Indexed In
- Open J Gate
- Genamics JournalSeek
- Smithers Rapra
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 13, Issue 3
Efficacy of Fenton Based Electrochemical Treatment in Rotating Disc Electrode Reactor for the Removal of Total Organic Carbon (TOC) from Pharmaceutical Wastewater through Different Modes
Kavitha Nagarasampatti Palani*, Balasubramanian N, Asha Mathew and Jhanani KarunagaranReceived: 23-Jan-2023, Manuscript No. ACE-23-19644 ; Editor assigned: 27-Jan-2023, Pre QC No. ACE-23-19644 (PQ); Reviewed: 09-Feb-2023, QC No. ACE-23-19644 ; Revised: 10-Apr-2023, Manuscript No. ACE-23-19644 (R); Published: 17-Apr-2023, DOI: 10.35248/2090-4568.23.13.286
Abstract
The pharmaceutical effluent was remediated by employing batch and Batch Recirculation (BR) of fenton, Electro Oxidation (EO) and Electro Fenton (EF) processes to detach the organic compounds from the pharmaceutical wastewater using rotating disc reactor. Synthetic wastewater was modelled with Total Organic Carbon (TOC) of 298 mg/L. EF process showed enhanced results which could be attributed to the generation of large amount of hydroxyl radicals by which the generation of ferrous ions by the reduction of ferric ions reacted with hydrogen peroxide along with the anode. The optimum conditions for EF process were Fe2+=0.2 g/L, H2O2=0.9 mM, Current Density (CD)=15 mA/cm2, rpm=500 rpm, pH=3.5 and Fe2+=0.2 g/L, H2O2=0.9 mM, CD=10 mM/cm2, pH=3.5, rpm=500, flow rate=60 L/h for batch and for BR mode. This study shows outstanding results for EF process than Electro Oxidation (EO) and fenton process for the pharmaceutical effluent treatment.
Keywords
Fenton; Electrofenton; Electro oxidation; Pharmaceutical wastewater; Total organic carbon; Batch recirculation
Introduction
The wastewater generated from pharmaceutical industries has strenuous color and bad odour which is harmful and poisonous to the living beings. Pharmaceutical chemicals make adverse impact on the human kind and ecosystem [1]. The conventional methods like biological and chemical methods are well appropriated only for low molecular weight organic pollutants. Furthermore, these conventional methods become unproductive for wastewater containing high molecular refractory compounds (resistant for biological treatment) and organic pollutants. Advanced Oxidation Process (AOP) has been considered to overcome this problem [2]. In this scenario, fenton process is highly promising due to its high organic removal percentage when compared to other process. The enhanced efficiency could be due to the generation of highly reactive hydroxyl radicals (*OH) [3]. Hydroxyl radicals (*OH) are generated on the anode surface and represented in the equations 1 to 4 [4].

The two major limitations of conventional Fenton processes are the transportation risk associated with H2O2 that leads to the reactive activity loss and production of sludge. This can be knobbed by a tailored process called the electro fenton process [5]. EF has several advantages such as high hydroxyl generation rate and low time consumption when compared to fenton and electro oxidation process.

Electrochemical science plays a significant role in offering ecofriendly technology for chemical synthesis, separations, fuel cells etc. The uniqueness of electrochemical technology is adaptability, ecofriendly, higher energy efficiency and low cost [6]. Electrochemical technology has been contributing towards zero discharge in chemical process industries by means of providing advanced treatment process such as electro coagulation, EO, electro deposition etc. [7].
Different electrochemical reactors are used in the chemical process industries for various applications from small scale to large scale commercial reactors. In general, electrochemical reactors are classified based on their electrode configuration and geometry. The selection and design of an appropriate reactor is the foremost criteria in electrochemical process with which the geometry of the reactor plays a vital role in the selectivity and yield of the process. Further, electrochemical reactors are classified based on flow pattern or mode of electrical network. Rotating Disc Electrochemical reactor (RDE) has received considerable attention owing to its facile operation. Rotating motion is being imparted to the electrode rather than a separate agitation assembly. Spinning disc drags the solution and drifts away from the core of the electrode owing to the centrifugal and centripetal force. The bulk solution replenish the transfer of solution to surface of the electrode in each and every single rotation. This results in improved mass transportation which could be owing by the forced convection and angular velocity of the electrode [8].
