Indexed In
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 9, Issue 3
Enhancing Virtual Colonoscopy: Exploring Electronic Colon Cleansing Strategies and Methodologies
Mehrnaz Mostafavi1* and Mahtab Shaabani22Department of Medical Science, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Received: 27-Dec-2023, Manuscript No. JCMS-23-24511; Editor assigned: 29-Dec-2023, Pre QC No. JCMS-23-24511 (PQ); Reviewed: 12-Jan-2024, QC No. JCMS-23-24511; Revised: 03-Apr-2025, Manuscript No. JCMS-23-24511 (R); Published: 10-Apr-2025
Abstract
The rapid advancements in Computed Tomography (CT) technology have positioned CT Colonography (CTC), commonly known as Virtual Colonoscopy (VC), as a promising tool for early detection of colon cancer. Despite more than 57,000 annual deaths in the United States attributed to colon cancer, CTC stands out as a recommended radiological examination for diagnosing colorectal neoplasia, especially in cases of incomplete or contraindicated colonoscopies. This method involves low-dose CT scans of the cleansed and distended colon in both supine and prone positions. This paper provides a comprehensive insight into CTC procedures and discusses key aspects of Electronic Colon Cleansing (ECC), aiming to segment the colon lumen from CT images with an oral contrast agent. The study outlines various ECC algorithms, their advantages, drawbacks, and technological intricacies extracted from reviewed literature. ECC plays a pivotal role in processing CT data, particularly in cleansing the colon's interior for effective polyp identification, a crucial step in virtual colonoscopy. The paper delves into multiple ECC methodologies, including thresholding, Markov random field models, segmentation using segmentation rays, and non-linear transfer functions combined with morphological operations. These methodologies vary in their approaches, performance, and computational requirements. For instance, while thresholding offers speed, it struggles with partial voxel segmentation and sensitivity to threshold alterations. In contrast, the Markov random field model integrated into the Expectation Maximization (EM) algorithm simultaneously estimates model parameters and segments voxels. Additionally, segmentation rays accurately detect partial volume regions and allow for their removal, enhancing accuracy in colon cleansing. The paper also discusses non-linear transfer functions and morphological operations, amalgamating two techniques to boost the precision of colon cleansing. This detailed analysis aims to present a nuanced understanding of ECC techniques, providing researchers with a comprehensive overview of these methodologies. The insights derived from this paper contribute to the ongoing exploration of effective ECC strategies for optimizing virtual colonoscopy and improving colon cancer screening outcomes.
Keywords
Electronic colon cleansing; Virtual colonoscopy; Polyp detection; CT colonography
Introduction
CT Colonography (CTC), also recognized as Virtual Colonoscopy (VC), has emerged as a promising approach for early colon cancer detection due to advancements in Computed Tomography (CT) technology [1-3]. Despite over 57,000 annual colon cancer deaths in the United States, CTC is highly recommended for diagnosing colorectal neoplasia, particularly in cases of incomplete or contraindicated colonoscopies [4,5]. This radiological method involves a low-dose, thin-section CT scan capturing a cleansed and distended colon in both supine and prone positions [6].
The procedure of CTC involves multiple phases, including colon cleansing and inflation with CO2, followed by a helical abdominal CT scan covering the entire colon [7,8]. This scan produces numerous 512 × 512 slices, which are reconstructed into a 3D volume ranging from 100-250 MB [9]. Subsequently, this volumetric data undergoes preprocessing stages crucial for virtual navigation, with the precise segmentation of the colon lumen being paramount. Virtual navigation within the colon's interior, crucial for polyp detection, is facilitated by meticulous volume rendering. The initial step in CT data processing, known as Electronic Colon Cleaning (ECC), becomes essential, particularly if other methods fail to extract fecal contents [10].
Both modern colonoscopy techniques, including virtual colonoscopy, necessitate a pristine colon lumen for accurate polyp identification, preventing misinterpretation of residual materials as part of the colon. Various computer algorithms have been proposed for ECC, aiming to eliminate voxels reflecting contrast and residual nutrients. These algorithms vary in image pre processing steps, local image characteristics, feature reduction procedures, applied modeling, segmentation techniques, and classification methods [11]. This paper seeks to concisely outline the strengths, weaknesses, and technical nuances of these ECC methods.
