Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 7
Effect of Sugarcane Juice Pre-Treatment on the Quality and Crystallization of Sugarcane Syrup (Treacle)
Waled M. Abdel-Aleem*Received: 22-Jun-2020 Published: 22-Jul-2020, DOI: 10.35248/2157-7110.20.11.834
Abstract
Treacle (black honey) is liquid syrup produced by heating and evaporation of sugarcane juice. It is rich in sugars including, sucrose, glucose, and fructose, which may crystallize during storage, especially at low temperatures. The crystallization of treacle was the main problem facing treacle producers in the Egyptian traditional food industry and affected negatively in the quality and consumer acceptability. Consequently, the aimed to investigate the effect of sugarcane juice pre-treatments, including the addition of citric acid at concentrations of (1, 2, and 3) g/l alone or in a combination with preheating at (60 or 70)°C for 1 h on physiochemical properties and crystallization of sugarcane syrup during storage for 60 days at room temperature. The results showed that these pre-treatments significantly affected the quality and properties of sugar cane syrup. The combination of the citric acid addition at a concentration of 1 g/l and preheat treatment at 70°C for 1 h resulted in syrup with the greatest overall acceptability. Also, these pretreatments prevented the crystallization of the produced syrup during the storage for 60 days at room temperature (20 ± 5)°C. Therefore, pre-treatment of the sugar cane syrup with a combination of the citric acid addition and heating can be suggested as a promising method for producing a high-quality sugar can syrup and preventing syrup crystallization during storage and handling.
Keywords
Citric acid; Colour; Sugars content; Physicochemical characteristics; Sensorial evaluation
Introduction
Sugarcane honey is syrup produced by evaporation of the sugarcane juice at (105-110)°C to total solids content between 65% and 75% [1]. Sugarcane syrup is a carbohydrate-rich, mainly constituted of inverted sugars and sucrose, with viscous, translucent, and light or shiny brown color, or amber yellow color. It is a nutritious food and a good source of nutrients such as minerals, including iron, calcium, potassium, sodium, phosphorus magnesium, and chlorine. Also, it contains natural antioxidants such as flavonoids and vitamins [2].
The sugarcane syrup has interesting attributes both in the food aspect because it is considered a nutritious and energetic food and has important nutrients, as in the economic and social issue, since it generates income for small and medium producers, Egypt's black honey (Treacle) production increased from 984,000 tons in 2014 to 1984,000 tons in 2015 [3].
In some foods, crystallization is controlled to give the desired texture (fondant, fudge, panned candies, and so on) or appearance (frosted cereals, icing, and so on). However, in other foods such as hard candies, spray-dried juices, and so on the crystallization of sugars is prevented, and crystallization upon storage is considered a defect due to giving the product a coarse, gritty texture as in sugarcane syrup [4]. The high concentration (Brix) and pH, the crystallization in the treacle is evident, due to an incorrect inversion of sucrose. An adequate combination of the two variables allows stabilizing the product, suppressing the crystallization. The flavor acceptance (acidity) depends on pH in its average value 4 ± 0.2 and the adequate concentration of the product (76 ± 0.5) Brix, and temperature (106°C ± 0.8)°C [5].
The citric acid is used as a preservative, antioxidant, and suppressor of the browning in doses of (0.05 to 2)% and to brown sugar liquid [6]. In order to obtain cane honey or molasses, the environment must be stable during 8 until 10 days, 0.04% citric acid is used to avoid crystallization and show an attractive color, 1% of potassium metabisulphite and 0.5% of benzoic acid as preservatives [7].
It is widely known that fructose, glucose, and corn syrup depress the solubility concentration of sucrose [8] by competing with the sucrose molecules for available water. When sucrose is replaced with corn syrup in a confectionery formulation, not only is there less sucrose, but the saturation concentration also decreases, changing the crystallization process [9].
The main objective of this investigation was to evaluate the effect of pre-treatments, including the addition of citric acid and preheat treatment of sugarcane juice on the crystallization and quality of the produced sugarcane syrup (Treacle).
Materials and Methods
Materials
Freshly harvested sugarcane was obtained from Mallawy Agricultural Research Station, Minia, Egypt. Ten commercial treacle samples were collected from markets in Mallawy, El- Minia governorate. All chemicals used in this investigation were of analytical grade and purchased from Sigma and El-Naser Pharmaceutical Chemicals Co. Ltd., Egypt.
