (%) | ND | ND | ND | 4.46 | 4.32 | 3.42 |
| C14:0 (%) | ND | 4.1 | 3.85 | ND | ND | ND |
| C18:0 (%) | 5.39 | ND | ND | ND | ND | ND |
| C18:1n9c (%) | 23.88 | ND | ND | ND | ND | ND |
| C22:6n3 (%) | ND | ND | 0.16 | 0.8 | 0.88 | 1 |
| SFAs (%) | 76.72 | 99.99 | 99.19 | 99.81 | 99.09 | 98.97 |
| MUFAs (%) | 23.88 | ND | ND | ND | ND | ND |
| PUFAs (%) | ND | ND | 0.16 | 0.8 | 0.88 | 1 |
| Phase A: nitrogen phase, phase B: nitrogen depletion phase, ND: not detected, SFAs: saturated fatty acids, MUFAs: monounsaturated fatty acids, PUFAs: polyunsaturated fatty acids. | ||||||
Table 4: Fatty acid profile employing sodium nitrate in a C/N ratio of 60.
It is observed that the fraction of fatty changes from the depletion of sodium nitrate, from the predominance of saturated fatty acids (76.72%) and monounsaturated (23.88), to almost totalitarian composition of saturated fatty acids (98.97%) after depletion.
The biomass composition depends strongly of the cultures conditions, so the manipulation of conditions can be a strategy to obtain desired. An example of strategy may be the use of depletion of the nitrogen source, which besides being used to induce the lipid accumulation, is also used to direct the production of a saturated fatty acid fraction, as well as the production of intracellular starch [24-28]. The SFAs of a chain length from C10 to C18 induce an increase of properties as cetane number and decline of viscosity, and also lower emission of pollutants that make them eminent for biodiesel production. Lipids profiles with mixtures of SFAs and MUFAs also are recommended for use in biodiesel production, when compared to long-chain polyunsaturated fatty acids [29-32].
The properties of biodiesel, show in Table 5, were obtained for the two phases of cultures. Only the parameters oxidation stability and kinematic viscosity, for both phases, does not show conformity to national and international standardization [33-35]. The parameters that have inconsistency with international standards are directly correlated in the formation of organic acids, water, peroxides and polymerization products, responsible engine attack and, consequently, reduction in life span.
| Properties | Phase A (120 hours) | Phase B (240 hours) |
|---|---|---|
| DU | 23.88 | 2 |
| SV | 395.07 | 432.56 |
| IV | 21.47 | 4.64 |
| CN | 55.28 | 57.87 |
| LCSF | 2.7 | 0.00 |
| CFPP (°C) | -7.99 | -16.48 |
| CP (C°) | -4.99 | -4.99 |
| APE | 23.88 | 5.00 |
| BAPE | 0.00 | 5.00 |
| OS (hours) | 0.00 | 0.00 |
| HHV | 33.56 | 32.31 |
| U (mm2/s) | 0.00 | -0.35 |
| ρ (g/cm3) | 0.88 | 0.88 |
| DU: degree of unsaturation; SV: saponification value; IV: iodine value; CN: cetane number; LCSF: lon-chain saturated factor; CFPP: cold filter plugging point; CP: cloud point; APE: allylic position equivalents; BAPE: bis-allylic position equivalents; OS: oxidation stability; HHV: higher heating value; U: kinematic viscosity; ρ: density. | ||
Table 5: Fuel properties of biodiesel.
Non-conformities can be circumvented by the addition of antioxidants and additives (natural or synthetic) or by blending biodiesel with petro-diesel, which improves the quality properties of biofuel [36-39].
Conclusion
From the study of the sources of nitrogen used under different carbon/nitrogen ratios (C/N), it is concluded that sodium nitrate under C/N ratio of 60 showed better performances in the production of lipids in heterotrophic cultivation of cyanobacteria Phormidium autumnale in systems employing cassava starch as a source of organic carbon. The strategy of nitrogen source depletion demonstrates the feasibility of using strategies that employ the manipulation of the culture medium in the induction of higher yields lipid. The lipid obtained showed high prospect for the production of biodiesel, which currently shows a growth in the international market through its merger with regular diesel.
Acknowledgements
Funding for this research was provided by São Paulo Research Foundation (FAPESP, Brazil).
REFERENCES
- Burja AM, Banaigs B, Abou-Mansour E, Burgess JG, Wright PC. Marine cyanobacteriaa prolific source of natural products. Tetrahedron. 2001;57:9347-9377.
- Burja AM, Dhamwichukorn S, Wright PC. Cyanobacterial postgenomic research and systems biology. Trends Biotechnol. 2003;21:504-511.
- Hong SJ, Lee CG. Evaluation of central metabolismo based on a genomic database of Synechocystis PCC6803. Biotechnol Bioprocess Eng. 2007;12:165-173.
- Francisco EC, Franco TT, Zepka LQ, Jacob-Lopes E. From waste-to-energy: the process integration and intensification for bulk oil and biodiesel production by microalgae. J Environ Chem Eng. 2015;3:482-487.
- Alagesan S, Gaudana SB, Krishnakumar S, Wangikar PP. Model based optimization of high cell density cultivation of nitrogen-fixing cyanobacteria. Bioresour Technol. 2013;148:228-233.
