Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Hypothesis - (2021) Volume 11, Issue 9
Multivitamin (Thiamine) Responsive Basal Ganglia Disease Mimicking Acute Encephalitis Syndrome among Infants of Bodo Tribe Assam: A Retrospective Study
Jemin Webster*Received: 30-Jun-2021 Published: 30-Sep-2021, DOI: 10.35248/2161-0509.21.11.152
Abstract
Background: Bilateral symmetrical basal ganglia infarcts were observed among infants, who presented with features of Acute Encephalitis Syndrome (AES). Thiamine is successfully tried in the management of the neurological condition, with basal ganglia involvement. Here we are comparing the treatment and outcome differences between infants who received multivitamins (thiamine), with infants who received only supportive care.
Methodology: In this retrospective study, done from the hospital medical record of a secondary level hospital in Assam, Northeast India, between 2011-2015; 50 infants had bilateral basal ganglia infarcts, encompassing our study population. Depending on the exposure to multivitamins (thiamine), introduced in May 2014; 27 infants were grouped in the non-exposure group (Sep 2011-April 2014), and 23 infants were grouped in the exposure group (May 2014-Sep 2015).
Results: Common presenting symptoms included seizures (100%), lethargy (90%), fever (70%), and feeding difficulties (76%). In the exposure group 1 (3.7%) infant died and in the non-exposure group 20 infants (86.9%) died (relative risk, 0.04; 95% Confidence Interval [CI], 0.006 to 0.29; P=0.00013). The infants in the exposure group have 96% less risk of death, compared with the non-exposure group. Two infants in subsequent follow-up, in the exposure group, did not have any neurological sequelae.
Conclusions: Intravenous multivitamin (thiamine) supplementation may be associated with less risk of death in infants who had bilateral symmetrical basal ganglia infarct in the brain and presented with symptoms of Acute Encephalitis Syndrome (AES). The study suggests the possibility of subclinical thiamine deficiency, mitochondrial diseases, or SLC19A3 gene mutation in this population.
Keywords
Acute Encephalitis Syndrome (AES); Thiamine; Multivitamins; Mitochondrial diseases
Introduction
Acute Encephalitis Syndrome (AES) is a public health problem in India. It is characterized by acute onset of fever, change in mental status with new-onset seizures. Acute Encephalitis Syndrome has a very complex etiology with similar neurologic manifestations caused by several different viruses, bacteria, fungus, parasites, spirochetes, chemicals, or toxins. The case fatality of this disease is very high (25%) and most of those who survive have neurologic sequelae [1]. Recurrent epidemics of encephalitis of unknown etiology have occurred in the country. Between 2008 and 2014, there have been more than 44,000 cases and nearly 6000 deaths from encephalitis in India, particularly in Uttar Pradesh and Bihar [2]. In 2016, there has been a rise in encephalitis, with over 125 children reported to have died in one hospital in Gorakhpur alone [3]. While the Japanese Encephalitis Virus (JEV) is the leading diagnosed cause of acute encephalitis, in many cases, however, no etiological agent is determined, and such cases are categorized broadly as Acute Encephalitis Syndrome (AES).
We observed bilateral symmetrical basal ganglia infarct among infants who presented with acute encephalitis-like syndrome. They had mild prodromal symptoms of fever, inconsolable cry, generalized tonic-clonic seizure, and altered mental status. Etiological workup done for some of the infants included Japanese encephalitis, scrub, malaria, blood cultures were negative. They were managed conservatively with antiepileptics and antibiotics and were associated with high mortality. The other differentials considered were a hypoxic insult, Leigh's disease.
Thiamine is successfully tried in the management of the neurological condition, with basal ganglia involvement like infantile Leighlike SLC19A3 gene defect, THTR2 deficiency, Biotin thiamine responsive basal ganglia disease [4]. Thiamine serves as a cofactor for numerous enzymes, predominantly with mitochondrial localization. Moreover, the brain is extremely vulnerable to thiamine deficiency due to its dependence on mitochondrial ATP production, and even more so is basal ganglia as they are highly metabolically active and are rich in mitochondria [5,6].
