Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 15, Issue 5
Evaluation of the Effectiveness of Nano-Hydroxyapatite Particles in Wound Healing in an Animal Study
Sahar Sadat Lalehzar1*, Rokhsareh Meamar2, Ardeshir Talebi3 and Mehrafarin Fesharaki22Department of Clinical Toxicology, Isfahan University of Medical Sciences, Isfahan, Iran
3Department of Pathology, Isfahan University of Medical Sciences, Isfahan, Iran
Received: 26-Nov-2022, Manuscript No. BLM-22-19007; Editor assigned: 29-Nov-2022, Pre QC No. BLM-22-19007 (PQ); Reviewed: 12-Dec-2022, QC No. BLM-22-19007; Revised: 22-Feb-2023, Manuscript No. BLM-22-19007 (R); Published: 01-Mar-2023, DOI: 10.35248/0974-8369.23.15.536
Abstract
Background: The main activity of the skin is to create a protective barrier against damage. Loss of the skin due to injury or disease and failure to regenerate the affected area may result in disability, infection, or even death. We conducted the animal study to evaluation of the effectiveness of nano-hydroxyapatite particles in wound healing.
Method: This animal study performed in Isfahan university of medical science animal lab. Experiments were performed on 30 wistar in 5 groups. Biopsies 5 mm × 5 mm were obtain of abdominal, and were transferred to the cell culture laboratory into the Phosphate Buffer Saline (PBS). The cell proliferation was determined using the colorimetric MTS assay. The type and approach of this animal study is to create a deep skin wound and try to treat the wound with drug (nano hydroxyapatite 10%, nano hydroxyapatite 40%, combination of nickle ion with nanohydroxyapatite 10%, and 40%) intervention on an animal model of rat. Macroscopic evaluation and pathological examination were done. For pathological and histological examination of the wound, sampling was done on the seventh and fourteenth days after ulcer induction. All continuous and categorical data are presented as mean ± Standard Deviation (SD) and frequency (percentage), respectively. Paired sample T-test and repeated measure Analysis of Variance (ANOVA), chi-squared test was used.
Results: During this study, MTS assay was carried out to evaluate the proliferation of mice fibroblast on the gelatin without hydroxyapatite, and with 10%, 40% hydroxyapatite after 1 day, 2 days and 3 days of culture. Significant enhancement of cell proliferation was observed in nano HA 10%, 40% and nano HA 10% with nickel in comparison when the cells seeded on gelatin and HA 10%. The best result was shown in 24 hours after seeding the cells in gelatin in comparison with 48 hours and 72 hours. Indeed, after 48 hours and 72 hours, the cell proliferation on gelatin decreased. In evaluation of wound area with image j soft ward, the wound area between 3rd day, 7th day and 14th day of treatment after wound induction there were no significant difference between groups. In microscopic study and analysis for evaluation and comparing wound length with the michrome camera and mosaic soft ward, there were no significant relation in time (p1=0.77). There is a difference is close to significant between the groups (p2=0.065). There was no significant difference between time and group (p3=0.323). In day 14 the wound length between groups had significant difference (p4=0.049).
Conclusion: In conclusion, hydroxyapatites and its combination with nickle ion have significant effect on wound healing and cell proliferation.
Keywords
Nano-hydroxyapatite; Wound healing; Animal study; Nickle ion; Cell proliferation
Introduction
The main activity of the skin is to create a protective barrier against damage caused by contact with the environment around the body [1]. Loss of the skin due to injury or disease and failure to regenerate the affected area may result in disability, infection, or even death [1]. Every year in the United States, more than 1.25 million people suffer from burns and 6.5 million suffer from bed sores, circulatory ulcers, or diabetes [2].
Recent advances in life sciences have made it possible to understand the effective processes in wound healing and to take effective steps towards better wound care [3]. Wound healing is a vital process for humans and many animals. One of the important goals of skin wound care is the process of rapid wound closure [4].