- Liu W, Huang Z, Li P, Xia J, Chen B. Formation of triacylglycerol in Nitzschia closterium f. minutissima under nitrogen limitation and possible physiological and biochemical mechanisms. J Exp Mar Biol Ecol. 2012;418:24-29.
- Bernard O. Hurdles and challenges for modelling and control of microalgae for CO2 mitigation and biofuel production. J Process Control. 2011;21:1378-1389.
- Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains, Bioresour Technol. 2012;124:217-226.
- Damiani MC, Popovich CA, Constenla D, Martinez AM, Doria E. Leonardi, Triacylglycerol content, productivity and fatty acid profile in Scenedesmus acutus PVUW12. J Appl Phycol. 2014;26:1423-1430.
- Spoehr HA, Milner HW. The chemical composition of Chlorella; effect of environmental conditions. Plant Physiol. 1949;24:120-149.
- Ruangsomboon S, Ganmanee M, Choochote S. Effects of different nitrogen, phosphorus, and iron concentrations and salinity on lipid production in newly isolated strain of the tropical green microalga, Scenedesmus dimorphus KMITL. J Appl Phycol. 2013;25:867-874.
- Zhu S, Huang W, Xu J, Wang Z, Xu J. Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour Technol. 2014;152:292-298.
- Perez-Garcia O, Escalante FM, De-Bashan LE, Bashan Y. Heterotrophic cultures of microalgae: metabolism and potential products. Water Research. 2011;45:11-36.
- Borowitzka MA. Algal biotechnology products and processes matching science and economics. J Applied Phycol. 1992;4:267-279.
- Rippka R, Derueles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1-61.
- APHA, AWWA, WEF (American Public Health Association, American Water works Association, Water Environmental Federation). Standard Methods for the Examination of Water and Wastewater. Prot City Press, Baltimore, Maryland, 2005.
- Bligh EG, Dyer JW. A rapid method of total lipid extraction and purification. Can J Biochem Phisiol. 1959;37:911-917.
- Hartman L, Lago RCA. A rapid determination of fatty acid methyl esters from lipids. Laboratory Practice. 1976;22:475-476.
- Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, Jaramillo-Jacob ADR. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel. 2012;91:102-111.
- Talebi AF, Mohtashami SK, Tabatabaei M, Tohidfar M, Bagheri A, Zeinalabedini M, et al. Fatty acids profiling: A selective criterion for screening microalgae strains for biodiesel production. Algal Research. 2013;2:258-267.
- Shi XM, Zhang XW, Chen F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzym Microbiol Technol. 2000;27:312-318.
- Suali E, Sarbatly R. Conversion of microalgae to biofuel. Renew Sust Energy Rev. 2012;16:4316-4342.
- Hsieh CH, Wu WT. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol. 2009;100:3921-3926.
- Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol. 2009;100:833-838.
- Guschina IA, Harwood JL. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res. 2006;45:160-186.
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. The Plant Journal. 2008;54:621-639.
- Klok AJ, Verbaanderd JA, Lamers PP, Martens DE, Rinzema A. A model for customising biomass composition in continuous microalgae Production. Bioresour Technol. 2013;146:89-100.
- Rochetta I, Mazzuca M, Conforti V, Ruiz L, Balzaretti V. Effect of chromium on the fatty acid composition of two strains of Euglena gracilis. Environ Pollut. 2006;141:353-358.
- Francisco EC, Neves DB, Jacob-Lopes E, Franco TT. Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol. 2010;85:395-403.
- Knothe G. “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuel. 2008;22:1358-1364.
- Knothe G. Production and properties of biodiesel from algal oils. In: Borowitzka MA, Moheimani NR (eds.) Algae for biofuels and energy. Springer, Dordrecht. 2013:207-221.
- McCormick RL, Graboski MS, Alleman TL, Herring AM, Tyson KS. Impact of biodiesel source material and chemical structure on emissions of criteria pollutants from a heavy-duty engine. Environ Sci Technol. 2001;35:1742-1747.
- ANP. Brazilian Biodiesel Standard ANP (National Agency of Petroleum, Natural Gas and Biofuels). 2014.
- ASTM. Standard Specification for Biodiesel Fuel (B100) Blend Stock for Distillate Fuels. 2002.
- UNE-EN. Automotive Fuels, Fatty Acid Methyl Esters (FAME) for Diesel Engines, Requirements and Test Methods. 2003.
- Filho MGR. Cardanol e Eugenol Modificados – Uso Como Antioxidante no Controle do Processo Oxidativo do Biodiesel de Algodão. PhD Dissertation. Federal University of Paraiba. 2010.
- Litwinienko G, Kasprzsycka-Guttman T, Jamanek D. DSC. Study of Antioxidant Properties of Dihydroxyphenols. Thermochimica Acta. 1999;331:79-86.
- Hui YH. Handbook of Food Science, Technology and Engineering. CRC Press, Boca Raton, FL, USA. 2006.
- Chu JM, Xu XQ, Zhang YL. Production and properties of biodiesel produced from Amygdalus pedunculata Pall. Bioresource Technology. 2013;134:374-376.
Citation: Francisco EC, Jacob-Lopes E, Vieira KR, Franco TT (2019) Nitrogen Starvation of Assessment in the Production of Single Cell Oils and Biodiesel Quality in Heterotrophic Cultures of Cyanobacteria Phormidium autumnale. J Adv Chem Eng 9:192. doi: 10.35841/2090-4568.9.192
Copyright: 2019 Francisco EC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