Moreover, segmentation rays demonstrate remarkable prowess in accurately identifying partial volume regions and facilitating their elimination, thereby augmenting precision in colon cleansing. The paper further navigates through non-linear transfer functions and morphological operations, amalgamating these techniques to enhance the precision of colon cleansing. This comprehensive analysis endeavors to furnish researchers with an intricate understanding of ECC techniques, thereby contributing significantly to the quest for effective strategies in optimizing virtual colonoscopy and ameliorating outcomes in colon cancer screening.
Materials and Methods
Thresholding
The thresholding operation principle depends on the division of voxels into two classes that use a predefined threshold value.
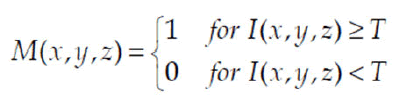
Where: M(x,y,z) is a binary mask (1-indicates an object, in this case a colon, 0-background), and I(x,y,z) indicates a CT. Typically, the threshold value is calculated on the basis of a CT histogram or general knowledge of the values assigned to anatomical structures.
The reduced resolution of the detectors and the soft property of the reconstruction kernel is the product of a boundary between contrast and tissues (Figure 1), undesirable but typically acquired after threshold operation.
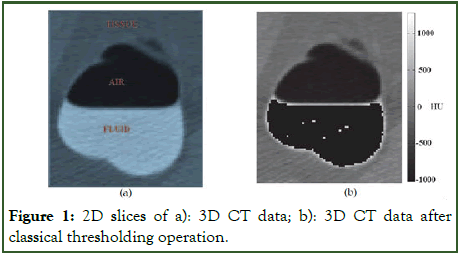
Figure 1: 2D slices of a): 3D CT data; b): 3D CT data after classical thresholding operation.
While thresholding is recognized for its speed, it accompanies several notable limitations. Firstly, this method fails to eliminate partial voxel content, as depicted in Figure 1b, where the strength profile along a vertical line reveals voxels with intensities straddling two distinct regions. These voxels are problematic as they are inaccurately classified during thresholding. For instance, in Figure 2, the voxels lie within the soft-tissue range between fluid and air, making their removal as soft-tissue voxels impossible. This misclassification notably impacts segmentation accuracy [12].
Moreover, although high-density fluid or stool is successfully removed, residual artifacts, such as a thin soft-tissue-like boundary, persist where it shouldn't exist.
Secondly, the sensitivity of thresholds to slight variations across different intensity spectrums, especially concerning the inner surface contour of the colon, significantly influences segmentation outcomes. Altering these thresholds can lead to varying segmentation results, making the method highly sensitive and prone to changes in outcomes.
Additionally, despite successfully removing high-density fluid or stool, thresholding often results in aliasing effects at the inner colon boundary. Upon closer inspection of the segmented volume, notable shifts in pressure values from soft tissue to air ranges become apparent, presenting undesired outcomes from a volume rendering perspective.
Furthermore, this approach causes the loss of the thin colonic mucosa present on the inner surface, leading to the creation of sharp borders that obstruct the diagnosis of polyps. Consequently, removing this colonic mucosa becomes undesirable, posing a challenge in accurately identifying and diagnosing polyps.
Results and Discussion
Markov random field model
Image segmentation: The algorithm is based on the framework of Maximum Posterior Probability (MAP), including a priori neighborhood membership information defined by the Randomly Filed Markov (MRF) theory.
It iteratively estimates the model parameters in an interleaved manner through the Expectation Maximization (EM) algorithm and segments the voxels by MAP, converging to a solution where the parameters of the model and voxel labels are estabilized within a given criterion. The algorithm's implementation consists of two steps: Parameter estimation and segmentation of the MAP [13].
Initial parameters: The algorithm operates under the framework of Maximum Posterior Probability (MAP) and incorporates a priori neighborhood membership details outlined by the theory of Randomly Filed Markov (MRF). It undergoes iterative estimation of model parameters using the Expectation Maximization (EM) algorithm, interleaved with voxel segmentation via MAP. This iterative process converges to a solution where model parameters and voxel labels stabilize within a specified criterion. The algorithm's execution involves two primary steps: Initial parameter estimation and MAP segmentation.
