Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 7
Kinetics and Thermodynamic Properties of Drying Winter Melon Seeds (Benincasa hispida)
Gabriel Henrique Horta de Oliveira1*, Ana Paula Lelis Rodrigues de Oliveira1, Leandro Fagundes Mançano2, Meire Sanne Aparecida Andrade2, Mônica Aparecida De Campos2 and Débora Cristina Tavares Campos e Silva22Department of Food Science and Technology, Federal Institute of Education, Science and Technology of Minas Gerais, Manhuaçu, Brazil
Received: 10-Jul-2023, Manuscript No. JFPT-23-22056; Editor assigned: 13-Jul-2023, Pre QC No. JFPT-23-22056 (PQ); Reviewed: 26-Jul-2023, QC No. JFPT-23-22056; Revised: 02-Aug-2023, Manuscript No. JFPT-23-22056 (R); Published: 10-Aug-2023, DOI: 10.35248/2157-7110.23.14.1031
Abstract
Seeds of winter melon can be used in the production of by-products or consumed in natura. To do so, it requires correct handling and processing, which drying process is inevitable. Thus, this work aimed to evaluate and model drying process of winter melon seeds and determine thermodynamic properties in three different temperatures (40°C, 50°C and 60°C). Winter melon seeds were acquired with initial water content of 1.04 d.b. and submitted to drying until constant weight. Experimental data were fitted to six mathematical models usually used to represent drying of agricultural products. Two Term Exponential presented the best fit to predict the drying curves of winter melon, except for the 40°C temperature. At this temperature, no model was able to predict water content. Thermodynamic properties presented a high correlation with drying temperature, in which higher temperature resulted in lower values of entropy and enthalpy values. On the other hand, higher temperatures increased values of Gibbs free energy. Activation energy of drying process was 53.9 kJ mol-1.
Keywords
Mathematical modeling; Activation energy; Enthalpy; Entropy
Introduction
The growing process of revision of environmental values of the population has been occupying a privileged place in all human activities due to the emergence of the ecological crisis and the recent environmental paradigms. While society becomes more aware of environmental issues, it is essential to assume new behaviors in relation to this green theme [1,2].
Global competitiveness and population growth at higher rates than food production require increases in production and productivity in agricultural areas, but there is no point in producing more if post-harvest losses reach undesirable levels. According to the UN (2015), some world problems would reduce if there were no food losses and waste [3].
Deivanayagame et al. (2020) evaluated the osmotic dehydration of Benincasa hispida in saline solutions, at 40°C, 50°C and 60°C [4]. Mondal et al. (2019) studied the effect of drying in a greenhouse and with intermittent drying air on the nutritional parameters of some vegetables from India, including, Benincasa hispida [5]. No studies were found on the drying of seeds of Benincasa hispida, which can be used in the production of by-products or in natura, often being discarded during the processing steps.
According to Vieira et al. (2019), discarded portions of fruits and vegetables, such as seeds and peel, have been used for nutritional purposes in the human diet and to reduce food losses [6]. Thus, vegetable seeds become alternative sources of protein and other nutrients for nutrition, such as melon seeds [7]. These seeds are rich in lipids, proteins, amino acids, fibers and antioxidant compounds; however, they are usually discarded [8,9].
The commercialization of melon seeds requires prior and careful studies on its storage, drying process for preservation and quality control of the components of interest [10]. It is noticed that the drying process is extremely important for maintaining the conservation of melon seeds, and in the elaboration of new products [11]. Still, understanding the drying process and the movement of molecules inside the product, through thermodynamic properties, is also essential. Therefore, the present work was developed with the objective of statistically adjusting and modeling the mathematical models for the drying kinetics of winter melon, as well as determining the thermodynamic properties of this process.
Materials and Methods
This study was conducted at the Federal Institute of Education, Science and Technology of Southeastern Minas Gerais, Campus Manhuaçu.
Raw material
The winter melons (Benincasa hispida) were obtained in the Zona da Mata region of Minas Gerais, harvested at the stage of green maturation. After transporting the melons, the seeds were removed and the initial water content was measured (1.04 ± 0.05 d.b.), according to the greenhouse nb b method, in triplicate [12].