In March 2014, intravenous multivitamin was added to our treatment protocol. In this study, we are comparing the treatment and outcome differences who received multivitamin; with infants who received only supportive care before the introduction of the multivitamin.
Methods
We retrospectively reviewed the hospital records of the infants with bilateral basal ganglia infarct, from a secondary level hospital in Tezpur, Assam, Northeast India.
There were 195 infants, who underwent CT scan brain imaging from the year 2011 to 2015, for various indications. Among them, 50 infants had features of bilateral basal ganglia infarcts comprising our study population. The infants are grouped depending on the exposure to multivitamins (thiamine). The infants before the introduction of the multivitamin injection (Sep 2011-April 2014) comprised the non-exposure group and the infants who received the multivitamin injection (May 2014-Sep 2015) comprised the exposure group. We collected the demographic, clinical, treatment, and outcome details of the infants. The clinical data we collected were presenting complaints and syndromic presentation. The treatment details included antibiotics, antiepileptic, multivitamins, and supportive treatment. We looked at mortality. Moribund leave against medical advice was considered dead.
Exposure:
In 2014, since thiamine was not separately available, an intravenous multivitamin injection with the highest thiamine concentration was added to the therapy. The constituent was Vit B-1(thiamine) 100 mg, Niacin 100 mg, Vit B-12 1000 mcg, Vit B-2(riboflavin) 5 mg, VitB-6 (pyridoxine)100 mg, d-panthen 50 mg/3 ml. It was given as a once-a-day infusion during the hospital stay. At discharge, multivitamin syrup was given, at a dose of 5 ml twice daily. Each 5 ml of the syrup contained Vit B-1 0.5 mg, Vit B-2 0.6 mg, Vit B-3 6.75 mg, Vit B-5 1.25 mg, Vit B-6 0.5 mg, Vit B-12 0.45 mg, Vit A 500IU, Vit D-3 100IU and Zinc 4.25 mg. The study was approved by the Emmanuel Hospital Association Institutional Ethics Committee (No. 233; 22-07-2020). The data were collected and analysed during August-December 2020.
Statistical methods:
Data are presented as mean and Standard Deviation (SD) for normally distributed variables and as frequency (percentage) for categorical variables. Characteristics of the study subjects were compared across the groups. The Pearson Chi-square test was used to find out the association between outcome (dead/alive) and vitamin supplement status. Firth logistic regression was used to assess risk association between vitamin supplement and binary outcome and estimates were presented as Odds Ratios (OR) and 95% Confidence Intervals (CI). All tests were two-sided at the α=0.05 level of significance. All statistical analysis was done using the SAS package (SAS® Institute Inc., USA, version 9.2 or higher) will be used for statistical evaluation.
Results
Among the 195 infants who underwent a CT scan during the study period between 2011 to 2015, for various indications. Bilateral basal ganglia infarct was noticed in 50 infants. Among the 50 infants, 23 were in the non-exposure group and 27 in the exposure group.
The baseline demographic characteristics of the study subjects were studied (Table 1).
