Indexed In
- Open J Gate
- Genamics JournalSeek
- Smithers Rapra
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 14, Issue 3
Kinetic and Thermodynamics Studies of the Biosorption of Pb(II), Cd(II) and Zn(II) Ions from Aqueous Solution Using (Thaumatococcus danielli) Biomass
Adeniyi John Ademoyegun* and Adesola BabarindeReceived: 30-Sep-2020, Manuscript No. ACE-23-6666; Editor assigned: 05-Oct-2020, Pre QC No. ACE-23-6666 (PQ); Reviewed: 19-Oct-2020, QC No. ACE-23-6666; Revised: 16-Aug-2024, Manuscript No. ACE-23-6666 (R); Published: 13-Sep-2024, DOI: 10.35248/2090-4568.24.14.342
Abstract
This study evaluated the kinetics, isotherms and thermodynamics of heavy metal ions sorption onto Thaumatococcus danielli (popularly known as the moimoi leaf). Different reaction conditions such as contact time, initial metal ion concentration and also temperature were investigated for the removal of Pb(II), Cd(II), Zn(II), ions from the aqueous solutions.
The batch biosorption study of the biomass onto metal ions revealed that the equilibrium time was reached at 150 mins for Cd(II) and Zn(II) and 180 mins for Pb(II) at optimum pH of 7. Contact time, initial metal ion concentration, biosorbent dosage and temperature are other reaction conditions which are found to influence the biosorption process.
The FT-IR study showed the presence of ionisable (-OH, C=C, C≡C, PH3 and C-H) functional groups which could participate in the binding of metal ions in solution.
The kinetic rates were modelled using the pseudo-first-order, pseudo-second-order, Elovich and intraparticle diffusion. The experimental data fitted well to the pseudo-second-order kinetics with correlation R2 values of 0.9935, 0.9963 and 0.9971 for Pb(II), Cd(II) and Zn(II) respectively. The experimental equilibrium biosorption isotherm were analysed with Langmuir, Freundlich, Temkin and Dubini-Radushkevich (D-R) isotherm models. The Freundlich isotherm model gave the best fit with the highest correlation coefficient R2 with respective value of 0.9769, 0.8902 and 0.9717 for Pb(II), Cd(II) and Zn(II). The thermodynamics analyses indicate that the metal ions sorption on Thaumatococcus danielli is feasible, spontaneous and exothermic for both Pb(II) and Cd(II) but endothermic for Zn(II) ions.
These results indicate that Thaumatococcus danielli has potential for the uptake of Pb(II) and Cd(II) from polluted aqueous solution.
Keywords
Thaumatococcus danielli; Biosorptions; Pb(II); Cd(II); Zn(II); Kinetic and isotherm models
Introduction
The need for the use of water in virtually all human activities, whether urban, rural or industrial in nature, cannot be overemphasized. However the presence of heavy metals in water bodies does not only affect human beings but also affects plants as well due to their bioaccumulation tendency and toxicity and therefore render it unfit for use. Cadmium has been regarded as an element of high toxicity and mobility in the environment. Harmful effects of cadmium on man include renal dysfunction, bone degeneration, lung insufficiency, liver damage and hypertension. The main consequences in the environment are the poisoning of aquatic organisms and severe changes in the aquatic fauna and flora.
Xu et al., reported that Pb(II) is one of the major harmful pollutant to the biosphere and found to pose a detrimental risk to human health even at trace amounts of it. Although lead is an essential element for the normal metabolism of many living organisms, it is believed that excess lead may be harmful to mankind. For humans, constant exposure to lead causes oedema, learning disabilities in children, damage to organs such as the liver, kidneys and heart and immune system disorders.
Similarly, zinc is a metal ions commonly found in effluents of a large number of industries though it is an essential element for life and a micronutrient in trace amounts. However, a chronic exposure to Zn ion is detrimental for human health. At higher concentration, zinc has been found to cause damages to the pancreas, upset protein metabolism and cause arteriosclerosis. The toxicity of this pollutant has been well documented in literature and its presence in water and wastewaters is a potential risk for the environment and public health. Consequently, there is a need to reduce zinc concentrations in waste water to acceptable concentration levels.
There are various techniques which have been reported for the removal of heavy metals from aqueous effluents aimed at minimizing their impact among which are chemical precipitation, coagulation, solvent extraction, electrolytic processes, membrane separation, ion-exchange, reverse osmosis and ultra-filtration.
The disadvantages associated with these conventional methods includes but not limited to like incomplete metal removal, expensive cost, time consumption, ineffectiveness, high reagent and energy requirements, generation of toxic sludge and so on [1]. Thus it is of necessity to search for alternative methods that have low cost, simple and sludge free and eco-friendly.
Hence, adsorption has been universally accepted as one of the most effective pollutant removal processes, with low cost, ease in handling, low consumption of reagents, as well as scope for recovery of value added components through desorption and regeneration of adsorbent.
In the present study, dead biomass of Thaumatococcus danielli leaf was used as biosorbent for the removal of Pb(II), Cd(II) and Zn(II) ions from aqueous solution. The aim of the present investigation was to study the sorption kinetics, to establish the kinetic rate coefficients for sorption of Pb(II), Cd(II) and Zn(II) using Thaumatococcus danielli leaf, biosorption equilibrium employing different two-parameter isotherm models as well as thermodynamic studies.