There are many ways for accelerating the skin wound healing such as aloe vera, collagen from marine source, on demand release of CO2 from photothermal hydrogels, ozone oil, and one of them is hydroxyapatites [5-9]. Hydroxyapatite with the formula Ca10(PO4)6(OH)2, as one of the biocompatible and bioactive substances containing calcium, is known for use in the regeneration of damaged tissues [10]. HA is containing calcium and it is one of the most essential ions in the wound healing mechanism and wound healing is mediated by calcium ions. They are also involved in the regeneration process [11]. The more calcium ions released from HA, the better the regulation of skin homeostasis, proliferation and differentiation of skin cells [12]. The effect of HA is to reduce the size of the wound by releasing calcium ions, which is one of the important criteria for wound healing. The key to initiating the wound healing process is blood coagulation, and one of the main factors causing blood coagulation is calcium [13-15].
Hydroxyapatite nanoparticles have been demonstrated as eminent nanomaterials because of their upgraded bioactivity, non-toxicity and biocompatibility properties [16]. There have been many studies on its use in medicine in various fields. For example, it has been used for bone repair, cartilage regeneration, and as an implant coating due to its hard tissue structure [17]. Since it has a stimulating effect on angiogenesis and cell proliferation, it is also used in soft tissue engineering [18]. The ionic products released by the HA reaction with the surrounding tissues are thought to cause an initial inflammatory reaction, which can accelerate wound healing and result in more satisfactory healing [19].
Also, in some new studies hydroxyapatite doped with ions for example, porous copper and lithium doped nano hydroxyapatite, rubidium, magnesium and nickle, and all these was used for evaluating cytocompatibility and its antibacterial effect [20-22]. They show that by using this combination in animal and human wounds rehabilitation was significantly accelerated.
Also, many other ions of some metals were doped with HA, and they find out they all had accelerated the rehabilitation of human tissue injury or not [23].
Due to the very valuable biological properties of HA and its role in decrease inflammation, cell proliferation, blood clotting and other important stages of wound healing, in this study, a new gel is produced by gelatinizing the nano particle of hydroxyapatites doped with nickle ion as a novel, and compare its effect on skin wound rehabilitation with nano particle of hydroxyapatites.
Materials and Methods
Study design
The animal study performed in 2019 in Isfahan university of medical sciences. The study is containing in vitro cell study, MTS assay, DAPI staining, preparation of drug, animal study, and animal study.
Materials
Gelatin type A (G 2500) and penicillin streptomycin were purchased from Sigma (USA) were obtained from Merck (Germany). All chemicals used for the cell culture study were obtained from Sigma, St. Louis, MO, USA, unless stated otherwise. DMEM, FBS, PBS, and trypsin EDTA were purchased from Bioidia corporation, Iran. (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2-H-tetrazolium) (MTS) was obtained from Promega all other chemicals and reagents were of analytical grade.
In vitro cell study
Biopsies 5 mm × 5 mm were obtain of abdominal, and were transferred to the cell culture laboratory into the Phosphate Buffer Saline (PBS). The fragments were washed extensively by sterile PBS at least three times. Then cut into 1 mm × 1 mm pieces and then cultivation in tissue culture medium (Including DMEM/Ham’s F12, modified Eagle’s medium (Gibco BRL, Paisley, UK) containing Fetal Bovine Serum (FBS) (12%) (Gibco), 1% streptomycin/penicillin solution (CM Media) and incubated at 37°C humidified incubator with 5% CO2 environment and were defined as passage 0 (P0). Culture media replaced every 3 days until 80% confluence. At confluence, cells were split with 0.0 5% Trypsin/0.02% EDTA and sub culture for more passages.
MTS assay
The cell proliferation was determined using the colorimetric MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2-H-tetrazolium) assay. The MTS assay was performed as previously described. After one, two and three days of cells seeding (5 × 105 cells/well) in 24 well plates, cells were washed with PBS and incubated with 20% of MTS reagent containing serum free medium, after incubated plates were in the dark for 3 hours at 37°C, in 5% CO2 aliquots were pipette. Transfer to 96 well plate. The absorbance of each well was detected at 540 nm. Using a spectrophotometric plate reader (Hyperion MPR 4+ Germany).