| Parameters | Total (n=50) | Non-Intervention Group (n=23) | Intervention Group (n=27) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (months)a | 6.7 (2.7) | 6.8 (2.9) | 6.6 (2.8) | ||||||
| Sex | |||||||||
| Boys | 25 (50.0) | 13 (56.5) | 12 (44.4) | ||||||
| Girls | 25 (50.0) | 10 (43.5) | 15 (55.6) | ||||||
| Weight for Age (Z score below -2) | 17 (34.0) | 6 (26.1) | 11 (40.7) | ||||||
| BodoTribe | 46 (92.0) | 21 (91.3) | 25 (92.6) | ||||||
| Other Tribes | 4 (8.0) | 2 (8.7) | 2 (7.4) | ||||||
| Chief Complaints | |||||||||
| Fever | 36 (72.0) | 15 (65.2) | 21 (77.8) | ||||||
| Cough/Coryza/Breathing difficulty | 24 (48.0) | 8 (34.8) | 16 (59.3) | ||||||
| Loose stool/vomiting | 6 (12.0) | 3 (13.0) | 3 (11.1) | ||||||
| Neurological complaints | |||||||||
| Seizures | 50 (100.0) | 23 (100.0) | 27 (100.0) | ||||||
| Lethargy | 45 (90.0) | 20 (87.0) | 25 (92.6) | ||||||
| Feeding Difficulties | 38 (76.0) | 16 (69.6) | 22 (81.5) | ||||||
| Associated Illness | |||||||||
| Respiratory tract Infection | 22 (44.0) | 8 (34.8) | 14 (51.9) | ||||||
| Acute Gastro Enteritis | 6 (12.0) | 3 (13.0) | 3 (11.1) | ||||||
| Other Illness | 1 (2.0) | 1 (4.3) | 0 (0.0) | ||||||
| Treatment | |||||||||
| Antibiotics | 44 (88.0) | 21 (91.3) | 23 (85.2) | ||||||
| Antiviral | 2 (4.0) | 2 (8.7) | 0 (0.0) | ||||||
| Antipyretics | 34 (68.0) | 15 (65.2) | 19 (70.4) | ||||||
| Intubated | 8 (16.0) | 4(17.4) | 4 (14.8) | ||||||
| Seizure medication | |||||||||
| One anti-epileptic (Phenytoin) | 19 (38.0) | 6 (26.1) | 13 (48.1) | ||||||
| More than one antiepileptic | 31 (62.0) | 17 (73.9) | 14 (51.9) | ||||||
Table 1: Demographic and other characteristics of the study.
The mean age at presentation was 6 months. There were an equal number of male and female children in the study group. These infants were born to non-consanguineous parents. It was observed that most of the children belonged to a low socio-economic group (new landers) of an indigenous tribe of Assam (North-East India) called the “Bodo” tribe.
Common clinical presentations included generalized tonic-clonic seizure (100%), lethargy (90%), fever (72%), and feeding difficulties (76%). Associated complaints included respiratory difficulties (48%) and loose stools (12%). There was a preceding illness like fever, lower respiratory tract infection, or acute diarrheal disease in 38(76%) infants. There was no statistically significant difference between the two groups concerning epidemiology, clinical features.
Lab parameters of the infants in both groups are shown in Table 2. There was no significant difference between the two groups. CSF analysis was done for 8 infants which were unremarkable (6 in the non-exposure group and 2 in the exposure group) (Table 2). Serum lactate was done for only 4 infants in the exposure group and the mean was 1.95 mmol/L (Normal 0.7-2.1 mmol/L). CRP was done for 5 infants in the exposure group and the mean was found to be 3.24 mg/L (normal 0-10 mg/L).
| Variables | Non-exposure group n-23 | Exposure group n- 27 | |||||
|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 9.4 (0.9) | 9.3 (1.0) | |||||
| Platelets (lakh. Cell/ mm3) | 4.81 (1.47) | 5.35 (1.5) | |||||
| Total counts (cell/mm3) | 12,560 (6895) | 14,434 (7438) | |||||
| Polymorph (%) | 56 (17) | 58 (18) | |||||
| Lymphocytes (%) | 35 (16) | 32 (16) | |||||
| Sodium (mmol/L) | 131 (7.5) | 134 (7) | |||||
| Potassium (mmol/L) | 5 (0.6) | 5.1 (0.5) | |||||
| Creatinine (mg/dl) | 0.65 (0.15) | 0.53 (0.27) | |||||
| CSF n = (8/50) | n=6/23 | n=2/27 | |||||
| Total counts (cell/mm3) | 7 (1.5) | 4 (2.8) | |||||
| Neutrophils (%) | Nil | Nil | |||||
| Lymphocytes (%) | 100% | 100% | |||||
| Total protein (mg/dl) | 45.2 (21.3) | 38.4 (10) | |||||
| Glucose (mg/dl) | 82 (40) | 95 (30) | |||||
| Values are presented as Mean (SD), CSF- Cerebrospinal fluid. | |||||||
Table 2: Shows the laboratory parameters between the two groups.