The aim of the current research work is to remediate the pharmaceutical wastewater in order to meet the effluent standards of Tamil Nadu Pollution Control Board (TNPCB), where total organic carbon is less than 50 mg/L, by the fenton, EO and EF process in batch and batch recirculation process through a well-designed novel reactor to analyze the capability with the conventional process. The energy consumption was estimated along with the kinetic studies in order to evaluate the reactor for scale up studies.
Materials and Methods
Synthetic wastewater was prepared from tablet waste and the characteristics are presented in Table 1. The effluent treatment was carried out in the Rotating Disc Reactor (RDR) as shown in Figure 2. The RDR mainly consists of plexiglass cylindrical reactor with the volume of 2.75 liters, diameter and height of 100 mm and 500 mm respectively as depicted in Figure 1a. The electrodes mounted in the reactor rotate with stainless steel cathode rings (5) of 2 mm thick (outer dia. 50 mm, inner dia. 8 mm) and static anode rings (5 nos.) of outer diameter 85 mm, 15 mm thickness and inner diameter of 65 mm with a gap of 5 mm which are located vertically and parallel to each other. The cathode is held and attached by a bearing and holder at the bottom and top of the vessel. The rotational speed was measured by a digital tachometer. The anode rod is mounted at the centre connected to the power supply and attached with the anode rings of 365 cm2 surface area. The experimental setup has provisions to operate the desired mode by adjusting the valves.
| S. No | Parameters | Values |
|---|---|---|
| 1 | pH | 6.9 |
| 2 | Total Solids (TS) | 2490 mgL-1 |
| 3 | Total Dissolved Solids (TDS) | 2410 mgL-1 |
| 4 | Total Suspended Solids (TSS) | 80 mgL-1 |
| 5 | Phenol content | 25 mgL-1 |
| 6 | Ammonical nitrogen content | 1075 mgL-1 |
| 7 | Chloride content | 425 mgL-1 |
| 8 | Biological Oxygen Demand (BOD) | 260 mgL-1 |
| 9 | Oil content | 15 mgL-1 |
| 10 | Total Organic Carbon (TOC) | 298 mgL-1 |
Table 1: Initial characteristics of pharmaceutical effluent.
The observed peaks in FTIR analysis as shown in Figure 1b at 1654 cm-1 and 1700 cm-1 are due to the CO stretching in enols and cis-cinnamic acid. These are imperative ingredients for anaesthetic and anti-allergic pharmaceutical formulations. The UV spectra shown in the inset Figure 1b inset was increased steadily with decreasing wavelength in the visible range, which reveals the influence of particles. The effluent with turbidity could be due to the presence of dissolved organic substances as inferred from the finger print at 280 mm.
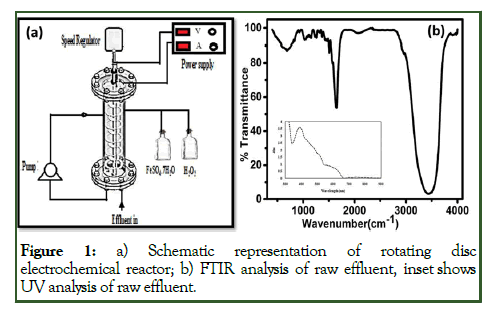
Figure 1: a) Schematic representation of rotating disc electrochemical reactor; b) FTIR analysis of raw effluent, inset shows UV analysis of raw effluent.
Analytical methods
FTIR (PerkinElmer spectrum RX11.60) and UV spectrophotometer (shimadzu) were employed to study the functional groups present in the untreated and treated wastewater. Total Organic Carbon (TOC) was analyzed by UV chemical oxidation.