DAPI staining
The staining of the cells with DAPI (4', 6-diamidino-2-phenylindole), after 1 day and 7 day of cell seeded scaffolds, the cells were fixed using paraformaldehyde at room temperature for 30 min, and washed with PBS thoroughly. After the permeabilization of the cells with Triton x-100 for 10 min, 5 μg/ml DAPI was added to each well and incubated in the dark for 5 min at room temperature. Finally, the samples were washed, dried and observed by a fluorescence microscope (Olympus BX51, Japan) equipped with an Olympus DP70 camera.
Preparation gelatin, nanoparticle of hydroxyapatite and nickle ion
After preparation gelatin 12%, we prepared a gel containing high (40%) and low dose (10%) of nano particle of HA. More information and the preparation method of this drug was characterized in previous study. Then combination of these percentage of HA nanoparticle with 500 ppm, 1000 ppm and 2000 ppm of nickel ion was prepared, which 1000 ppm of this ion has the most antibacterial effect than other doses. The under treatment groups were defined as control (only gel), HA 10%, HA 40%, HA 10% + Ni and HA 40%/Ni.
Animal study
This animal study has been approved in by Isfahan university of medical science with the ethic code: IR.MUI.MED.REC. 1398.338
The type and approach of this animal study is to create a deep skin wound and try to treat the wound with drug intervention on an animal model of rat. Experiments were performed on 30 wistar rats (220 ± 20 g) in accordance with the European communities council directive of 24 November 1986 (86/609/EEC). The rats were kept for a week in the animal nest of the biosafety laboratory of the department of biology, University of Isfahan, with suitable facilities to get used to the conditions of the nest. With the necessary facilities, the conditions of the nest environment with a suitable temperature of 22°C ± 2°C are maintained. In animal nests, a 12 hour cycle of darkness and light is established and the relative humidity is set at about 40%. The animal had free access to water and food for rats. All rats were distributed to five groups and all rats in a standard cage.
Animal group was classified as five groups including six rats in each group, containing control group, nano hydroxyapatite 10%, nanohydroxyapatite 40%, combination of nickle ion with nanohydroxyapatite 10% and 40%.
According to the IACUC guidelines, to cause wounds, animals are anesthetizing intraperitoneal with (ketamine 100 and xylazine 10 mg/kg) and for reducing the pain injection 300 mg/kg of acetaminophen intraperitoneal.
After anesthesia, the hair was shaved between the two shoulders, and the place was disinfected. Then by using a punch, a wound measuring 4 cm2 × 4 cm2 and a depth of 1 cm was applied remove all the skin layers (epidermis, dermis and hypodermis). We considered the surgery day as day zero and the day after the operation (the first day) based on the grouping of nanohydroxy gel and no additional dressing was used.
7 days and 14 days after wound induction and the treatment, we killed the animals and prepared a biopsy of their wound.
Biopsy was fixed in 10% formalin buffer for subsequent light microscopic examination. And processed by conventional methods for subsequent histology.
Macroscopic evaluation
After inducing the wounds, they were photographed on days 3, 7, and 14. A millimeter ruler was used as a reference wound area was measured in cm (length and width) using image j software.
Pathological examination
For pathological and histological examination of the wound, sampling was done on the seventh days and fourteenth days after ulcer induction. For skin sampling from the area around the wound with a margin of about 2 mm from the wound with a scalpel razor incision was made and the entire skin of the margin and wound was taken as a square with dimensions of 1 cm to 2 cm. The sample collected included both the central part and the healthy part of the wound margin.
Samples fixed in 10% formalin were sent to the pathology laboratory for slurry and staining. 5 micron sections were prepared and stained by hematoxylin and eosin.
To interpret the resulting images of the slides, we used the michrome camera and mosaic soft ward.
Statistical analysis
All continuous and categorical data are presented as mean ± Standard Deviation (SD) and frequency (percentage), respectively. Paired sample T-test was used for analysis of differences within groups and independent sample T-test and repeated measure Analysis of Variance (ANOVA) for analysis between groups in each time. Chi-squared test was used to compare categorical data between groups. A value of p ≤ 0.05 was considered statistically significant. All statistical analyses were conducted using the SPSS software version 15 (SPSS Inc., USA). The image j, michrome camera and mosaic soft ward was used for measurement of length and area of the wounds in macroscopic and microscopic study.