All the infants did not get all the etiological workup done. Among the 50 infants, JEV-specific IgM in CSF by IgM-capture ELISA was done for 4 infants, Scrub Typhus IgM Rapid for 6 infants, Malaria Parasite Antigen Rapid for 20 infants, and Automated blood culture & sensitivity for 10 infants; all were negative.
The need for more than one antiepileptic medication was lesser in the exposure group 14/27 (51.85%) than the non-exposure group 17/23 (73.91%). All infants received antibiotics. Eight infants required intubation; out of that, 4 infants were in the exposure group and all of them survived, whereas, the 4 infants who were intubated from the non-exposure group died. Weight for age parameter was calculated to assess the nutritional status of the study population. It showed that 17(34%) infants had a Z score of less than 2 SD; 6 in the non-exposure group and 11 in the exposure group.
Moribund leave against medical advice was considered dead. There was 16 (n=27) moribund leave against medical advice infants, in the non-exposure group and 1(n=23) in the exposure group. In the exposure group 1 (3.7%) infant died and in the non-exposure group 20 infants (86.9%) died (relative risk, 0.04; 95% confidence interval [CI], 0.006 to 0.29; P=0.00013) (Table 3). The infants in the exposure group have 96% less risk of death, compared with the non-exposure group. Thus, bringing out the evidence so evident that multivitamin (thiamine supplementation) administration is associated with improved survival in these groups of infants.
| Variables | Dead n (%) | Alive n (%) | RR (95%CI) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure group | 1 (3.7) | 26 (96.3) | 0.04 (0.006-0.29) | 0.0013 | |||||||
| Non-Exposure group | 20 (86.9) | 3 (13.0) | --- | --- | |||||||
Table 3: Analysis of association between outcome and vitamin supplement.
There were subsequent OPD follow-up data available for 7 patients available in the exposure group and none in the non-exposure group (Table 4). Among them, two infants had normal development, and all others had neurological sequelae. Among the infants with neurological sequelae, two infants were able to walk with support.
| Infants | Age At Admission In Months | Age At Last Available OPD Follow-Up In Months | Follow-Up Details |
|---|---|---|---|
| A | 12 | 16 | Normal development |
| B | 7 | 15 | With Neurological Sequelae, can walk with support, was verbalizing mama, didi. |
| C | 2 | 4 | Normal development |
| D | 6 | 9 | Neurological sequelae present -Spastic Diplegia |
| E | 6 | 26 | Neurological sequelae present- Failure to thrive, tone increased in all limbs |
| F | 8 | 9 | Neurological sequelae present |
| G | 12 | 16 | Neurological sequelae present- was able to walk with support. |
Table 4: Follow up data the infants.
Discussion
Our data showed significantly less risk of death among the infants who received multivitamin (thiamine) supplementation. These infants presented clinically with features of acute encephalitis syndrome, whose etiological workup were negative for Japanese encephalitis, scrub typhus, malaria, bacteremia and the CSF finding was also unremarkable.
Most of the infants were appropriate for age, weight for age indices did not reflect the underlying malnutrition. We noticed the requirement of anti-epileptic to control seizures reduced in the exposure group.
Further, among the infants who had come for subsequent OPD follow-up in the exposure group, two infants had no neurological sequelae. Suggesting that with prompt identification and treatment the sequelae were preventable. The other 5 infants had neurological sequelae.
Figure 1 showed the CT brain of the infants showing bilateral symmetrical infarcts involving the caudate, putamen, globas pallidus, and medial thalamus. In biotin responsive basal ganglia disease, MRI brain features included the involvement of hypointensity of the striatum in T1-weighted images and hyper-intensity on T2-weighed images of the same region. These areas correspond to bilateral necrosis in the central part of the caudate heads and all or part of the putamen [7].