Materials and Methods
Preparation of biomass
Thaumatococcus danielli (Ewe eran) leaves used in the study were plucked from a small farm in Ago-Iwoye, Ogun State, Nigeria. The leaves were thoroughly washed with distilled water to remove dust and other impurities. The leaves were air dried at room temperature for two weeks and then stored till the time of use.
Apparatus
The analysis of the metal ions was carried out using a Flame Atomic Absorption Spectrophotometer (FAAS) equipped with a flame burner. The concentration before and after biosorption of each metal ion was determined using a Perkin-Elmer analyst 700 (FAAS) with deuterium background corrector. Operational parameters for the metals under study were those recommended by the manufacturer. All the metals were measured under optimised operating conditions by FAAS with an air-acetylene flame. Fourier Transform Infrared (FTIR) spectra of dried unloaded biomass and metal loaded biomass were recorded at 400 cm-1-4000 cm-1, using a Shimadzu FTIR model 8400S spectrophotometer.
FT-IR analysis
The functional groups present on the surface of TD would give insight to the biosorption capacity of the biomass. These groups would form active sites for sorption on the biomass. Therefore, the FT-IR spectra of dried unloaded, Cd-loaded, Pb-loaded and Zn-loaded TD were taken to obtain information on the nature of possible interactions between the functional groups of plantain flower and the metal ions.
Batch biosorption study
The biosorption study was conducted by batch experiments under different conditions for a period of time (between 0-3 h) in a test tube. The biosorption study was conducted at 27°C using thermostated water bath to determine the effect of pH, contact time, biosorbent dosage and initial metal ion concentration of the biosorbent on each metal ion [2]. The study of the biosorption of Cd (II), Pb(II) and Zn(II) on TD leaf, using the time interval of 5 to 300 mins at fixed concentration of 100 mgL-1 was done by contacting 0.5 g of TD leaf with 25 mL of 100 mgL-1 of each of the metal ions solution at optimal pH.
The leaf was left in the solution for different periods of time, between 5-300 mins. At the predetermined time, the glass tubes were withdrawn from the bath and the residual Cd(II), Pb(II) and Zn(II) concentration in solution were analyzed using FAAS. The amount of metal ion biosorbed from solution was determined using the following equation:
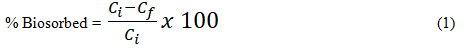
Where Ci is the initial metal ion concentration and Cf is the final metal ion concentration. For the determination of the effect of varying biomass dosage on biosorption, different biomass dosage were used in this study (0.2 g to 2.0 g) while the metal ions concentration were fixed at 100 mgL-1. The effects of sorbent dose on the uptake of Cd(II), Pb(II) and Zn(II) were carried out by contacting different masses of TD leaf with 25 mL of 100 mgL-1 solution of the metal ion at optimal pH and time.
Statistical analysis
The curve fittings of the data obtained were performed using origin 8.0 software.
Results and Discussion
The FT-IR spectra of the TD before and after biosorption of the metal ions were recorded using a mid-range FT-IR with wave number range between 400 cm-1-4000 cm-1. The FT-IR spectra of dried leaves unloaded and loaded with metals ions are shown in Figure 1.
The following functional groups were shown by IR of the moimoi leaf; OH stretching of the carboxylic group at 3264 cm-1, C-H stretch at 2927.45 cm-1, phosphine group at 2348.67 cm-1, C=C at 1647.11 cm-1 and C≡C at 2113.02 cm-1.
Analysis of the FT-IR spectra showed the presence of ionizable and electron rich functional groups (O-H, C=C, C≡C) which are able to interact with positive ions (cations) of the metals [3]. The FT-IR spectra characteristics of Thaumatococcus danielli leaf before and after biosorption of Pb(II), Cd(II) and Zn(II) is shown in Table 1.
| Metal ion | Absorption band (cm-1) | |||
|---|---|---|---|---|
| Before | After | Difference | Assignment | |
| Cd(II) | 3264.06 | 3252.42 | 11.64 | O-H stretch |
| Pb(II) | 3264.06 | 3275 | 10.94 | O-H stretch |
| Zn(II) | 3264.06 | 3285.96 | -21.9 | O-H stretch |
| Cd(II) | 2927.45 | 2923.33 | 4.12 | C-H stretch |
| Pb(II) | 2927.45 | 2929.31 | -2.22 | C-H stretch |
| Zn(II) | 2927.45 | 2923.78 | 3.67 | C-H stretch |
| Cd(II) | 2348.67 | 2348.47 | 0.2 | Phosphine |
| Pb(II) | 2348.67 | 2348.31 | 0.36 | Phosphine |
| Zn(II) | 2348.67 | 2350.58 | -1.91 | Phosphine |
| Cd(II) | 1647.11 | 1650.42 | -3.31 | C=C |
| Pb(II) | 1647.11 | 1542.29 | 104.82 | C=C |
| Zn(II) | 1647.11 | 1661.13 | -14.02 | C=C |
| Cd(II) | 2113.02 | 2128.82 | -15.8 | C≡C |
| Pb(II) | 2113.02 | 2121.29 | 8.27 | C≡C |
| Zn(II) | 2113.02 | 2122.59 | -9.57 | C≡C |
Table 1: FT-IR spectra characteristic of TD leaf (before and after) biosorption of Cd(II), Pb(II) and Zn(II).