Results and Discussion
Fenton process in batch and recirculation mode
This method is non-selective and oxidizes the organic compounds efficiently by means of hydroxyl radicals [9]. The parameters such as rotational speed, H2O2, ferrous sulphate concentration and flow rate were optimized in the Fenton process as shown in Figure 2A. The rotational speed was varied between 0, 250, 500, 750 and 1000 rpm. The mixing rate enhanced with increase in the rotational speed as a result, uniform consumption of fenton reagent was achieved in order to remove the organic particles efficiently. The rotational speed of 500 rpm was found to be optimum as the maximum removal of organic compounds was achieved in 4 hours for batch and BR mode. It is inferred that there is only marginal improvement in the removal percentage with increase in the rotational speed. Similarly, H2O2 and ferrous sulphate was varied such as 0, 0.3, 0.6, 0.9, 1.2, 1.5 mM and 0, 0.05, 0.1, 0.15, 0.2, 0.25 gL-1.The optimum conditions were observed at 0.9 mM and 0.2 gL-1. The flow rate was adjusted to bring an axial and radial dispersion in the reactor in order to achieve efficient organic pollutant removal in BR mode. The optimum flow rate was 60 L/h due to its high removal efficiency and also increase in flow rate increase in energy loss. The pH was not varied because the degradation can takes place effectively in acidic pH. The results in Figure 3a shows TOC values and % TOC removal at 4 hr time period in batch and BR mode were 94% and 95% of TOC removal respectively. This implied that fenton process can consume more time like conventional process in the removal of organic compound [10].
Electro oxidation in batch and recirculation mode
The reactor was operated effectively by covering the wide range of significant parameters to find the optimum conditions in electro oxidation process. So, pH was varied as 3,5,7 and 10 for both batch and BR mode as shown in Figure 2A. The maximum organic removal was observed at pH 3.5 and 7 for batch and BR modes, this is due to the increase in chlorine evolution which leads to the degradation of pollutants by indirect oxidation. The cathode rotational speed varied from 0, 250, 500, 750 and 1000 rpm for batch and BR mode and implied that increase in rotational speed increases the turbulence which in turn increases the mass transfer coefficient. The optimum speed was observed as 500 rpm based on energy considerations. CD was varied from 5, 10, 15 and 20 mA/cm2 and the optimum CD was found to be 15 and 10 mA/cm2 for batch and BR mode because increase in CD increases the temperature inside the reactor.
The flow rate was adjusted for BR mode from 15, 30, 60 and 120 L/h and the organic removal was maximum at 60 L/h and 120 L/h because of increase in mass transfer rate on the surface of the anode. Although, 60 L/h was chosen as optimum for EO process due to an energy conservation. The optimized results for batch and BR modes were pH 7, cathode rotational speed 500 rpm, CD 15 and 10 mA/cm2 and flow rate 60 L/h with the organic removal efficiency of 92.3 %. The % TOC removal for batch and BR mode in 2 hours which shows significant effect when compared to conventional Fenton process without any formation of sludge is shown in Figure 2A.
Electro fenton in batch and recirculation mode
Fenton process was integrated with electro oxidation to generate high amount of hydroxyl radicals to remove organic pollutants effectively in this study. CD was varied from 5, 10, 15 and 20 mA/cm2. The optimized dosage of FeSO4. 7H2O2 and H2O2 were 200 mg/L and 0.9 mM exhibited in Figures 2B. The removal efficiency were 92% and 96% at pH 3.5, cathode rotational speed 500 rpm, CD 15 and 10 mA/cm2, H2O2 0.9 mM and FeSO47H2O2 was 200 mg/L and flow rate 60 L/ h for batch and BR modes respectively in 1 hour. The attained results revealed that electro fenton process is most promising and suitable for pharmaceutical wastewater treatment in scale up process with lower energy consumption.

Figure 2: (A) % TOC Removal of a) Fenton process; b) Electro oxidation process and; c) Electro fenton process, inset shows TOC removal. (B) Electro fenton process optimization of a) FeSO4.7H2O2 batch; b) FeSO4.7H2O batch recirculation; c) H2O2 batch and; d) H2O2 batch recirculation.