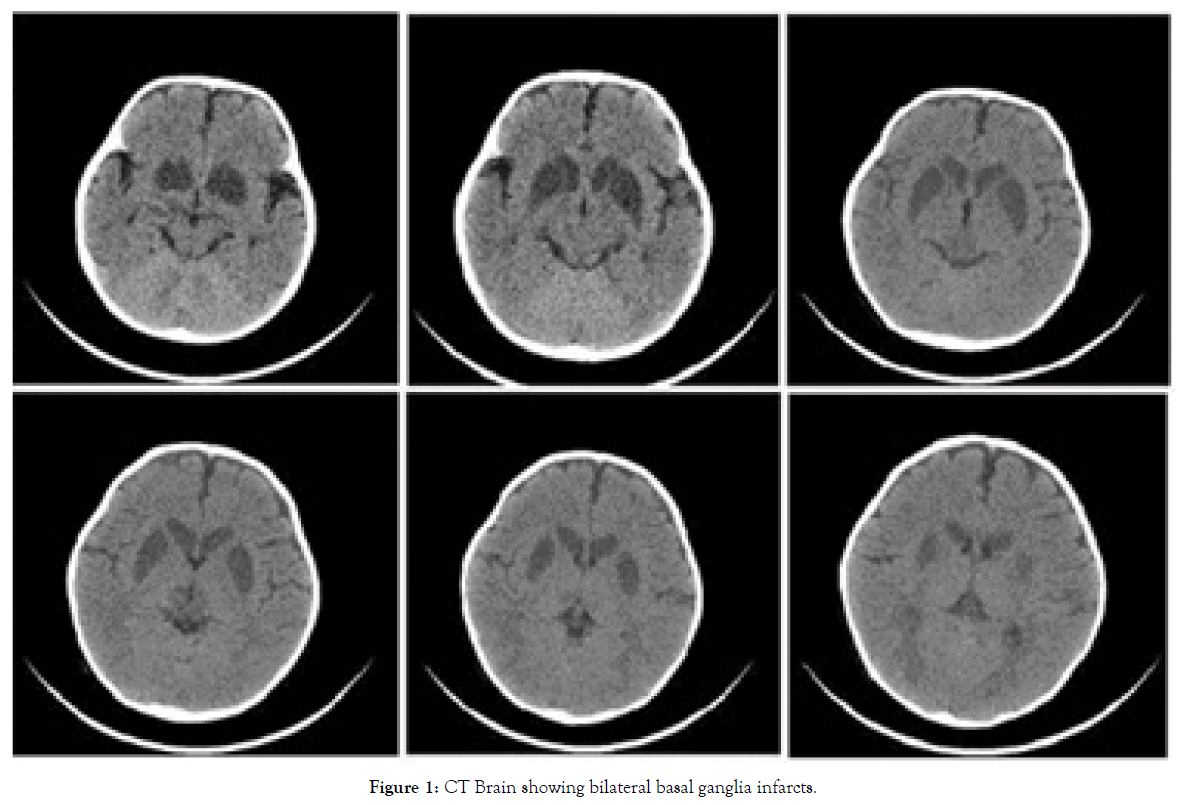
Figure 1: CT Brain showing bilateral basal ganglia infarcts.
Basal ganglia are prone to hypoxia, toxic poisoning, metabolic, mitochondrial diseases. Basal ganglia are highly metabolically active, and their metabolism is unique in several ways. They are rich in mitochondria and have a high oxygen consumption [8,9]. Thiamine plays an important role in oxidative phosphorylation and energy metabolism. Key enzymes in these metabolic pathways are mitochondrial pyruvate dehydrogenase, Alpha-Ketoglutarate Dehydrogenase (alpha KDH), and Cytosolic Transketolase.
KDH was identified as a rate-limiting enzyme of cerebral glucose utilization. A low enzyme activity caused by reduced intracellular Thiamine Diphosphate (TDP) concentration results in lactic acidosis, cerebral dysfunction, and neuronal cell degeneration [10].