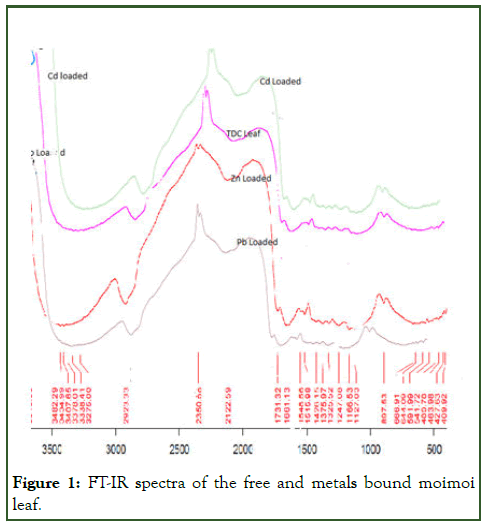
Figure 1: FT-IR spectra of the free and metals bound moimoi leaf.
Effect of solution pH on biosorption
The effect of pH on the biosorption of Cd(II), Pb(II) and Zn(II) was carried out within pH 1-7 by contacting 0.5 g of the leaf biomass with 25 ml of each metal ions solution. Higher pH is avoided as it leads to the precipitation of the metal hydroxides. The optimal pH was found to be pH 7 for Pb(II) and Cd(II) and pH 6 for Zn(II), amount of metal ions uptake decreases at higher pH value.
At lower pH, the active sites of the biomass were protonated; hence hydrogen ions being positive ions compete with the metal ions of same positive ions for the exchange (active) sites of the biomass resulting in low biosorption due to the presence of excess hydrogen ions. Literature also reveals that biosorbent cell wall ligands are saturated with hydroxonium ions and repulsive forces preventing the effective biosorption of metal ions onto the cell wall. Optimum biosorption in this present study was found to be 92% for Pb(II), 81% for Cd(II) and 70% for Zn(II) at pH 7 for Pb(II) and Cd(II) and pH 6 for Zn(II) [4]. These results were in agreement with the results obtained by Babarinde et al., on using (Musa paradisiaca) flower for the biosorption of these respective metals ions (Figure 2).
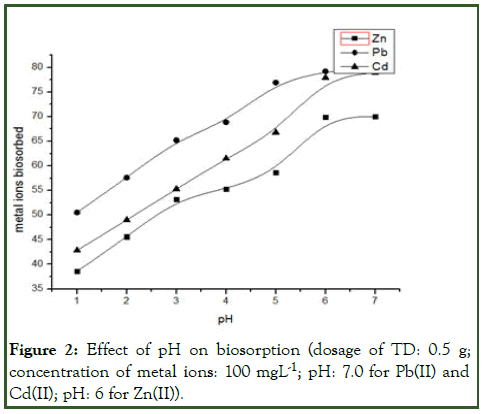
Figure 2: Effect of pH on biosorption (dosage of TD: 0.5 g; concentration of metal ions: 100 mgL-1; pH: 7.0 for Pb(II) and Cd(II); pH: 6 for Zn(II)).
Kinetics study
Biosorption kinetics was carried out so as to have a good understanding of the biosorption process with time. The biosorption of the metal ions onto TD was studied at various time intervals (0-300 min) and at the concentration of 100 mgL-1. The kinetics of sorption is an important factor in predicting the rate at which sorption takes place for a given system and also very essential in understanding the mechanism of the process. However, sorption kinetics has been found to vary with different biosorbent as the sorption process shows a large dependence on the physical and/or chemical characteristics of the sorbent material, which also influences the sorption process and the mechanism.
The equilibrium time required for biosorption of Cd(II), Pb(II) and Zn(II) on TD obtained by studying biosorption of each metal ion at various contact times.
The results show that the biosorption of the three metal increases with increase in the contact time. The biosorption of the metal ions by the biosorbent was initially rapid for the initial 5 mins [5]. The fast initial uptake of the metal ions by the sorbent has been suggested to be the availability of free binding sites on the moimoi leaf.
The rate of biosorption of the metal ions becomes relatively steady as time progresses and then fairly constant at 150 mins for Zn(II) and Cd(II) while the rate of biosorption was fairly constant at 180 mins for Pb(II) (Figure 3).
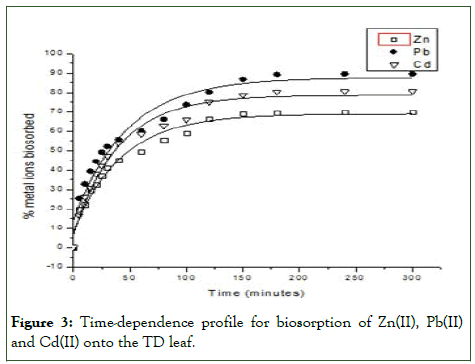
Figure 3: Time-dependence profile for biosorption of Zn(II), Pb(II) and Cd(II) onto the TD leaf.
To establish the mechanisms of the biosorption of the metal ions onto the Thaumatococcus danielli leaf, four twoparameters kinetic models were employed viz., pseudo-first order, pseudo-second order, Elovich and intra-particle diffusion kinetic models and the kinetic parameters for different models are presented in Tables 2 and 3 (Figures 4-7).
| S/N | Kinetics model | Linearized equation |
|---|---|---|
| 1 | Pesudo-first order | 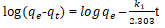 |
| 2 | Pseudo-second order | 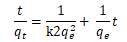 |
| 3 | Elovich | 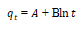 |
| 4 | Intra-particles | 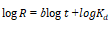 |
Table 2: Kinetic sorption models.