Results
Evaluation of cell proliferation
MTS assay was also carried out to evaluate the proliferation of mice fibroblast on the gelatin without hydroxyapatite, and with 10%, 40% hydroxyapatite after 1 day, 2 days and 3 days of culture. Significant enhancement of cell proliferation was observed in nano HA 10%, 40% and nano HA 10% with nickel in comparison when the cells seeded on gelatin and HA 10%. The best result was shown in 24 hours after seeding the cells in gelatin in comparison with 48 hours and 72 hours. Indeed, after 48 hours and 72 hours, the cell proliferation on gelatin decreased. In addition, significantly difference was observed under treatment groups when compared HA 10%/Ni, HA 40% and HA 10% with HA 10% during 24 hours after seeding cells. However, such results were shown only for HA 40% and HA 10% in comparison with HA 10% during 48 hours (Figure 1).
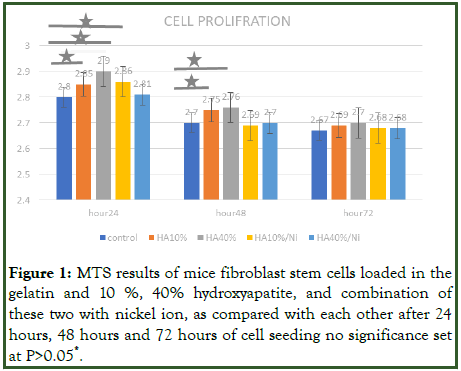
Figure 1: MTS results of mice fibroblast stem cells loaded in the gelatin and 10%, 40% hydroxyapatite, and combination of these two with nickel ion, as compared with each other after 24 hours, 48 hours and 72 hours of cell seeding no significance set at P>0.05*.
In cell proliferation the best effects (and significantly different with control) were in 24 hours after seeding the cells in gelatin in HA 40%. These effects were also seen in HA 10% and Ni 10% in compared with control, but the best effect was observed in HA 4% when compared with control group (Figure 2).
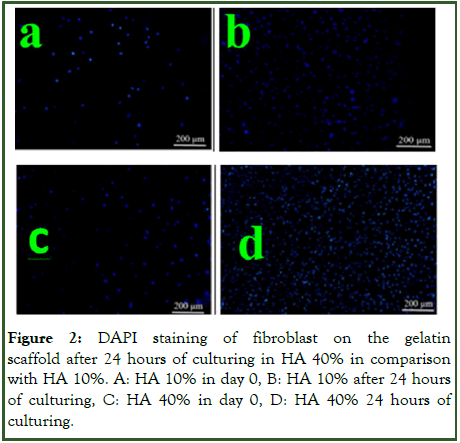
Figure 2: DAPI staining of fibroblast on the gelatin scaffold after 24 hours of culturing in HA 40% in comparison with HA 10%. A: HA 10% in day 0, B: HA 10% after 24 hours of culturing, C: HA 40% in day 0, D: HA 40% 24 hours of culturing.
Macroscopic study: In evaluation of wound area with image j soft ward, the wound area between 3rd day, 7th day and 14th day of treatment after wound induction there were no significant difference between groups (Figure 3 and Table 1).
| Day | Day 3 | Day 7 | Day 14 |
|---|---|---|---|
| Group (mean ± SD) | |||
| Control | 1.10 ± 0.28 | 0.86 ± 0.50 | 0.66 ± 0.01 |
| HA 10% | 0.95 ± 0.23 | 0.44 ± 0.27 | 0.42 ± 0.49 |
| HA 40% | 0.93 ± 0.18 | 0.30 ± 0.10 | 0.05 ± 0.05 |
| Ni/1HA 10% | 0.67 ± 0.09 | 0.21 ± 0.07 | 0.09 ± 0.07 |
| Ni/HA 40% | 0.74 ± 0.19 | 0.48 ± 0.06 | 0.63 ± 0.03 |
| Total | 0.88 ± 0.24 | 0.46 ± 0.30 | 0.25 ± 0.29 |
| P value | 0.064 | 0.265 | 0.157 |
Table 1: Comparative macroscopic study of mean and standard deviation of wound area in undertreated groups and control.