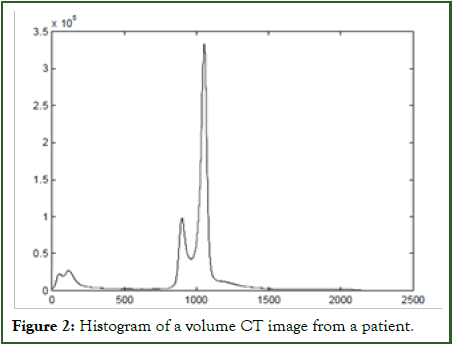
The histogram depicted in Figure 2 represents a standard histogram extracted from a patient's abdominal CT image, delineating distinct features present in all abdominal CT scans. Three prominent peaks in the histogram correspond to the intestine's air, soft tissue, and muscle, while other elements like strengthened stool, fluid, and bone manifest as a smaller peak beyond the muscle range. Notably, partial volume effects are notable between air and soft tissue, as well as between muscle and simulated products within these pressure ranges.
Traditionally, initial parameter estimates were derived from histograms using threshold-based methods, which were somewhat heuristic. However, an alternative approach, involving online vector quantization, was proposed. This technique relies on primary component analysis for local feature vectors of each voxel, followed by classification into classes based on the nearest neighbor norm. This novel method stands out due to its independence from initial values. As part of this initial parameter calculation, the vector quantization approach offers a preliminary segmentation outcome. It then proceeds to concentrate on segmenting the tagged stool/fluid area, leveraging a hidden MRF model to address issues of non-uniformity [14,15].
Figure 2: Histogram of a volume CT image from a patient.
Parameter estimation: Assume that an image consists of L classes (or tissue types) and each class l is characterized by a Gaussian parameter vector θ1(μ1, ν1). Let ρ1(Yijk | θ1) be the probability distribution of voxel Yijk which is associated with class l. The likelihood for each voxel Yijk, falling into L distinct classes, is described by a mixture functional as
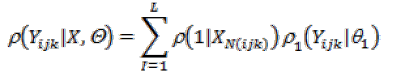
Where ρ(1|XN(ijk)) is the locally-dependent probability when Xijk is equal to l, and XN(ijk) reflects the labels of thosenearby voxels.
Assuming a set of initial estimation {μ(0), ν1 (0)}L I=1 and applying the EM algorithm, that have, at each iteration n,
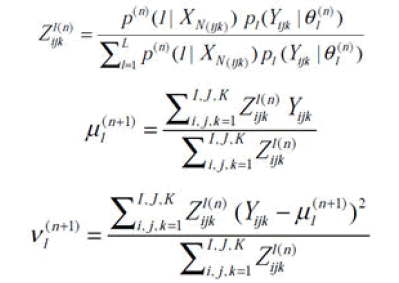
Where Z1(n) ijk is the conditional probability that voxel Yijk belongs to class l, which represents the tissue percentages withinthat voxel. The calculation of tissue percentages {Z1(n) ijk} within each voxel requires determination of the conditional probability ρ(n)(1|X(ijk)) at the nth iteration. The determination of ρ(n)(1|X(ijk)) requires estimation of the classlabels {l}, which can be obtained by the following MAP segmentation method.
MAP segmentation method: A MRF prior is constructed to reflect the neighborhood information. The assignment of labels over the voxel array is performed by the MAP criterion. A Markov random field prior can be constructed to reflect the neighborhood information by.
To represent the neighborhood details, a prior MRF is constructed. The MAP criteria executes the distribution of labels over the voxel series [16,17]. A prior Markov random field can be constructed to represent the neighborhood data by
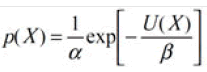
Where α is a constant of normalization and b is a constant parameter. The U(X) energy function defines the degree of penalty levied on the neighbors and, in three dimensions, can be specified as
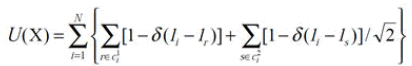
Where δ(0)=1, the voxel names of the neighbors are δ((≠0)=0 and {li}. The r index runs over the 6 neighbors of the first order and s runs over the 12 neighbors of the second order.