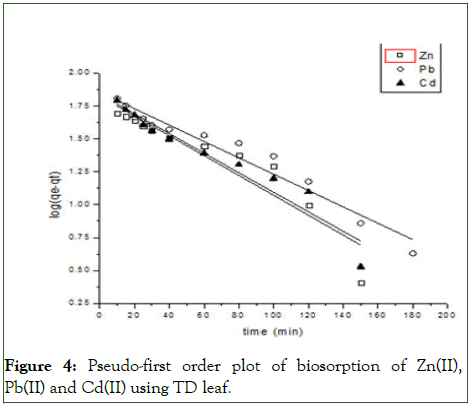
Figure 4: Pseudo-first order plot of biosorption of Zn(II), Pb(II) and Cd(II) using TD leaf.
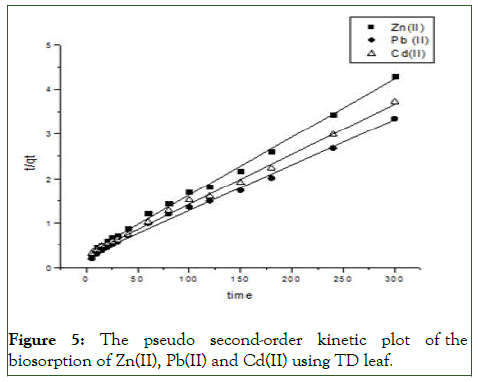
Figure 5: The pseudo second-order kinetic plot of the biosorption of Zn(II), Pb(II) and Cd(II) using TD leaf.
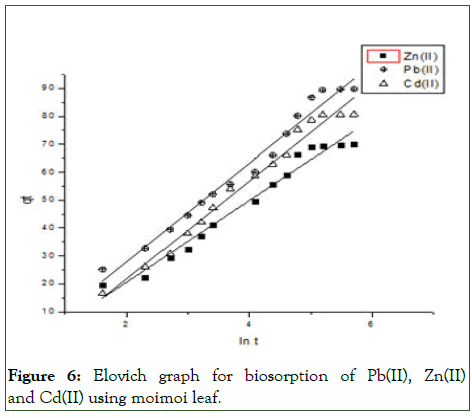
Figure 6: Elovich graph for biosorption of Pb(II), Zn(II) and Cd(II) using moimoi leaf.
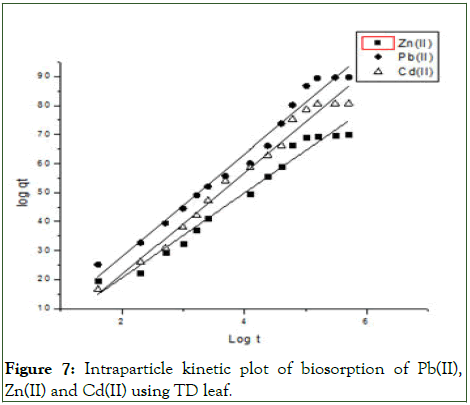
Figure 7: Intraparticle kinetic plot of biosorption of Pb(II), Zn(II) and Cd(II) using TD leaf.
| Kinetic model | Parameters | Pb(II) | Zn(II) | Cd(II) |
|---|---|---|---|---|
| Pseudo-first order | qe(mgg-1) | 71.43 | 67.11 | 68.79 |
| k1(min-1) | 1.432 x 10-2 | 1.741 x 10-2 | 1.711 x 10-2 | |
| R2 | 0.9512 | 0.8659 | 0.9297 | |
| Pseudo-second order | qe cal(mgg-1) | 98.04 | 76.92 | 89.21 |
| k1(gmg-1min-1) | 3.951 x 10-4 | 5.18 x 10-4 | 4.18 x 10-4 | |
| R2 | 0.9935 | 0.9971 | 0.9963 | |
| Elovich | A | -7.5709 | -8.5988 | -7.5709 |
| B | 17.71 | 14.62 | 17.71 | |
| R2 | 0.9769 | 0.9717 | 0.9812 | |
| Intraparticle diffusion | kd(mgg-1min-1) | 2.685 X 10-8 | 2.52 X 10-9 | 6.03 X 10-14 |
| B | 17.708 | 14.615 | 17.502 | |
| R2 | 0.9769 | 0.9717 | 0.9812 |
Table 3: Kinetic parameters for the biosorption of Pb(II), Zn(II) and Cd(II) using the TD leaf.
From the Table 3, it can be inferred that the kinetics studies is best explained with the pseudo-second order and Elovich model with the highest residual correlation (R2) as shown in Table 3 suggesting that the biosorption process proceeded via chemisorption [6]. The equation defining the Elovich model based on the kinetic principle with the assumption of adsorption sites increase exponentially with adsorption, which implies a multilayer adsorption. The data obtained also showed that the amount of metal ions absorbed at equilibrium for the sorption processes were respectively 98.04mgg-1, 89.21mgg-1 and 76.92mgg-1 for Pb(II), Cd(II) and Zn(II).