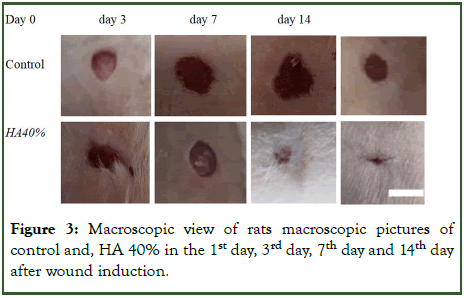
Figure 3: Macroscopic view of rats macroscopic pictures of control and, HA 40% in the 1st day, 3rd day, 7th day and 14th day after wound induction.
Microscopic study: In microscopic study and analysis for evaluation and comparing wound length with the michrome camera and mosaic soft ward, there were no significant relation in time (p1=0.77). There is a difference is close to significant between the groups (p2=0.065). There was no significant difference between time and group (p3=0.323). In day 14 the wound length between groups had significant difference (p4=0.049) (Table 2 and Figure 4).
| Wound size (mean ± SD) | ||||||
|---|---|---|---|---|---|---|
| Main effect interaction | ||||||
| Day 7 | Day 14 | P1 Time | P2 Group | P3 Time × Group | ||
| Treated group | HA 10% | 7228.9000 ± 881.01262 | 6681.4650 ± 3300.11685 | 0.776 | 0.065 | 0.323 |
| HA 40% | 6138.8350 ± 1313.51449 | 2479.8550 ± 662.83483 | ||||
| Ni/HA 10% | 5631.6100 ± 2722.14898 | 4034.2800 ± 2908.16049 | ||||
| Ni/HA 40% | 8778.3700 ± 1184.71499 | 15683.4800 | ||||
| Control group | 9552.15 | 5587.3450 ± 193.90989 | ||||
| P4 | 0.338 | 0.049 | ||||
Table 2: Summary of analysis of repeated variance for the average of wound size in treated and control group in microscopic view P1, P2 and P3 based on repeated measure ANOVA and P4 based on sample T-test, all variable is presented as mean ± SD.
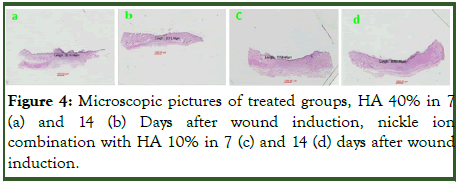
Figure 4: Microscopic pictures of treated groups, HA 40% in 7 (a) and 14 (b) Days after wound induction, nickle ion combination with HA 10% in 7 (c) and 14 (d) days after wound induction.
Discussion
In this study, in the cell culture study, in cell proliferation the best effects were reported in the first 24 hours and the best result were observed in microscopic and macroscopic evaluation the best result was observed in HA 40% and then HA 10%/Ni.
Hydroxyapatite has long been among the most studied biomaterials in the medical field for both its proven biocompatibility and for being the main constituent of the mineral part of bone and teeth. Hydroxyapatite is also an important source of calcium and phosphate, very important for the remineralization of demineralized enamel areas [24]. HA also uses for wound healing. Healing of skin wounds involves a cascade of molecular cellular events including inflammation, angiogenesis, migration, cell proliferation, and regeneration of damaged tissues. After injury, various phenomena occur that cause tissue reconstruction and repair. This dynamic process requires the coordination of epidermal cells, arteries, fibroblasts, inflammatory cells, and extracellular matrix. Hydroxyapatite with the formula Ca10(PO4)6(OH)2 known as hydroxyapatites, as one of the biocompatible and bioactive substances containing calcium, is known for use in regenerating damaged tissues. The more calcium ions released from HA, the better the regulation of skin homeostasis, proliferation and differentiation of skin cells. The effect of HA is to reduce the size of the wound by releasing calcium ions, which is one of the important criteria for wound healing [25].
In an article which used hydroxyapatites reported morphological, antibacterial, and cell attachment of cellulose acetate nanofibers containing modified hydroxyapatite for wound healing utilizations [26]. Another study was showed the vascularization effect on the human wound healing and accelerated the re-epithelialization of the cutaneous wounds and decreasing the wound size with using HA as a novel dressing for wound healing [27].