The segmentation is carried out iteratively, where at the (n+1)th iteration, the current voxel is allocated to the corresponding tissue type l by,
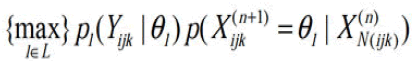
Segmentation using segmentation rays
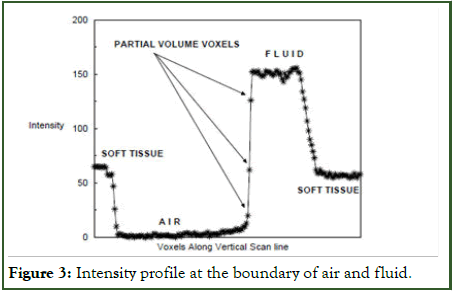
Need for a sophisticated segmentation: Following the recent bowel preparation, the acquired CT data is notably more intricate compared to that obtained from an uncleansed bowel. This complexity arises from the substantial accumulation of fluid and stool within the colon (Figure 3). Despite efforts to address these unwanted residues, their lack of a distinct boundary persists, attributed to the partial volume effect.
Moreover, this issue is exacerbated by the finite resolution of the CT scanner and its inadequate contrast capabilities. This renders naive segmentation methods impractical due to their inability to effectively address these challenges.
Figure 3: Intensity profile at the boundary of air and fluid.
This approach's core concept lies in identifying a unique property existing at the junction of regions with distinct densities. As it moves towards this junction, it encounters the Partial Volume Effect (PVE) voxels present in this transition. By recognizing these voxels and removing them accurately, the method aims to address this challenge.
The approach involves a preliminary analysis and storage of individual strength profiles specific to various intersections. These profiles are characteristic of the region intersections and remain consistent across different CT scanning protocols. The subsequent step involves identifying intersections by comparing intensity profiles along cast rays with those of various intersections. When a match occurs, the ray successfully detects the intersection.
Further refinement of this algorithm prevents ray casting where no intersection is expected, leveraging the presence of air within the colon. This is accomplished by outlining the rough contour of the colonic interior using air voxels from a seed point within the colon. The algorithm continues its tasks until a ray detects an intersection. This typically involves the extraction and processing of PVE voxels at the intersection, either through classification or by altering their strength values to reconstruct the mucosa. Consequently, most PVE voxels are identified and removed using this method.
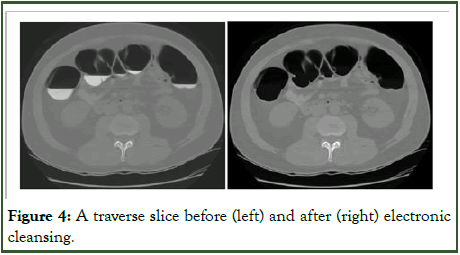
Additionally, the algorithm applies volumetric contrast enhancement to residual PVE voxels found at the fluid-soft tissue intersection, a technique akin to contrast enhancement in image processing (Figure 4). This process facilitates mucosal regeneration and extraction of residual fluid from the colon, compensating for areas missed due to high fluid density [18].
Figure 4: A traverse slice before (left) and after (right) electronic cleansing.
Using non-linear transfer function and morphological operations
The proposed ECC method relies on a combination of nonlinear value adjustments and the manipulation of morphological voxels. Additionally, if the CT data lacks offset Hounsfield Unit (HU) values, an approach involves augmenting voxel values by 1024 to obtain an unsigned 16-bit fixed-point integer data format, effectively reducing computational time [19,20].
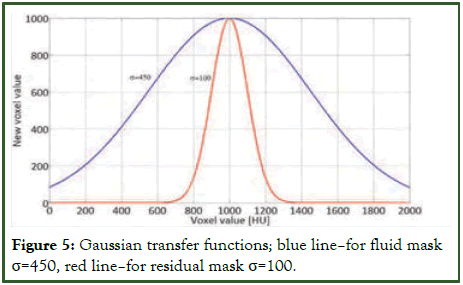
Identification and removal of contrast-reflecting voxels in the CT data require the computation of two binary masks: A fluid mask and a residual mask. The fluid mask, created through the thresholding process outlined in section 3, assigns a value of 1 to voxels with values exceeding 1600. Similarly, the residual mask targets values greater than 1350 and equal to or smaller than 1600, encompassing voxels representing stool and fluid within this range. Subsequently, dilation is applied to all masks using regular hexahedrons of size 3. Voxels marked with a mask value of 1 undergo processing via two transfer functions illustrated in Figure 5, demonstrating a Gaussian transformation of intensity.