Thiamine deficiency diminishes the thiamine-dependent enzyme activity, alters mitochondrial function, impairs oxidative metabolism, and causes selective neuronal death. This deficiency can be caused by inadequate consumption of thiamine, increased need, or impaired absorption [11].
SLC19A3 gene mutation is implicated in the deterioration of thiamine transport in neurons via thiamine transporter 2 (hTHTR2). Depending on the age of the patients, the SLC19A3 gene mutation has 4 different clinical pictures. Firstly, during neonatal period presents as Leigh syndrome-like phenotype is characterized by acute encephalopathy and lactic acidosis. Secondly, presents as a severe disease characterized by epileptic spasms, bilateral thalamic, and basal ganglia lesion, during the early infancy period. Thirdly, as Biotin- thiamine responsive basal ganglia disease during childhood. Finally, Wernicke’s encephalopathy-like condition in the second decade of life. Biotin and thiamine have been found successful in the management of BRBGD [4].
Most of the infants (n=46/50-92%) presented were from the same tribe called the Bodo tribe in Assam. Whether there is an underlying genetic cause or an environmental or a dietary deficiency needed to be investigated. The Bodo community daily diet consisted of polished rice, taken three times a day with lentil soup, tubers and meat. The rice is taken along with boiled vegetables and leaves. They are known to consume a lot of tea as most of these tribal people live among tea gardens. Heat-stable thiamine antagonists, including polyphenol, occur in several plants; ferns, tea, coffee, betel nut, red beets, red cabbage [12]. They react with thiamine to yield the non-absorbable thiamine disulfide. Thiamine deficiency in Thailand was reported to be related to tea drinking and chewing of fermented tea leaves; tannins being the major component having an anti-thiamine activity [13]. Maternal thiamine deficiency during pregnancy often leads to infantile beriberi in such communities [14].
Infantile beriberi, if not treated in time, may almost always result in death, and is thought to account for a large proportion of the high infant mortality rates found in the Philippines, Burma, Cambodia, Laos, Vietnam, and probably also in other rice-eating countries [13]. Infantile cardiac beriberi responding to thiamine is also reported in northeast rural India. Where 23 (92%) infants presenting with acute cardiac failure recovered with thiamine administration [15].
There are case reports on Vitamin B12 deficiency causing bilateral globus palidus abnormalities in adults. Methylmalonic acid's effect on succinate-supported mitochondrial oxygen consumption is the proposed mechanism for the degeneration. In the myriads of presentation of vitamin b12 deficiency, the basal ganglia involvement is one of the rarest associations [16]. Among infants, Infantile tremor syndrome is associated with Vitamin B12 deficiency or mutations affecting the metabolic pathway of the vitamin b12- utilization/binding of the vitamin cofactor. Furthermore, cortical atrophy and prominence of ventricular involvement and subarachnoid space are the frequently involved radiological involvement [17].
With many etiological hypotheses that are upcoming for AES, like lychee-associated hypoglycemic syndrome in undernourished children, etiologies other than infection need to be looked up [18,19].
Our study, therefore, raises an important question regarding the status of thiamine deficiency in India. Patients reaching our hospital from the tribal region, traveling long distances may represent just the tip of an iceberg, with more undiagnosed unrecognized disease prevalent in this group of people. There is a need for a large community-based study to identify thiamine deficiency, mitochondrial diseases, or SLC19A3 gene mutation. And multivitamin (Thiamine) supplementation should be part of the management protocol in infants presenting with similar presentations.
Limitation of this study:
This survey had several limitations, including the use of retrospective hospital data. The confounding factor associated with the administration of thiamine as an intravenous multivitamin injection along with other vitamins (Vitamin B2, B3, B7 & B12) as separate intravenous thiamine was not available. Lack of facilities for testing thiamine levels, MRI scan, and genetic testing.