HA has a structure very similar to bone structure, so it is often preferred to other biocompatible materials for orthopedic treatment. There have been many studies on the use of HA in medicine in various fields. HA, for example, has been used for bone repair, cartilage regeneration, and as an implant coating due to its hard tissue structure [28]. There have been many studies on HA and bone tissue engineering [29,30]. Since HA has a stimulating effect on angiogenesis and cell proliferation, HA is also used in soft tissue engineering [31]. The ionic products released by the HA reaction with the surrounding tissues are thought to cause an initial inflammatory reaction, which can accelerate wound healing and result in more satisfactory healing. [32]. Recent studies on HA have also identified primary inflammatory reactions due to it, which is considered as the first stage of wound healing [33]. In addition, in another study, HA was used in combination with other substances, including silk fibrin, to treat skin wounds [34].
Some studies reported that the nano particle of hydroxyapatites is better than HA. Nano particle of HA is the inorganic component of bone or teeth is nanocrystalline HA, which provides the toughness and ability to withstand pressure [35,36]. The nano-hydroxyapatite has a strong ability to bond with proteins, as well as with fragments of plaque and bacteria. New articles showed that nano particle of HA can exactly increase the wound rehabilitation. The calcium phosphate is stacked and aligned with the organic matrix formed by collagen fibers, glycoproteins, and mucopolysaccharides for conferring the elasticity and resistance to the structure. Some studies used it as a human bone as an organic inorganic composite having the components of collagen fibrils containing embedded and well disposed, nanocrystalline Hydroxyapatite (HA) [37]. Nanocrystalline HA powders exhibit improved sinter ability and enhanced densification due to greater surface area, which may improve fracture toughness, as well as other mechanical properties [38]. Moreover, nano-HA, compared to coarser crystals, is expected to have better bioactivity [39]. These reported properties make it better than primitive HA.
In this study, in addition to using nano particle of HA, we also used a combination with nickel to understand whether they had synergism effects on increasing wound healing or not. It was done for the first time and we don’t have any similar study to compare. However, in a study which used Ni, was reported that when it was doped with HA, it would significantly increase the rehabilitation and wound healing. It seems that it’s because of antiapoptotic and also synergic effects with HA. In compare we discuss that the HA results in wound rehabilitation was the best but the combination of HA/Ni effects were competitable in clinic and treatment not only in the level of in vitro but in in vivo.
Our study has the limitation if sample size. Therefore, for further studies and further review of the results, we need a larger sample size and a larger study to be able to net the results.
Conclusion
In conclusion, hydroxyapatites and its combination with nickle ion have significant effect on wound healing and cell proliferation.
Ethics Approval and Consent to Participate
This research has been performed in accordance with the declaration of Helsinki and has been approved by the ethics committee of Isfahan University of medical Sciences. The animal study has been approved in by Isfahan University of medical science with the ethic code: IR.MUI.MED.REC. 1398.338.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of Interest
The authors declared there is no conflict of interest.
Funding
Financial support and sponsorship: The project was supported financially by the Isfahan University of medical Sciences for the design of the study and collection, analysis, and interpretation of data (Research project number: IR.MUI.MED.REC. 1398.338).
Acknowledgements
We would like to thank the colleagues and personnel of the department of pathology, the clinical toxicology department and laboratory of faculty of pharmacy.
Author’s Contribution
Conception and design
Rokhsareh Meamar and Sahar sadat Lalehzar
Data collection
Rokhsareh Meamar, Sahar Sadat Lalehzar, Ardeshir Talebi, Mehrafarin Fesharaki
Data analysis and interpretation
Rokhsareh Meamar, Ardeshir Talebi, Mehrafarin Fesharaki
Writing the original draft
Sahar sadat Lalehzar, Rokhsareh Meamar,
All authors approved the final version of the manuscript prior to submission and have agreed to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
References
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738-746.
[Crossref] [Google Scholar] [PubMed]
- Daley J, Khuri SF, Henderson W, Hur K, Gibbs JO, Barbour G, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: Results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):328-340.