Drying process
The product was dried in an oven with forced air circulation (new ethics, model 400/5ND), at temperatures of 40°C, 50°C and 60°C. During the drying process, the trays with the samples were weighed periodically using a digital scale (Shimadzu, model BL3200H) of 0.01 g resolution. Drying was completed when the mass of the trays with the seeds did not vary more than 0.01 g in three consecutive weighing’s.
Mathematical modeling
To determine the Moisture Ratios (MR) of winter melon seeds during drying, under different air temperature conditions, Equation 1 was used:

Where, MR is the moisture ratio, dimensionless; Ut* is the water content of the product at t time, decimal dry basis; Ue* is the equilibrium water content of the product, decimal dry basis; Ui* is the initial water content of the product, decimal dry basis. The drying rate during drying of winter melon was calculated by Equation 2:

Where: DR is Drying Rate, kgH2O kgms-1 h-1; Ut+dt * is water content at t+dt time, % d.b.; t is time, h.
Different models proposed in the literature were used to predict the drying kinetics of winter melon seeds: Page, Logarithm, Modified Midilli, Diffusion Approximation, Two-term Exponential and Henderson and Pabis (Table 1).
| Model name | Equation | Reference |
|---|---|---|
| Page | MR=exp(-ktn) | Page (1949) |
| Logarithm | MR=a exp(-kt)+c | Chandra and Singh (1995) |
| Modified Midilli | MR=exp(-ktn)+bt | Midilli et al. (2002) |
| Diffusion Approximation | MR=a exp(-kt)+(1-a)exp(-kbt) | Kassem (1998) |
| Two-term Exponential | MR=a exp(-kt)+(1-a)exp(-kat) | Sharaf-Eldeen, Blaisdell e Hamdy (1980) |
| Handerson e Pabis | MR=a exp(-kt) | Henderson and Pabis (1961) |
| Note: Where: a, b, c, n are coefficients of the models, dimensionless; k is the drying constant, h-1; and t is the time, h. | ||
Table 1: Mathematical models used to predict the drying phenomenon.
For the adjustment of the mathematical models, nonlinear regression analysis was performed by the Gauss Newton method, using the STATISTICA 14.0 software®. The choice of the best model was based on the coefficient of determination (R2), Standard Deviation of the Estimate (SDE), in the Relative Mean Error (MRE) and the calculation of the last two was performed using the following equations [13].
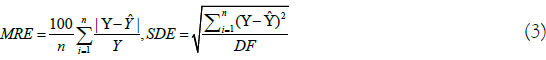
Where: P=relative mean error, %; n=number of observed data; Y=observed value, % d.b.; Ŷ=value estimated by the model, % d.b.; SDE=Standard Deviation of the Estimate, % d.b; and, DF=Degrees of Freedom of the model.
Effective diffusion coefficient
To calculate the effective diffusion coefficient, Fick's second law (Equation 4) was used, with an approximation of eight terms, considering the seeds as oblate spheroids, considering the moisture boundary condition on the surface of winter melon seeds and disregarding volumetric contraction during drying [14].
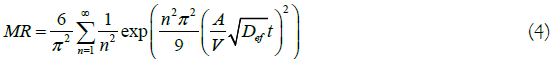
Where, Def=Effective Diffusion Coefficient, m2 s-1; A=surface area of the product, m2; V=volume of the product, m3; n=number of terms.
To calculate the volume (Equation 5) and area (equation 6) of winter melon seeds, measurements of length (a), width (b) and thickness (c) were performed using a digital caliper (Vonder, model PDV1500) with an accuracy of 0.01 mm, in 10 seeds dried at each temperature.
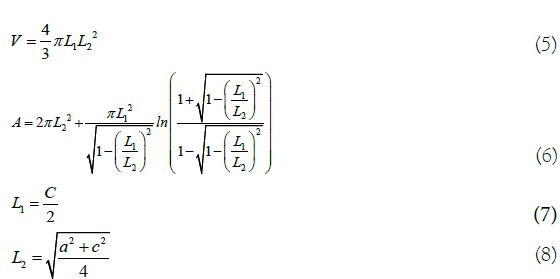
Where, L1 and L2=dimensions of oblate spheroid, m.