Effect of biomass dosage
Biomass provides binding sites for the sorption of metal ions, hence its concentration strongly affects the sorption of metal ions from the solution. Hence varying the biomass dosage is an important parameter in adsorption studies because it determines the capacity of adsorbent for a given initial concentration of metal ion in solution. In this present study, there is an increase in three metal ions biosorbed with increase in biomass dosage due to the increased binding sites on the biomass available for biosorption, although with different biosorption as shown in Figure 8.
The difference in biosorption capacity q (mg/g) at the same initial metal ion concentration and contact time can be suggested to be difference in their affinities and ion-exchange capacity with respect to the chemical functional group on the surface of the biosorbent.
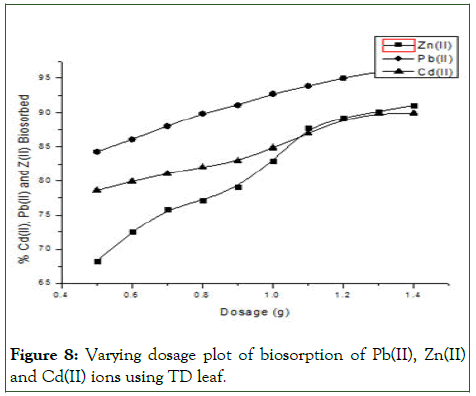
Figure 8: Varying dosage plot of biosorption of Pb(II), Zn(II) and Cd(II) ions using TD leaf.
Effect of initial metal ion concentration and biosorption isotherm
The initial concentration of metal ions in the solution plays a key role as a driving force to overcome the mass transfer resistance between the aqueous and solid phases. It is generally agreed that the biosorption capacity increases as the initial metal ion concentration in the solution increases, whereas the removal efficiency decreases by increasing the metal ion initial concentration.
By principle, increasing the initial metal concentration results in an increase in the biosorption capacity because it provides a driving force to overcome mass transfer resistance between the biosorbent and the biosorption medium.
In the present study, sorption characteristic indicated that surface saturation was dependent on initial metal ion concentration, at low concentration, adsorption sites took up the available metal more quickly. When the concentration became higher, the rate of diffusion became slow, this was probably due to the fact that metal needed to diffuse to the biomass surface by intraparticle diffusion and greatly hydrolyzed ion will diffuse at a lower rate (Figure 9) [7].
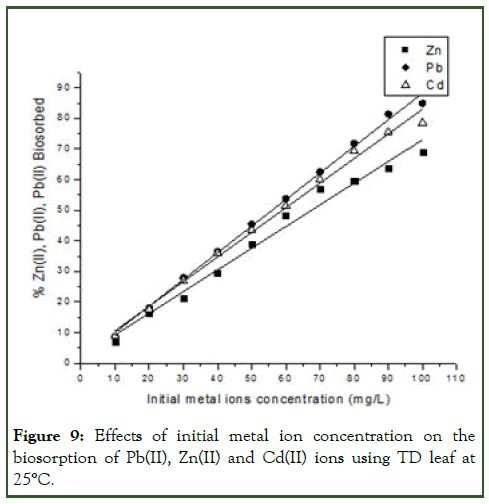
Figure 9: Effects of initial metal ion concentration on the biosorption of Pb(II), Zn(II) and Cd(II) ions using TD leaf at 25°C.
The experimental equilibrium biosorption isotherm were analysed with Langmuir, Freundlich, Temkin and Dubini- Radushkevich (D-R) isotherm models. The Freundlich isotherm model gave the best fit with the highest correlation coefficient R2 with respective value of 0.9769 and 0.8902 for Pb(II) and Cd(II) indicating that biosorption mechanism occur via multilayer diffusion of the metal ions on the biosorbent surface (Tables 4 and 5) (Figures 10-13) [8].
| S/N | Isothermal model | Linearized equation |
|---|---|---|
| 1 | Freundlich | 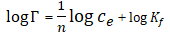 |
| 2 | Langmuir | 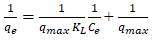 |
| 3 | Temkin | 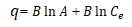 |
| 4 | Dubini-Radushkevich (D-R) | 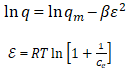 |
Table 4: Two-parameter sorption isotherm models.
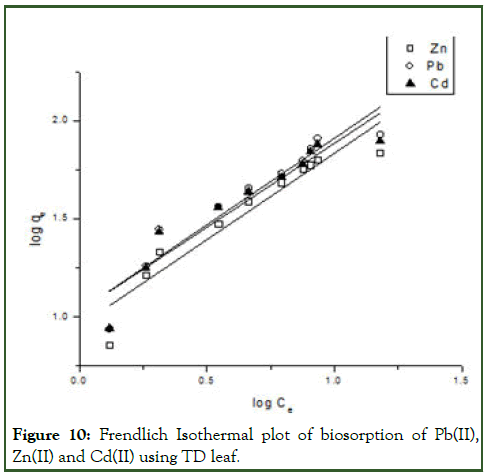
Figure 10: Frendlich Isothermal plot of biosorption of Pb(II), Zn(II) and Cd(II) using TD leaf.