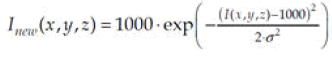
With σ=450 for the fluid mask and 100 for the residual one. This operation is desirable due tonecessity to keep smooth changes of intensity on the border between colon and soft tissues.
Figure 5: Gaussian transfer functions; blue line–for fluid mask σ=450, red line–for residual mask σ=100.
In the subsequent step, a binary dataset (MBin) is generated, assigning 0s to air voxels (v<300) and 1s to the remaining sections. Subsequently, a sequence of two morphological procedures is implemented: Firstly, a 3D erosion operation is applied to each volume utilizing a three-cubic matrix, followed by a dilation process on the resulting data from the erosion step.
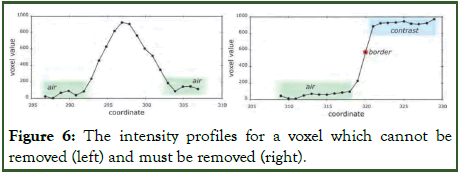
Additionally, it is imperative to validate whether the voxels acquired from the subtraction belong to the boundary. Given the patient's positioning either on their back or abdomen during CT scanning, the boundary often aligns parallel to the body's surface (Figure 6). The entire procedure is summarized by the following theorem [21,22].
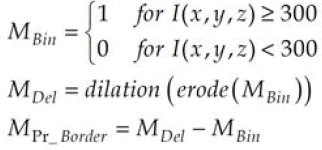
Figure 6: The intensity profiles for a voxel which cannot be removed (left) and must be removed (right).
After subtraction, with each voxel equal to 1 in the MPr-Border volume, the strength profile must be tested in the usual direction of the body surface (Figure 6): If the profile includes voxels belonging to the stool or comparison, this voxel is omitted.
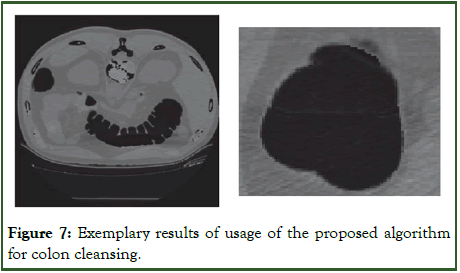
Figure 7 demonstrates excellent performance from the implementation of the proposed algorithm for electronic colon cleansing.
Figure 7: Exemplary results of usage of the proposed algorithm for colon cleansing.
Conclusion
The landscape of medical imaging has been revolutionized by the rapid advancements in Computed Tomography (CT) technology. Within this realm, CT Colonography (CTC), also known as Virtual Colonoscopy (VC), has emerged as a promising non invasive tool for the early detection of colon cancer. Despite the staggering toll of over 57,000 annual deaths attributed to colon cancer in the United States alone, CTC has emerged as a recommended radiological examination, particularly in scenarios where conventional colonoscopies remain incomplete or are contraindicated. This modality revolves around the acquisition of low-dose CT scans capturing the purified and distended colon in both supine and prone positions.
This paper endeavors to provide an exhaustive exploration into CTC procedures while delving into the critical domain of Electronic Colon Cleansing (ECC). ECC assumes a pivotal role in the segmentation of the colon lumen from CT images utilizing an oral contrast agent. This study meticulously scrutinizes diverse ECC algorithms, meticulously extracting their advantages, limitations, and technological intricacies from an extensive review of relevant literature. ECC assumes paramount importance in the processing of CT data, particularly in ensuring a pristine interior of the colon for accurate identification of polyps, a fundamental requirement in virtual colonoscopy.