Specific Energy Consumption (SEC) and cost analysis
The performance of electro oxidation is characterized by its consumption of energy in terms of TOC removed (kWh/kg). It plays a very important role as it confines the commercial applicability of the electrochemical process. It is described as

Where ‘V’ the applied voltage in volts, ‘I’ the current in milliampere,‘t’ the electrolysis time in hours. ‘ΔC’ the difference in TOC expressed in mg/L, ‘Ve’ the volume of effluent treated in liters. Table 2 shows energy consumption and energy cost of EO and EF process. The energy consumption EF is very low compared to EO for the removal of organics because it requires very shorter time. The energy cost (kWh m-3) for the removal of TOC for each process was estimated by applying the below equation.

Where ‘V’ Voltage (V), ‘I’ applied current (A), ‘t’ electrolysis time (h) and ‘Vol’ volume of solution (L).
SEC and energy cost for electro fenton BR mode was 50 % lesser than electro oxidation BR mode which was due to the integrated and lesser time taken for electrolysis as shown in Figure 3b. The energy consumption was less when compared to some integrated Fenton process as UV illumination consumes more energy when compared to EO. The cost is considerably less when related with other Fenton based research works and is depicted in Table 2. Therefore, EO integrated Fenton process is feasible for all type of effluent treatment with high organic loading and is appropriate for scale up studies as well.
| S. No | Process and modes | SEC (KWh/Kg TOC) | Energy cost (kWh/m3) |
|---|---|---|---|
| 1 | Electro oxidation | ||
| Batch | 41 | 12.23 | |
| Batch recirculation | 22 | 6.48 | |
| 2 | Electro fenton | ||
| Batch | 20 | 6 | |
| Batch recirculation | 11 | 3.099 | |
Table 2: Specific energy consumption and cost analysis.

Figure 3: FTIR analysis of (A) Batch process and; a) Electro fenton; b) Electro oxidation and; c) Fenton; b) Specific energy consumption of (B) Batch recirculation process for; a) Electro oxidation; b) Electro fenton energy cost of; c) Electro oxidation and; d) Electro fenton process.
Instrumental analysis
The presence of alkyne groups at 2070 cm-1, alkenes at 1653 cm-1, ketone groups at 1508 cm-1 and amine groups at 3976 cm-1 in Figure 1b. The bands that are produced by several contaminants, existing enols, alkynes and alcohol functional groups have been found to disappear after treatment and further, confirm that the degradation was by oxidation. Thetreated water in batch and BR mode by fenton, EO and EF for one hour operating time is shown in Figures 3A.
UV fingerprints of treated and untreated water by fenton, EO and EF process in batch and BR mode is shown in Figures 4 A. The decrease in absorbance at 380 nm shows that the organics were effectively removed by AOP. EF process successfully removed the maximum amount of pollutants in both modes within one hour. The slight decrease in the peak intensity signify the decolourization and TOC decrease. The attained results were in good agreement with the FT-IR spectral results.

Figure 4: (A) UV analysis of all the three process in a) Batch and; b) Batch recirculation. (B) a) Kinetics and; b) Performance analysis of all the three process in batch and recirculation mode for one hour treatment.
Kinetics and performance analysis
Kinetics of all the three processes of fenton, EO and EF in batch and BR mode with rate constants of 0.009, 0.0011, 0.018.0.20, 0.019 and 0.021 min-1. The rate constant for EF batch recirculation was high which assures the higher organic removal. The kinetics also revealed that integration of both EO and fenton process elevated the reaction rate constant higher as compared to the individual process. The performance of all the three process were determined under the best operating condition for one hour treatment time period. It was observed from Figure 4B that at one hour treatment time electro fenton shows maximum organic removal and the pH was shifted from acidic condition to slightly neutral condition. This could be due to the complete mineralization of organics whereas in electro oxidation, there is no significant change as the process was carried out at neutral pH. Perhaps, it needs extra electrolysis time for the removal of organics. The performance of the fenton process is determined and it shows longer time period for complete mineralization of organics.