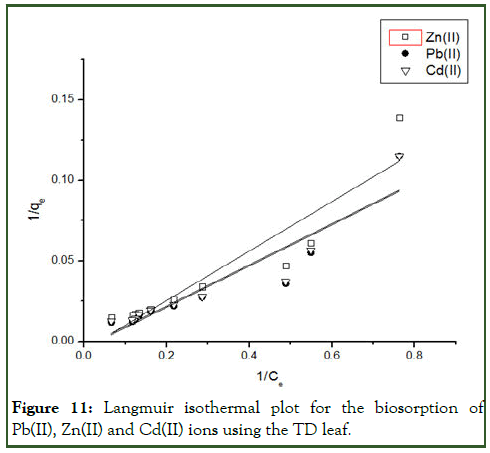
Figure 11: Langmuir isothermal plot for the biosorption of Pb(II), Zn(II) and Cd(II) ions using the TD leaf.
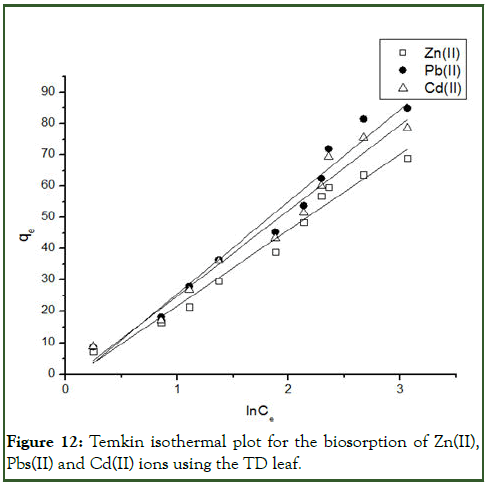
Figure 12: Temkin isothermal plot for the biosorption of Zn(II), Pbs(II) and Cd(II) ions using the TD leaf.
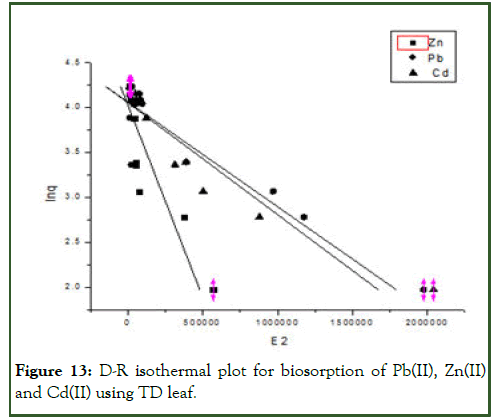
Figure 13: D-R isothermal plot for biosorption of Pb(II), Zn(II) and Cd(II) using TD leaf.
| Isothermal model | Parameter | Pb(II) | Zn(II) | Cd(II) |
|---|---|---|---|---|
| Freundlich | N | 1.135 | 1.136 | 1.169 |
| Kf | 10.683 | 8.978 | 10.692 | |
| R2 | 0.9769 | 0.9717 | 0.8902 | |
| S.D | 0.07404 | 0.07281 | 0.07299 | |
| Langmuir | qmax(mgg-1) | -230.95 | -190.48 | -284.09 |
| KL(Lmg-1) | -3.38 x 10-2 | -3.42 x 10-2 | 2.76 x 10-2 | |
| R2 | 0.8933 | 0.8516 | 0.8597 | |
| S.D | 0.00641 | 0.0077 | 0.00621 | |
| Temkin | A | 0.8797 | 0.9174 | 0.9113 |
| B | 29.4068 | 24.2185 | 27.2856 | |
| R2 | 0.9656 | 0.9753 | 0.9639 | |
| S.D | 0.7226 | 0.6915 | 0.6468 | |
| D-R | qm(mgg-1) | 4.1416 | 3.9209 | 4.0919 |
| β (mol2J-2) | -3.5415 | -3.4333 | -3.4538 | |
| E (Jmol-1) | 0.3757 | 0.3816 | 0.3805 | |
| R2 | 0.8373 | 0.7774 | 0.8425 | |
| S.D | 5.1479E-7 | 6.0288E-7 | 4.9265E-7 |
Table 5: Isothermal parameters of the biosorption of Pb(II), Zn(II) and Cd(II) using the moimoi leaf.
Where Kf and n are empirical Frendlich constants and indicative of adsorption capacity and adsorption intensity respectively, Ce is the equilibrium concentration of metal ion in solution (molL-1). Figure 10 shows the biosorption isotherm of Pb(II), Zn(II) and Cd(II) ions using TD leaf. The equilibrium biosorption capacity qe increases with an increase in the metal ions concentration. The Kf values of Cd(II), Pb(II) and Zn(II) are respectively 10.69, 10.68 and 8.98 suggesting that Cd(II) had a greater adsorption tendency toward the TD leaf biomass than Pb(II) and Zn(II) [9]. The isothermal parameters are shown in Table 5.
Also, from the Langmuir model employed to estimate the maximum adsorption capacity corresponding to complete monolayer coverage on the biomass surface. The model explains adsorption by assuming that an adsorbate behaves as an ideal gas at isothermal conditions. The qmax values are respectively 230.95mg/g, 190.48mg/g and 284.09mg/g for Pb(II), Zn(II) and Cd(II) as shown in Table 3. The value of RL indicate whether the isotherm will be irreversible (RL=0), favourable (0<RL<1), linear (RL=1) or unfavourable (RL>1) [10].
Similarly, from the D-R model, the constant β gives an idea about the mean free energy ε(Jmol-1) of biosorption per molecule of the biosorbate when it is transferred to the surface of the solid from infinity in the solution. E can be calculated from the relationship given below.