The manuscript meticulously dissects a spectrum of ECC methodologies, encompassing thresholding, Markov random field models, segmentation employing segmentation rays, and the fusion of non-linear transfer functions with morphological operations. These methodologies exhibit distinct approaches, performances, and computational requisites. For instance, while thresholding emphasizes speed, it grapples with challenges in segmenting partial voxels and susceptibility to alterations in thresholds. Conversely, the incorporation of the Markov random field model within the Expectation Maximization (EM) algorithm facilitates concurrent estimation of model parameters and voxel segmentation, showcasing promising potential.
Moreover, segmentation rays demonstrate remarkable prowess in accurately identifying partial volume regions and facilitating their elimination, thereby augmenting precision in colon cleansing. The paper further navigates through non-linear transfer functions and morphological operations, amalgamating these techniques to enhance the precision of colon cleansing. This comprehensive analysis endeavors to furnish researchers with an intricate understanding of ECC techniques, thereby contributing significantly to the quest for effective strategies in optimizing virtual colonoscopy and ameliorating outcomes in colon cancer screening.
The paper systematically unfolds the advantages and drawbacks of each ECC approach, illuminating the trade-offs inherent in these methodologies. Thresholding emerges as the fastest and simplest method, yet it faces challenges in handling partial voxels and exhibits sensitivity to intensity thresholds. Contrastingly, the integration of Markov random field models within the EM algorithm allows for simultaneous estimation of model parameters and voxel segmentation, leveraging spatial knowledge to address non-uniformity issues. Segmentation rays excel in accurately detecting and removing partial volume regions, while the fusion of non-linear transfer functions and morphological operations enhances the accuracy of colon cleansing.
The ECC approaches in virtual colonoscopy offer distinct advantages, each contributing uniquely to the accuracy and efficiency of the process. Thresholding stands out as the fastest and simplest method among the ECC strategies. Despite its speed, it faces challenges in handling partial voxels and sensitivity to intensity thresholds.
The integration of Markov random field models within the EM algorithm presents a significant advancement. This integration allows for the concurrent estimation of model parameters while segmenting voxels. This approach harnesses spatial knowledge effectively to address non-uniformity issues, enhancing the accuracy of colon cleansing significantly. Segmentation rays emerge as a powerful tool by accurately detecting partial volume regions within the colon. Their capability to identify and remove these regions, if necessary, marks a significant leap in precision, ensuring a clearer and more accurate depiction of the colon's interior.
Lastly, the fusion of non-linear transfer functions with morphological operations signifies a sophisticated enhancement in accuracy. By combining these methods, there's a notable increase in the precision of colon cleansing, improving the overall effectiveness of virtual colonoscopy in detecting polyps and abnormalities within the colon.
In conclusion, each ECC approach offers unique advantages, and their comparative analysis illuminates the ongoing quest for an optimized virtual colonoscopy method. This systematic exploration sets the stage for further advancements in ECC strategies and paves the way for improved colon cancer screening methodologies.
References
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50(1):7-33.
[Crossref] [Google Scholar] [PubMed]
- Harchegani HB, Mostafavi M, Shaabani M. Novel algorithm for image processing to detect cancerous morphology. Int J Anal Pharm Biomed Sci. 2015:161-165.
- ACS. Global Cancer: Facts and figures. 4th Ed. American Cancer Society, Inc., Atlanta, United States. 2003.
- Mostafavi M, Ghanavi J. Nanotechnology in proteomics: Current status, promises and challenges. Arch Adv Biosci. 2012;3(4).
- Yoshida H, NaÌ?ppi J, MacEneaney P, Rubin DT, Dachman AH. Computer-aided diagnosis scheme for detection of polyps at CT colonography. Radiographics. 2002;22(4):963-979.
[Crossref] [Google Scholar] [PubMed]
- Savareh BA, Bashiri A, Mostafavi M. Neighborhood matrix: A new idea in matching of two dimensional gel images. PBioSci. 2017;6(2):129-137.
- Ristvedt SL, McFarland EG, Weinstock LB, Thyssen EP. Patient preferences for CT colonography, conventional colonoscopy, and bowel preparation. Am J Gastroenterol. 2003;98(3):578-585.