Conclusion
This study highlights the possibility of subclinical thiamine deficiency, mitochondrial diseases, or SLC19A3 gene mutation in these regions. Our study shows the importance of thiamine supplementation in children with seizures and basal ganglia infarction. That multivitamin (thiamine) supplementation may be associated with less risk of death, compared with the infants who did not receive the supplementation. The neurological sequelae are preventable with early identification and prompt administration of multivitamins (thiamine), along with good seizure control and appropriate supportive management.
REFERENCES
- Jaiswal RK, Dhariwal AC, Sen PK, Lal S, Raina VK. National Programme for Prevention and Control of Japanese Encephalitis (JE)/Acute Encephalitis Syndrome (AES)-An update. J Commun Dis. 2014;46(1):119-127.
- Narain JP, Dhariwal AC, MacIntyre CR. Acute encephalitis in India: An unfolding tragedy. Indian J Med Res. 2017;145(5):584.
- Make dengue, malaria notifiable diseases: Harsh Vardhan to Delhi govt | Delhi News - Times of India.2019 October 28.
- Değerliyurt A, Gündüz M, Ceylaner S, Ünal Ö, Ünal S, Training HO. Neonatal form of biotin-thiamine-responsive basal ganglia disease. Clues to diagnosis. Turk J Pediatr Dis. 2019;61(2):261-266.
- Dhir S, Tarasenko M, Napoli E, Giulivi C. Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front Psychiatry. 2019;10:207.
- Davidson’s Principles and Practice of Medicine. Elsevier Health Sci UK. 2010.
- Ozand PT, Gascon GG, Al Essa M, Joshi S, Al Jishi E, Bakheet S, et al. Biotin-responsive basal ganglia disease: A novel entity. Brain: J Neurol. 1998;121(7):1267-1279.
- Ganong WF. Corticospinal & corticobulbar system: Basal ganglia. Rev Med Physiol. Mcgraw-hill. 2005.
- Colledge N. Molecular &genetic factors in disease: Functional anatomy & physiology; mitochondria and energy production. Davidson’s Princ Pract Med, Edinburgh; New York: Churchill Livingstone/Elsevier. 2010;44.
- Bitsch R. Thiamine Physiology. In B Caballero, L Trugo PF, editor Encycl Food Sci Nutr 3rd ed, London: Academic press. 2014;5772–5280.
- Bravatà V, Minafra L, Callari G, Gelfi C, Grimaldi LM. Analysis of thiamine transporter genes in sporadic beriberi. Nutrition. 2014;30(4):485-488.
- Hilker DM, Somogyi JC. Antithiamins of plant origin: Their chemical nature and mode of action. Ann NY Acad Sci.1982;378(1):137-145.
- World Health Organization. Thiamine deficiency and its prevention and control in major emergencies. WHO.1999.
- Mother and child nutrition in the Tropics and Subtropics. P;317–321.
- Thankaraj S, Koshy RM, Ismavel V. Infantile cardiac beriberi in rural North East India. Indian Pediatr. 2020;57(9):859-860.
- Sharrief AZ, Raffel J, Zee DS. Vitamin B12 deficiency with bilateral globus pallidus abnormalities. Arch Neurol. 2012;69(6):769-772.
- Holla RG, Prasad AN. Infantile tremor syndrome. Med J Armed Forces India. 2010;66(2):186.
- Dhariwal AC, Venkatesh S, Chauhan LS, Kumar A, Shrivastava A, Bhushan G, et al. Lychee-associated acute hypoglycaemic encephalopathy outbreaks in Muzaffarpur, India–Author's reply. Lancet Glob Health. 2017;5(9):861-862.
- John TJ, Das M. Acute encephalitis syndrome in children in Muzaffarpur: Hypothesis of toxic origin. Curr Sci. 2014;106(9):1184-1185.
Citation: Webster J (2021) Multivitamin (Thiamine) Responsive Basal ganglia Disease Mimicking Acute Encephalitis Syndrome Among Infants of Bodo Tribe Assam: A Retrospective Study. J Nutr Disorders Ther. J Nutr Disorders Ther, J Nutr Disorders Ther.vol 11: 152.
Copyright: © 2021 Webster J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.