Thermodynamic properties
The activation energy is defined as the energy that must be exceeded or the minimum energy required initiating a chemical reaction. Thus, the activation energy of the drying kinetics of winter melon seeds was calculated. The Arrhenius equation (Equation 9) was used, in which it reports the relationship between the activation energy and the speed at which the reaction occurs.

Where: A0 is the pre-exponential factor, h-1; Ea is the activation energy, J mol-1; R is the universal gas constant, 8.314 J mol-1 K-1, and; T is the temperature, K.
The thermodynamic properties of the drying process of winter melon seeds were obtained using the method described by Jideani and Mpotokwana (2009) [15]:

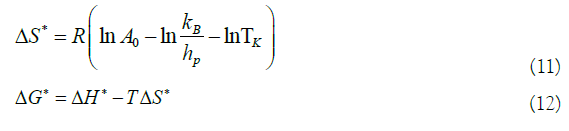
Where ΔH*: enthalpy, J mol-1; ΔS*: entropy, J mol-1; ΔG*: Gibbs free energy, J mol-1; kB: Boltzmann constant, 1.38 × 10-23 J K-1; hP: Planck constant, 6.626 × 10-34 J s-1.
Results and Discussion
Drying rate increased with temperature increment (Figure 1). Furthermore, at the beginning of drying, the drying rate is higher, mostly at temperatures of 50°C and 60°C. Corrêa Filho et al. (2015) also concluded this same trend, working with drying kinetics of figs [16].
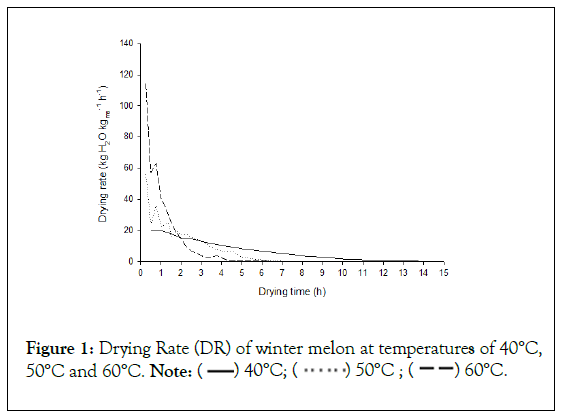
Figure 1: Drying Rate (DR) of winter melon at temperatures of 40°C,
50°C and 60°C. Note: (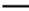 ) 40°C; (
) 40°C; (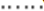 ) 50°C ; (
) 50°C ; (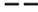 ) 60°C.
) 60°C.
It was not verified a period of constant drying rate at temperatures of 50°C and 60°C. This trend is explained by periods of lower velocities of drying, in which water molecules migration from the center to the outliers of winter melons occurs mainly by diffusion mechanism [17]. On the other hand, it was observed, at the temperature of 40°C, a slightly period of constant drying rate. This can be explained because of the lower temperature, in which requires more time to dry the product, thus providing higher time to the migration of water molecules throughout diffusion to occur.
Mathematical modeling of drying kinetics
Table 2 shows the parameters of the Page exponential, logarithm, modified Midilli, diffusion approximation, two-term exponential and Henderson and Pabis models obtained after adjustment to the experimental data.