[Crossref] [Google Scholar] [PubMed]
- Takeo M, Lee W, Ito M. Wound healing and skin regeneration. C Cold Spring Harb Perspect Med. 2015;5(1):a023267.
[Crossref] [Google Scholar] [PubMed]
- Clark RA. The molecular and cellular biology of wound repair. Springer Science and Business Media. 2013.
- Liang J, Cui L, Li J, Guan S, Zhang K, Li J. Aloe vera: A medicinal plant used in skin wound healing. Tissue Eng Part B Rev. 2021;27(5):455-474.
[Crossref] [Google Scholar] [PubMed]
- Cruz MA, Araujo TA, Avanzi IR, Parisi JR, de Andrade AL, Rennó AC. Collagen from marine sources and skin wound healing in animal experimental studies: A systematic review. Mar Biotechnol (NY). 2021;23(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Xie G, Zhou N, Gao Y, Du S, Du H, Tao J, et al. On-demand release of CO2 from photothermal hydrogels for accelerating skin wound healing. Chem Eng J. 2021;403:126353.
- Xiao WR, Wu M, Bi XR. Ozone oil promotes wound healing via increasing miR-21-5p-mediated inhibition of RASA1. Wound Repair Regen. 2021;29(3):406-416.
[Crossref] [Google Scholar] [PubMed]
- Qianqian O, Songzhi K, Yongmei H, Xianghong J, Sidong L, Puwang L, et al. Preparation of nano-hydroxyapatite/chitosan/tilapia skin peptides hydrogels and its burn wound treatment. Int J Biol Macromol. 2021;181:369-377.
[Crossref] [Google Scholar] [PubMed]
- Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267.
[Crossref] [Google Scholar] [PubMed]
- Munaron L, Pla AF. Endothelial calcium machinery and angiogenesis: Understanding physiology to interfere with pathology. Curr Med Chem. 2009;16(35):4691-4703.
[Crossref] [Google Scholar] [PubMed]
- Li M, Ke QF, Tao SC, Guo SC, Rui BY, Guo YP. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B. 2016;4(42):6830-6841.
[Crossref] [Google Scholar] [PubMed]
- Yu W, Jiang YY, Sun TW, Qi C, Zhao H, Chen F, et al. Design of a novel wound dressing consisting of alginate hydrogel and simvastatin-incorporated mesoporous hydroxyapatite microspheres for cutaneous wound healing. RSC Adv. 2016;6(106):104375-104387.
- Kawai K, Larson BJ, Ishise H, Carre AL, Nishimoto S, Longaker M, et al. Calcium-based nanoparticles accelerate skin wound healing. PLoS One. 2011;6(11):e27106.
[Crossref] [Google Scholar] [PubMed]
- JE L, BM B. Blood clotting; The inhibition by anions and the function of calcium. Biochem J. 1951;48(3):34.
[Google Scholar] [PubMed]
- Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence of events. Toxicol Pathol. 2007;35(6):767-779.
[Crossref] [Google Scholar] [PubMed]
- de Wilde EA, Jimbo R, Wennerberg A, Naito Y, Coucke P, Bryington MS, et al. The soft tissue immunologic response to hydroxyapatite-coated transmucosal implant surfaces: A study in humans. Clin Implant Dent Relat Res. 2015;17(Suppl 1):e65-e74.
[Crossref] [Google Scholar] [PubMed]
- Miguez-Pacheco V, Hench LL, Boccaccini AR. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015;13:1-5.
[Crossref] [Google Scholar] [PubMed]
- Galindo-Ferreiro A, Elkhamary SM, Maktabi A, Galvez-Ruiz A, Schellini SA. Chronic orbital inflammation associated to hydroxyapatite implants in anophthalmic sockets. Case Rep Ophthalmol. 2017;8(3):5745-80.
[Crossref] [Google Scholar] [PubMed]
- Li B, Lei Y, Hu Q, Li D, Zhao H, Kang P. Porous copper-and lithium-doped nano-hydroxyapatite composite scaffold promotes angiogenesis and bone regeneration in the repair of glucocorticoids-induced osteonecrosis of the femoral head. Biomed Mater. 2021;16(6):065012.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Tan Y, Wu J. Rubidium doped nano-hydroxyapatite with cytocompatibility and antibacterial. J Asian Ceram Soc. 2021;9(1):323-333.