BR mode was found to be better when compared to batch mode in terms of TOC removal at various operating parameters for all the three processes is shown in Figure 4B. The pollutant removal increased significantly with increase in the recirculation at an optimum condition of 60 lph. The reduction in flow rate limits the process by the reduction in transfer coefficients and an increase in the reactor volume causes greater capital investment. Thus, the recycle system of operation can be concluded as better performance among the conventional reactor configurations studied. The percentage TOC, COD and BOD removal for batch and batch recirculation mode in optimum operating conditions for all three processes as shown in Figure 4. It is concluded from the results that EF process showed maximum removal of pollutants at a period of one hour treatment time.
Conclusion
EF process using RDR was significantly effective for a shorter period of treatment time when compared to the conventional Fenton process. The % TOC removal efficiency for fenton, EO and EF processes in batch and BR modes were 94, 90, 92 and 95, 92 and 96% in 4, 2 and 1 hour respectively. EO and EF process have appreciable advantages such as shorter operating time, higher removal efficiency of organics which was ascertained by UV, FT-IR analysis and kinetic studies. Thus, EF is more feasible as Fe2+ ions prevent the peroxide overflow by means of continuous generation of hydroxyl radicals and remarkable advantage of shorter reaction time with high removal efficiency that satisfy the effluent standards of TOC despite high cost of EF when compared with conventional fenton method.
Acknowledgement
The first author acknowledges the financial support given by the department of chemical engineering, AC tech, Anna university, Chennai.
References
- Yu F, Zhou M, Zhou L, Peng R. A novel electro-fenton process with H2O2 generation in a rotating disk reactor for organic pollutant degradation. Environ Sci Technol Lett. 2014;1(7):320-324.
- Wang Y, Li X, Zhen L, Zhang H, Zhang Y, Wang C. Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate. J Hazard Mater. 2012;229:115-121.
[Crossref] [Google Scholar] [PubMed]
- Garza-Campos B, Morales-Acosta D, Hernandez-Ramirez A, Guzman-Mar JL, Hinojosa-Reyes L, Manriquez J, et al. Air diffusion electrodes based on synthetized mesoporous carbon for application in amoxicillin degradation by electro-fenton and solar photo electro-fenton. Electrochim Acta. 2018;269:232-240.
- Xu YL, Zhong DJ, Jia JP. Electrochemical-assisted photodegradation of allura red and textile effluent using a half-exposed rotating TiO2/Ti disc electrode. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;43(5):503-510.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Li S, Qin Q, Peng C. Sustainable and feasible reagent-free electro-Fenton via sequential dual-cathode electrocatalysis. Proc Natl Acad Sci USA. 2021;118(34):e2108573118.
[Crossref] [Google Scholar] [PubMed]
- Monteil H, Pechaud Y, Oturan N, Oturan MA. A review on efficiency and cost effectiveness of electro-and bio-electro-fenton processes: Application to the treatment of pharmaceutical pollutants in water. Chem Eng J. 2019;376:119577.
- Carter KE, Farrell J. Oxidative destruction of perfluorooctane sulfonate using boron-doped diamond film electrodes. Environ Sci Technol. 2008;42(16):6111-6115.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Li Y, Zhao Y, Li G, Zhang F. Carbon black oxidized by air calcination for enhanced H2O2 generation and effective organics degradation. ACS Appl Mater Interfaces. 2019;11(31):27846-27853.
[Crossref] [Google Scholar] [PubMed]
- Aboudalle A, Fourcade F, Assadi AA, Domergue L, Djelal H, Lendormi T, et al. Reactive oxygen and iron species monitoring to investigate the electro-Fenton performances. Impact of the electrochemical process on the biodegradability of metronidazole and its by-products. Chemosphere. 2018;199:486-494.
[Crossref] [Google Scholar] [PubMed]
- Friedrich JM, Ponce-de-Leon C, Reade GW, Walsh FC. Reticulated vitreous carbon as an electrode material. J Electroanal Chem. 2004;561:203-217.
Citation: Palani KN, Balasubramanian N, Mathew A, Karunagaran J (2023) Efficacy of Fenton Based Electrochemical Treatment in Rotating Disc Electrode Reactor for the Removal of Total Organic Carbon (TOC) from Pharmaceutical Wastewater through Different Modes. Adv Chem Eng. 13:286.
Copyright: © 2023 Palani KN, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