| Model | Temperature | Coefficients | R² | MRE (%) | SDE (× 10-5 % d.b.) | |||
|---|---|---|---|---|---|---|---|---|
| a | k | b | c | |||||
| Two-Term Exponential | 40˚C | 1.6638 | 0.3548 | - | - | 0.999 | 17.88 | 4.42 |
| 50˚C | 1.4172 | 0.5778 | - | - | 0.9973 | 4.23 | 9.19 | |
| 60˚C | 1.2863 | 1.2344 | - | - | 0.9988 | 3.37 | 5.43 | |
| Diffusion Approximation | 40˚C | 1 | 0.2723 | 1 | - | 0.9933 | 45.92 | 30.97 |
| 50˚C | 1 | 0.5064 | 1 | - | 0.9967 | 6.59 | 11.2 | |
| 60˚C | 1 | 1.1552 | 1 | - | 0.9987 | 4.11 | 5.7 | |
| Logarithm | 40˚C | 1.0765 | 0.244 | -0.0597 | - | 0.9988 | 24.99 | 5.51 |
| 50˚C | 1.0328 | 0.4453 | -0.0529 | - | 0.9984 | 4.38 | 6.34 | |
| 60˚C | 1.0013 | 1.1219 | -0.0087 | - | 0.9989 | 6.1 | 4.99 | |
| Modified Midilli | 40˚C | - | 0.2248 | -0.0019 | 1.1093 | 0.9995 | 13.13 | 2.2 |
| 50°C | - | 0.4874 | -0.0098 | 0.9483 | 0.9981 | 4.2 | 7.25 | |
| 60˚C | - | 1.1417 | -0.0025782 | 0.9882 | 0.9989 | 7.32 | 5.27 | |
| Page | 40˚C | - | 0.2192 | - | 1.1507 | 0.9989 | 17.02 | 4.87 |
| 50˚C | - | 0.4969 | - | 1.0252 | 0.9969 | 6.14 | 12.25 | |
| 60˚C | - | 1.155 | - | 1.0039 | 0.9988 | 3.89 | 5.68 | |
| Henderson and Pabis | 40˚C | 1.0371 | 0.2826 | - | - | 0.995 | 39.98 | 23.3 |
| 50˚C | 0.9939 | 0.503 | - | - | 0.9968 | 7.96 | 12.83 | |
| 60˚C | 0.9957 | 1.1502 | - | - | 0.9988 | 3.39 | 55.9 | |
Table 2: Parameters adjusted to empirical models at temperatures of 40°C, 50°C and 60°C.
According to Chicco, Warrens and Jurman (2021) and Souza et al. (2019), although the coefficient of determination (R²) is a good parameter for the selection of models that represent the experimental data, this alone is not presented as a good criterion, and the values of the Standard Deviation of the Estimate (SDE) and Mean Relative Error (MRE) are also used [13,18]. The observed values of MRE demonstrate the deviation of the experimental results in relation to the data estimated by the model, and it is recommended that its result be less than 10% in the selection of models [13].
Given the above, according to Table 2, it was observed that all the models studied presented good adjustments to the experimental data, with R² values greater than 0.99, however, at 40°C, no model obtained good MRE results, according to Souza et al. (2019) criterion, being this temperature disregarded for the continuation of the discussion in the present study. This situation can be explained since, with the lower temperature, the period of decreasing drying rate may have extended to the drying time, making the behavior of moisture loss linear and, thus, hindering modeling, which represents an exponential behavior [13,19]. This trend was observed in Figure 1.
With this, the Two Term Exponential model was chosen to represent the experimental data, since it was the model that presented MRE values, for temperatures of 50°C and 60°C, lower compared to the other models, as well as lower standard deviation values of the estimate (SE).
According to Table 2, for the Two Term Exponential model, it is possible to observe that the drying coefficient “k” increased with the increase in temperature, while the constant “a” reduced as a function of the drying temperature. As "k" is the constant of the drying rate, the result obtained in this experiment is consistent with what is expected for this parameter, since, with the increase in temperature, the rate of water loss from the product to the environment increases.
Leite et al. (2019), when adjusting the experimental data of jackfruit seed drying, found as the best model the exponential of two terms with excellent results of R² and mean square deviation [10]. While Costa et al. (2021), in their studies of drying the seeds of Amaranthus cruentus 'BRS Alegria', also noticed that the parameter "k" increased with the increase in temperature and "a" showed a downward trend as a function of the increase in drying temperature [20]. Such results from these literatures confirm the data obtained in this current study.
Figure 2 shows the drying curves of the samples at temperatures of 50°C and 60°C, adjusted by the Two Term Exponential model as a function of drying time. As shown in Figure 2, for all temperatures, a rapid reduction in product moisture is observed in the early stages of drying, and when the drying time approaches equilibrium moisture, the speed decreases sharply. In general, during drying, the speed of moisture loss is higher during the first stages, since the sample has high moisture contents (presence of free water), and, over time, there is a drastic drop in the speed of water removal, since the still existing water is strongly linked to the constituents of the food [19].