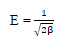
If the magnitude of ε is between 8 and 16 kJmol-1, the sorption process is supposed to proceed via chemisorption but if ε is less than 8 kJmol-1, the sorption process is of physical nature.
In the present study, the values of mean free energy E(J/mol) are as presented in Table 5 indicating that the process is of physical nature.
Biosorption efficiency
The biosorption efficiency (E) for each metal ion was calculated using equation shown in equation:
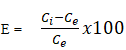
Where Ci and Ce are the initial and the equilibrium metal ion concentrations (mgL-1), respectively [11]. The effect of initial metal ion concentration on biosorption efficiency is shown in the Figure 14 below.
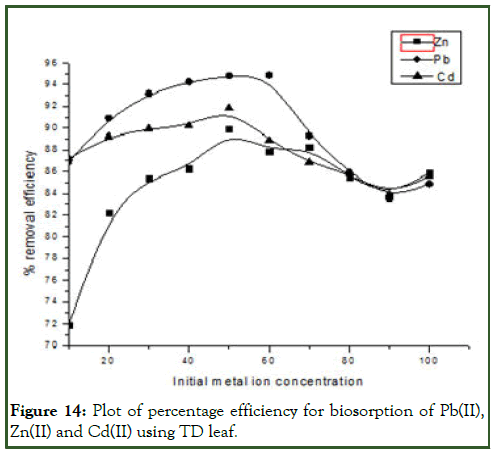
Figure 14: Plot of percentage efficiency for biosorption of Pb(II), Zn(II) and Cd(II) using TD leaf.
Biosorption thermodynamics
The biosorption of metal ions may involve chemical bond formation and ion exchange since a major factor affecting adsorption is temperature. The variation of temperature affects the biosorption of Pb(II), Zn(II) and Cd(II) surface of the moimoi leaves as shown in Table 4. The nature of each side of the equilibrium determines the effect of temperature on the position of equilibrium. In the present study, the biosorption experiments were carried out in the temperature range 20°C-40° C. Thermodynamic parameters were obtained by varying temperature conditions over the range 20°C-40°C by keeping other variables constant. The corresponding free energy change was calculated from the relation given below in equation.

Where T (K) is the absolute temperature, Kc is the equilibrium constant which can be calculated from the relationship as shown below in equation.
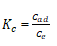
Where Ce and Cad are the equilibrium concentrations of the metal ions (mgL-1) in the solution and on the biosorbent respectively. If the free energy value is positive, it is endothermic and be favoured by increase in temperature while negative value of free energy value indicates exothermic process and be favoured by decrease in the temperature. Consequently, the thermodynamic behaviour of the biosorption Pb(II), Zn(II) and Cd(II) onto the moimoi leaf was calculated through the change in free energy (ΔG°), enthalpy (ΔH°) and the entropy (ΔS°).
The thermodynamic parameters like the enthalpy and entropy was obtained using Van’t Hoff equation. The change in free energy is related to other thermodynamic properties as expressed in the equations.

Where T(K) is the absolute temperature, R is the gas constant (8.314 Jmol-1K-1). The change in enthalpy was evaluated from the intercept and slope of the plot of T versus ΔG° as presented in Figure 15. The slopes of the plot is of free energy against the temperature are -14.3564 Jmol-1K-1, -10.7966 Jmol-1K-1 and -6.6136 Jmol-1K-1 for Pb(II), Cd(II) and Zn(II) respectively indicating that the respective values of the entropy while the intercepts values which indicates the enthalpy are -0.00429 kJmol-1K-1, -0.00743 kJmol-1K-1 and 0.06857 kJmol-1K-1 for Pb(II), Cd(II) and Zn(II) respectively. This indicates that the process is endothermic for both Pb(II) and Cd(II) but exothermic for Zn(II) with positive enthalpy value [12]. The sorption process was spontaneous with negative free energy values indicating the feasibility of the biomass in removing the metal ions from the aqueous solution. The thermodynamics parameters are presented in Table 6.
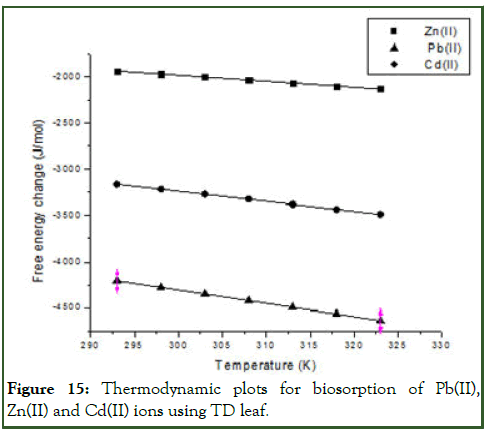
Figure 15: Thermodynamic plots for biosorption of Pb(II), Zn(II) and Cd(II) ions using TD leaf.
| Metal ion | ΔH (kJmol-1) | ΔS(Jmol-1) | R2 | A(kJmol-1) (298K) | A(kJmol-1) (313K) |
|---|---|---|---|---|---|
| Pb(II) | -0.00429 | 14.356 | 1 | 2.4776 | 2.6022 |
| Zn(II) | 0.06857 | 6.613 | 1 | 2.4775 | 2.6023 |
| Cd(II) | -0.00743 | 10.796 | 1 | 2.4776 | 2.6022 |
Table 6: Thermodynamic parameters of the biosorption of Pb(II), Zn(II) and Cd(II) onto TD leaf.