[Crossref] [Google Scholar] [PubMed]
- Lefere PA, Gryspeerdt SS, Dewyspelaere J, Baekelandt M, van Holsbeeck BG. Dietary fecal tagging as a cleansing method before CT colonography: Initial results-polyp detection and patient acceptance. Radiology. 2002;224(2):393-403.
[Crossref] [Google Scholar] [PubMed]
- Vining DJ. Technical feasibility of colon imaging with helical CT and virtual reality. Proc. Annual Meeting of American Roentgen Ray Society, 1994. 1994.
- Wang Z, Liang Z, Li X, Li L, Li B, Eremina D, et al. An improved electronic colon cleansing method for detection of colonic polyps by virtual colonoscopy. IEEE Trans Biomed Eng. 2006;53(8):1635-1646.
[Crossref] [Google Scholar] [PubMed]
- Cai W, Lee JG, Zalis ME, Yoshida H. Mosaic decomposition: An electronic cleansing method for inhomogeneously tagged regions in noncathartic CT colonography. IEEE Trans Med Imaging. 2010;30(3):559-574.
[Crossref] [Google Scholar] [PubMed]
- Lakare S, Chen D, Li L, Kaufman AE, Liang Z. Electronic colon cleansing using segmentation rays for virtual colonoscopy. Medical Imaging 2002: Physiology and Function from Multidimensional Images, San Diego, United States. 2002;4683:412-418.
- Chen D, Wax MR, Li L, Liang Z, Li B, Kaufman AE. A novel approach to extract colon lumen from CT images for virtual colonoscopy. IEEE Trans Med Imaging. 2000;19(12):1220-1226.
[Crossref] [Google Scholar] [PubMed]
- Li L, Chen D, Lu H, Liang Z. Segmentation of brain MR images: A self-adaptive online vector quantization approach. Medical Imaging 2001: Image Processing, San Diego, United States. 2001;4322:1431-1438.
- Leahy R, Hebert T, Lee R. Applications of Markov random fields in medical imaging. Prog Clin Biol Res. 1991;363:1-4.
[Google Scholar] [PubMed]
- Held K, Kops ER, Krause BJ, Wells WM, Kikinis R, Muller-Gartner HW. Markov random field segmentation of brain MR images. IEEE Trans Med Imaging. 1997;16(6):878-886.
[Crossref] [Google Scholar] [PubMed]
- Liang Z, MacFall JR, Harrington DP. Parameter estimation and tissue segmentation from multispectral MR images. IEEE Trans Med Imaging. 1994;13(3):441-449.
[Crossref] [Google Scholar] [PubMed]
- Lakare S, Wan M, Sato M, Kaufman A. 3D digital cleansing using segmentation rays. Proceedings Visualization 2000. VIS 2000 (Cat. No.00CH37145), Salt Lake City, USA. 2000:37-44.
- Skalski A, Socha M, Zielinski T, Duplaga M. Colon cleansing for virtual colonoscopy using non-linear transfer function and morphological operations. 2007 IEEE International Workshop on Imaging Systems and Techniques, Cracovia, Poland. 2007:1-5.
- Zalis ME, Perumpillichira J, Hahn PF. Digital subtraction bowel cleansing for CT colonography using morphological and linear filtration methods. IEEE Trans Med Imaging. 2004;23(11):1335-1343.
[Crossref] [Google Scholar] [PubMed]
- Grosu S, Wesp P, Graser A, Maurus S, Schulz C, Knösel T, et al. Machine learning-based differentiation of benign and premalignant colorectal polyps detected with CT colonography in an asymptomatic screening population: A proof-of-concept study. Radiology. 2021;299(2):326-335.
[Crossref] [Google Scholar] [PubMed]
- Blanes-Vidal V, Baatrup G, Nadimi ES. Addressing priority challenges in the detection and assessment of colorectal polyps from capsule endoscopy and colonoscopy in colorectal cancer screening using machine learning. Acta Oncol. 2019;58(sup1):S29-S36.
[Crossref] [Google Scholar] [PubMed]
Citation: Mostafavi M, Shaabani M (2025) Enhancing Virtual Colonoscopy: Exploring Electronic Colon Cleansing Strategies and Methodologies. J Clin Med Sci. 09:314.
Copyright: © 2025 Mostafavi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.