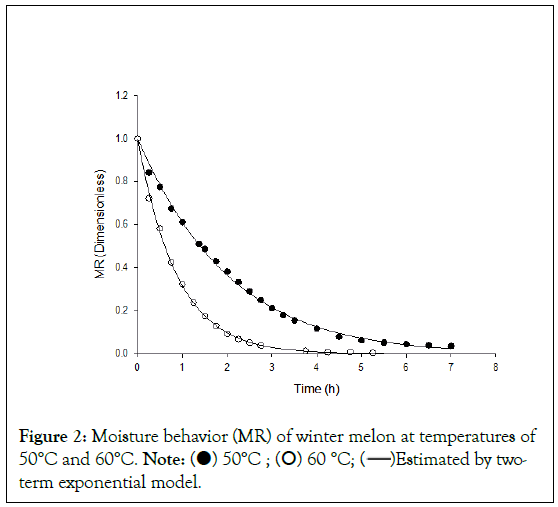
Figure 2: Moisture behavior (MR) of winter melon at temperatures of
50°C and 60°C. Note: ( ) 50°C ; (
) 50°C ; ( ) 60 °C; (
) 60 °C; ( )Estimated by two-
term exponential model.
)Estimated by two-
term exponential model.
This trend in humidity behavior was also observed by Santana et al. (2022) when evaluating the effects of separation methods on the drying kinetics of pitaya seeds; by Amini, Salehi and Rasouli (2021) when studying the drying kinetics of basil seeds; and by Keneni, Hvoslef-Eide and Marchetti (2019) when performing the mathematical modeling of pitaya seeds [19,21,22].
In addition, it was also observed that the drying time decreased when increasing the temperature from 50°C to 60°C, since the water pumpkin seeds reached the equilibrium value more quickly at the temperature of 60°C (approximately 4 hours), while at the temperature of 50°C this time was greater (approximately 6 hours). This behavior proves that the drying temperature exerts a great influence on the drying speed of the studied material, making the drying time shorter with increasing temperature.
Given the above, the increase in temperature creates, on the product to be dehydrated, a greater temperature differential between the food and the drying air, as well as a reduction in relative humidity. This type of condition accelerates heat transfer and, consequently, increases the rate of water loss from the product, since it increases the difference between the vapor pressure of the drying air and that of the seed samples [23].
Effective diffusion coefficient
Regarding the values of the effective diffusion coefficient, there is a direct relationship with the drying temperature, with values of 1.114 × 10-10 m²s-1; 1.472 × 10-10 m²s-1; 3.093 × 10-10 m²s-1 for temperatures of 40°C, 50°C and 60°C, respectively. With the increase in temperature, there is an increase in the effective diffusion coefficient, which was expected, since higher temperatures lead to a decrease in water viscosity, facilitating its removal from the seeds [24]. Jittanit (2011), working with pumpkin seeds (Cucurbita spp.), found values of 3.76 × 10-10 to 5.09 × 10-10 m²s-1 for temperatures 60°C, 70°C and 80°C. Uddin et al. (2016) reported values between 8.2 and 33.2 × 10-9 m²s-1 for drying pumpkin seeds in tray and fluidized bed, at 70°C, 80°C and 90°C and different air speeds (2 ms-1, 3 ms-1 and 4 ms-1) [25]. This variety of values denotes the importance of studying different products at different drying temperatures.
Thermodynamic properties
Activation energy regarding water diffusion of winter melon during the drying process was 53.9 kJ mol-1. Previous study reported that activation energy generally ranges from 12.7 kJ mol-1 to 110 kJ mol-1 for food [26]. Thus, value encountered at the present work is within the range. Table 3 presents the values of enthalpy, entropy and Gibbs free energy, regarding the drying process of winter melon.
| Temperature (˚C) | ∆H* (J mol-1) | ∆S* (J mol-1) |
|---|---|---|
| 40˚C | 51,331.80 | -82.12 |
| 50˚C | 51,248.66 | -82.38 |
| 60˚C | 51,165.52 | -82.63 |
Table 3: Values of thermodynamic parameters at different temperatures for the drying process of winter melon.