- Alshemary AZ, Akram M, Goh YF, Tariq U, Butt FK, Abdolahi A, et al. Synthesis, characterization, in vitro bioactivity and antimicrobial activity of magnesium and nickel doped silicate hydroxyapatite. Ceram Int. 2015;41(9):11886-11898.
- Sinulingga K, Sirait M, Siregar N, Abdullah H. Synthesis and characterizations of natural limestone-derived nano-hydroxyapatite (HAp): A comparison study of different metals doped HAps on antibacterial activity. RSC Adv. 2021;11(26):15896-15904.
[Crossref] [Google Scholar] [PubMed]
- Pepla E, Besharat LK, Palaia G, Tenore G, Migliau G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann Stomatol (Roma). 2014;5(3):108-114.
[Google Scholar] [PubMed]
- Broughton GII, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7S):12S-34S.
[Crossref] [Google Scholar] [PubMed]
- Elsayed MT, Hassan AA, Abdelaal SA, Taher MM, Ahmed KM, Shoueir KR. Morphological, antibacterial, and cell attachment of cellulose acetate nanofibers containing modified hydroxyapatite for wound healing utilizations. J Mater Res Technol. 2020;9(6):13927-13936.
- Namasivayam SK, Venkatachalam G, Bharani RA. Noteworthy enhancement of wound-healing activity of triphala biomass metabolite-loaded hydroxyapatite nanocomposite. Appl Nanosci. 2021;11:1511-1530.
- Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol. 2016;164:379-385.
[Crossref] [Google Scholar] [PubMed]
- Sutha M, Sowndarya K, Chandran M, Yuvaraj D, Bharathiraja B, Kumar PR. Synthesis of value added biomimetic material of hydroxyapatite using aqueous calcareous fish wastes. Waste to wealth: Springer, Singapore. 2018:59-64.
[Crossref] [Google Scholar] [PubMed]
- Aguirre A, Gonzalez A, Navarro M, Castano Linares O, Planell Estany JA, Engel Lopez E. Control of microenvironmental cues with a smart biomaterial composite promotes endothelial progenitor cell angiogenesis. Eur Cell Mater. 2012;24:90-106.
[Crossref] [Google Scholar] [PubMed]
- Arkudas A, Balzer A, Buehrer G, Arnold I, Hoppe A, Detsch R, et al. Evaluation of angiogenesis of bioactive glass in the arteriovenous loop model. Tissue Engineering Part C: Methods. 2013;19(6):479-486.
[Crossref] [Google Scholar] [PubMed]
- Gerhardt LC, Boccaccini AR. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials (Basel). 2010;3(7):3867-3910.
[Crossref] [Google Scholar] [PubMed]
- Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27(17):3249-3255.
[Crossref] [Google Scholar] [PubMed]
- Leu A, Leach JK. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm Res. 2008;25:1222-1229.
[Crossref] [Google Scholar] [PubMed]
- Zhao X, Ng S, Heng BC, Guo J, Ma L, Tan TT, et al. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch Toxicol. 2013;87:1037-1052.
[Crossref] [Google Scholar] [PubMed]
- Shi Z, Huang X, Cai Y, Tang R, Yang D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009;5(1):338-345.
[Crossref] [Google Scholar] [PubMed]
- Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7(7):2769-2781.
[Crossref] [Google Scholar] [PubMed]
- LeGeros RZ. Biodegradation and bioresorption of calcium phosphate ceramics. Clin Mater. 1993;14(1):65-88.
[Crossref] [Google Scholar] [PubMed]
- Stupp SI, Ciegler GW. Organoapatites: Materials for artificial bone. I. Synthesis and microstructure. J Biomed Mater Res. 1992;26(2):169-183.
[Crossref] [Google Scholar] [PubMed]
Citation: Lalehzar SS, Meamar R, Talebi A, Fesharaki M (2023) Evaluation of the Effectiveness of Nano-Hydroxyapatite Particles in Wound Healing in an Animal Study. Bio Med. 15:536.
Copyright: © 2023 Lalehzar SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