This is also supported by the increase in the value of biosorption capacity of the biosorbent with rise in temperature.
The negative value of the ΔS entropy as observed in the Table 6 suggests the degree of randomness of the process and the adsorption process involves an associative mechanism. It also suggests that there is no significant change in the internal structures of the adsorbent during the adsorption process. However, the values of the entropy is about five times lower compared to value reported by Babarinde et al., on using Musa parasidica flower for the removal of Pb(II), Cd(II) and Zn(II) ions from aqueous solution indicating the process is less spontaneous.
Generally, it has been reported that the standard free energy for the physiosorption is in the range of -20 kJmol-1 to 0 kJmol-1 and between -80 kJmol-1 and -400 kJmol-1 for chemisorption process.
In this present study, the values of the standard Gibbs free energy (ΔGo) ranges from -4.64 kJmol-1 to -1.94 kJmol-1. These results correspond to a spontaneous physical adsorption of the metal ions. The increase in the ΔGo with increase in temperature indicates that the process becomes more spontaneous with increase in temperature and more efficient biosorption at higher temperature.
Also, the magnitude of activation energy (A) gives an idea about the type of adsorption which is mainly diffusion controlled process (not diffusivity of solute through micro pore wall surface of a particle) or chemical reaction processes. The activation energy can be evaluated by the relation given below in equation 26:
A=ΔH°+RT
Energy of activation A below 42 kJmol-1 indicates diffusion controlled processes, and higher value suggests chemical reaction processes. Hence, the present study is diffusion controlled as given by the value of the activation energy as shown in Table 6.
Conclusion
From the results obtained in this present study, it can be concluded that pH is one of the key factors that affects the rate of biosorption. Maximum biosorption occurred between pH 6 and 7 for the biosorption of Pb(II), Zn(II) and Cd(II) using TD leaf.
Also, increasing the biosorbent dosage leads to increase in biosorption rate as a result of increased available biosorption sites. Maximum uptakes occurred at the dosage of 1.4 g for all the metal ions.
The equilibrium time for biosorption of Pb(II), Zn(II) and Cd(II) from aqueous solution was achieved within 180 mins and the experimental data were better described by pseudo second order model as shown by the correlation plots while the biosorption isotherm study showed that Frendlich model was found to provide the best fit of the experimental data.
The results of the present study suggest that Thaumatococcus danieli leaf is an efficient biosorbent for the removal of Pb(II), Zn(II) and Cd(II) from aqueous solution.
References
- Abdel-Ghani NT, Hefny M, El-Chaghaby GA. Removal of lead from aqueous solution using low cost abundantly available adsorbents. Int J Environ Sci Technol. 2007;4:67-73.
- Gouamid MO, Ouahrani MR, Bensaci MB. Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using date palm leaves. Energy Proced. 2013;36:898-907.
- Babarinde NA, Babalola JO, Adebisi OB. Kinetic, isotherm and thermodynamic studies of the biosorption of zinc (II) from solution by maize wrapper. Int J Phys Sc. 2008;3(2):50-55.
- Bhattacharya AK, Mandal SN, Das SK. Adsorption of Zn (II) from aqueous solution by using different adsorbents. Chem Eng J. 2006;123(1-2):43-51.
- Bueno BY, Torem ML, Molina FA, de Mesquita LM. Biosorption of lead (II), chromium (III) and copper (II) by R. opacus: Equilibrium and kinetic studies. Miner Eng. 2008;21(1):65-75.
- Dang VB, Doan HD, Dang-Vu T, Lohi A. Equilibrium and kinetics of biosorption of cadmium (II) and copper (II) ions by wheat straw. Bioresour Technol. 2009;100(1):211-219.
[Crossref][Google Scholar] [PubMed]
- Gode F, Pehlivan E. Removal of chromium (III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J Hazard Mater. 2006;136(2):330-337.
[Crossref] [Google Scholar] [PubMed]
- Gupta VK, Rastogi A. Biosorption of lead from aqueous solutions by green algae Spirogyra species: Kinetics and equilibrium studies. J Hazard Mater. 2008;152(1):407-414.
[Crossref] [Google Scholar] [PubMed]
- Ho YS, Ofomaja AE. Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent. Biochem Eng J. 2006;30(2):117-123.
- Iqbal M, Saeed A, Zafar SI. Hybrid biosorbent: An innovative matrix to enhance the biosorption of Cd (II) from aqueous solution. J Hazard Mater. 2007;148(1-2):47-55.
[Crossref] [Google Scholar] [PubMed]
- Patel KP, Tank SK, Patel KM, Patel P. Removal of cadmium and zinc ions from aqueous solution by using two type of husks. APCBEE Proced. 2013;5:141-144.
- Saurav K, Kannabiran K. Biosorption of Cd(II) and Pb(II) ions by aqueous solutions of novel alkalophillic Streptomyces VITSVK5 spp. biomass. J Ocean U China. 2011;10:61-66.
Citation: Ademoyegun AJ, Babarinde A (2024) Kinetic and Thermodynamics Studies of the Biosorption of Pb(II), Cd(II) and Zn(II) Ions from Aqueous Solution Using (Thaumatococcus danielli) Biomass. Adv Chem Eng. 14:342.
Copyright: © 2024 Ademoyegun AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