Enthalpy and entropy values of activation decreased, while the Gibbs free energy increased with increased drying temperatures in Table 3. As stated by Oliveira et al. (2016), water diffusion process for the product during drying responds to sensible heat and requires energy (ΔH*>0) to promote changes [20]. Higher values of enthalpy indicate a higher amount of energy required to remove moisture linked to the product during drying. As expected, this trend occurred at lower drying temperatures, indicating higher energy is required, so drying can occur, indicated by the higher required drying time. Negative values of entropy are attributed to the existence of chemical adsorption and/or structural alterations of the adsorbent in which entropy values decreased with temperature increment. This trend can be explained by the fact that whilst moisture is removed from the winter melon, moisture content decreases and the movement of water molecules become more restrict [27-34]. In other words, there are less available sites so diffusion mechanism can occur [20]. Gibbs free energy values were positive, indicating that drying is a non-spontaneous process. This trend is characteristic of endergonic reaction, in which needs an energy addition from the environment where the product is being dried, so the reaction can occur. This addition is made by the drying process, increasing air temperature.
Conclusion
The Two Term Exponential presented the best fit to predict the drying curves of winter melon, except for the 40°C temperature. Activation energy of drying process was 53.9 kJ mol-1. Thermodynamic properties presented a high correlation with drying temperature, in which higher temperature resulted in lower values of entropy and enthalpy values, whilst this increment increased values of Gibbs free energy.
Acknowledgments
The authors thank IF Sudeste MG for the financial support.
References
- Pinto LC. Aproveitamento de produtos derivados de levedura (Saccharomyces spp.) para o enriquecimento nutricional de alimentos à base de mandioca (Manihot esculenta Crantz). 2011.
- Thøgersen J, Alfinito S. Goal activation for sustainable consumer choices: A comparative study of Denmark and Brazil. J Consum Behav.2020;19(6):556-569.
- UN. Assembleia Geral das Nações Unidas. Transformando Nosso Mundo: A agenda 2030 para o desenvolvimento sustentável. 2015.
[Crossref]
- Deivanayagame T, Venkatachalam S, Jaganathan PM, Munusamy PM. Evaluation of mass transfer during osmo-convective drying of Benincasa hispida cubes in salt solution using regressional-desirability method. J Microbiol Biotechnol Food Sci. 2020;10(2):257-264.
- Costa PM, Bianchini A, Caneppele C, Azevedo PH, Silva AL. Drying kinetics of Amaranthus cruentus ‘BRS Alegria’seeds in natural and artificial methods. Revista Brasileira de Engenharia Agrícola e Ambiental. 2021;25:345-352.
- Vieira DM, Barros SL, de Alcântara Silva VM, Santos NC, Nascimento AP, Melo MO. Cinética de secagem e sua influência nas dimensões de sementes de abóbora sem casca. Revista Verde de Agroecologia e Desenvolvimento Sustentável. 2019;14(5):665-670.
- Bahramsoltani R, Farzaei MH, Abdolghaffari AH, Rahimi R, Samadi N, Heidari M et al. Evaluation of phytochemicals, antioxidant and burn wound healing activities of Cucurbita moschata Duchesne fruit peel. Iran J Basic Med Sci. 2017;20(7):798.
- Darrudi R, Nazeri V, Soltani F, Shokrpour M, Ercolano MR. Genetic diversity of Cucurbita pepo L. and Cucurbita moschata Duchesne accessions using fruit and seed quantitative traits. J applied research on medicinal and aromatic plants. 2018;8:60-6.
- do Amaral LF, Macedo Ferreira I, do Nascimento Santos LV, Silva AM, Araújo Fagundes A, de Carvalho MG. Biscuit with spices and corn flours and pumpkin seed: development and quality assessment. Food, Nutrition & Health. 2019;14.
- Leite DD, Queiroz AJ, Figueirêdo RM, Lima LS. Mathematical drying kinetics modeling of jackfruit seeds (Artocarpus heterophyllus Lam). Revista Ciência Agronômica. 2019;50:361-369.
- do Vale CP, Loquete FC, Zago MG, Chiella PV, Bernardi DM. Composição e propriedades da semente de abóbora. Fag Journal of Health. 2019;1(4):79-90.
- Brasilia. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. 2009:395.
- Souza JL, Oliveira DE, Plácido GR, Egea MB, Caliari M, da Silva MA. Thermodynamic and nutritional properties and drying kinetics of pequi (Caryocar brasiliense Cambess) mesocarp. Revista Brasileira de Engenharia Agrícola e Ambiental. 2019;23:655-661.
- Brooker DB, Bakker-Arkema FW, Hall CW. Drying and storage of grains and oilseeds. Springer Sci and Business Media. 1992.
- Jideani VA, Mpotokwana SM. Modeling of water absorption of Botswana bambara varieties using Peleg’s equation. J Food Eng. 2009;92(2):182-188.
- Corrêa Filho LC, Andrade ET, Martinazzo AP, D’Andrea EM, Sousa FA, Figueira VG. Cinética de secagem, contração volumétrica e análise da difusão líquida do figo (Ficus carica L). Revista Brasileira de Engenharia Agrícola e Ambiental. 2015;19:797-802.
- Santos DD, Leite DD, Lisbôa JF, Ferreira JP, Santos FS, Lima TL et al. Modelagem e propriedades termodinâmicas da secagem de fatias de acuri. Braz J Food Technol. 2019;22.
- Chicco D, Warrens MJ, Jurman G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Comput Sci. 2021;7(1):623.
- Keneni YG, Hvoslef-Eide AT, Marchetti JM. Mathematical modelling of the drying kinetics of Jatropha curcas L. seeds. Ind Crops Prod. 2019;132:12-20.
- Oliveira GH, Costa MR, Botelho FM, Viana JL, Garcia TR. Thermodynamic properties and kinetics of drying process of chia seeds (Salvia hispanica L). Res J Seed Sci. 2016;9(2):36-41.
- de Oliveira Santana FC, Panato K, Angonese M, Müller CM. Effect of separation methods on the drying kinetics of organic pitaya (Hylocereus undatus [Haw.] Britton and Rose) seed. LWT. 2022;153:112353.
- Amini G, Salehi F, Rasouli M. Drying kinetics of basil seed mucilage in an infrared dryer: Application of GA‐ANN and ANFIS for the prediction of drying time and moisture ratio. J Food Process Preserv. 2021;45(3):15258.
- Fellows PJ. Tecnologia do Processamento de Alimentos-: Princípios e Prática. Artmed Editora. 2018.
- Reis RC, Barbosa LS, Lima MD, Reis JD, Devilla IA, Ascheri DP. Modelagem matemática da secagem da pimenta Cumari do Pará. Revista Brasileira de Engenharia Agrícola e Ambiental. 2011;15:347-353.
- Jittanit W. Kinetics and temperature dependent moisture diffusivities of pumpkin seeds during drying. Agriculture and Natural Resources. 2011;45(1):147-158.
- Zogzas NP, Maroulis ZB, Marinos-Kouris D. Moisture diffusivity data compilation in foodstuffs. Drying technology. 1996;14(10):2225-2253.
- Moreira R, Chenlo F, Torres MD, Vallejo N. Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. J Food Eng. 2008;88(4):514-521.
- Chandra PK, Singh RP. Applied numerical methods for food and agricultural engineers. CRC Press. 1995.
- Henderson SM. Grain drying theory, I Temperature effect on drying coefficient. J Agr Eng Res. 1961;6(3):169-73.
- Kassem AS. Comparative studies on thin layer drying models for wheat in 13th International congress on agricultural engineering Morocco.1998.
- Midilli AD, Kucuk HA, Yapar Zİ. A new model for single-layer drying. Drying technology. 2002;20(7):1503-1513.
- Page GE. Factors influencing the maximum rates of air drying shelled corn in thin layers. Purdue University. 1949.
- Sharaf-Eldeen YI, Blaisdell JL, Hamdy MY. A model for ear corn drying. Transactions of the ASAE. 1980;23(5):1261-1265.
- Uddin Z, Suppakul P, Boonsupthip W. Effect of air temperature and velocity on moisture diffusivity in relation to physical and sensory quality of dried pumpkin seeds. Drying technology. 2016;34(12):1423-1433.
Citation: Oliveira GHH, Oliveira APLR, Fagundes LF, Andrade MSA, De Campos MA , Silva DCTC (2023) Kinetics and Thermodynamic Properties of Drying Winter Melon Seeds. J Food Process Technol.14:1031.
Copyright: © 2023 Oliveira GHH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