Methods
Extraction of sugarcane juice and treatments: The stripped stalks of sugarcane were passed through a three roller mill to extract the juice. The raw juice was screened through layers of clean cheesecloth to remove the large pieces of suspended matters [10], the juice was divided to three groups, each grouped to four treatments, as follows: the first group (Trade), the syrup was produced immediately after preparing the treatments as follow:
• Control • 1 g citric acid/l juice • 2 g citric acid/l juice • 3 g citric acid/l juice
The second and third groups were prepared in the same manner and placed in a water bath at a temperature of (60 and 70)°C for 1 h (1H60 and 1H70), respectively, then evaporated in open stainless steel pan (capacity 5 liters). The concentration process was carried using a hot plate (108 to 110)°C and the syrup was collected from the evaporator when its concentration reached 75° Brix, which was measured using a hydrometer and sugar [11].
During evaporation, the rising scum was eliminated with a ladle. The obtained syrup was screened and passed through several layers of clean cheesecloth and then filled hot in sterile brown glass containers (1 Kg). The syrup containers were rapidly cooled in water to terminate the effect of high temperature on the syrup characteristics.
Physicochemical characteristics
Total Soluble Solids (TSS%); pH value; Ash content and Moisture content were determined according to the methods of AOAC [12].
Acidity content was determined as citric acid according to Adekunte AO et al. [13].
Colour intensity value: The method used for optical density determination as follows: one gram from of honey sample was dissolved in 10 ml distilled water in 10 ml beaker them optical density of this solution was determined at 420 nm (absorbing) using Labomed, inc. Spectro UV-Vis R.S. spectrophotometer [14].
Determination of sugars content: The phenol-sulfuric acid method described by Dubois et al. was used in the determination of Total Sugars (TS) [15]. Reducing Sugars (RS) assessed by the DNS method [16]. Sucrose was calculated as the difference between the total sugars (TS)
%Sucrose=(TS-RS) × 0.95
Measuring the concentration time by measuring the concentration by refractometer and temperature every 30 minutes during produced of sugar cane syrup [11].
Determination of color: The color characteristics of samples were measured by a color difference meter (model color Tec- PCM, USA) using different color parameters (L, a, b) according to Francis [17].
Determination of the crystallization: Light microscopy was used to determine the crystallization kinetics. Images were taken with a 5X objective according to Laos et al. [18]
Sensory evaluation: For the color, texture, taste, odour, and overall quality was done to determine consumer acceptability. A numerical hedonic scale which ranged from 1 to 10 (1 is very bad and 10 for excellent) was used for sensory evaluation [19].
Statistical analysis
Data collected were subjected to two-way Analysis of Variance (ANOVA) to determine the overall effect of treatments, on physicochemical and quality attributes of samples. The differences were separated using the Least Significant Difference (LSD) [20].
Results and Discussions
Effect of sugarcane juice pre-treatment on pH value
Since the manufacturing of sugarcane syrup does not involve centrifugation, unlike what happens in the sugar as many of the micronutrients are removed in this process, the nutritional value of this syrup is considerably high, and therefore it is as an important food for society [21].
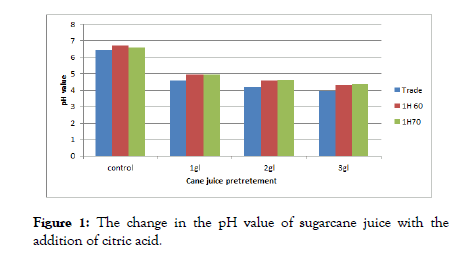
The data in Figure 1 show that the addition of citric acid to the sugarcane juice before the production of sugar cane syrup led to a significant decrease in the pH value as the amount of citric acid increased. The control without the pre-treatment recorded the highest pH value, followed by the citric acid treatment 1 g/l and the lowest treatment is the 3 g/l. Heating sugar cane juice at (60 and 70)°C for one hour before the production of sugar cane syrup due to a significant increase in the pH values for all treatments compared with the control sample. Concentration sugarcane juice the sucrose inversion starts at 80°C. It is evident at 100°C and it depends on factors such as pH, temperature, and boiling duration [22].
Figure 2: The change in the pH value of sugarcane juice with the addition of citric acid.
Effect of sugarcane juice pre-treatment on TSS (Brix)
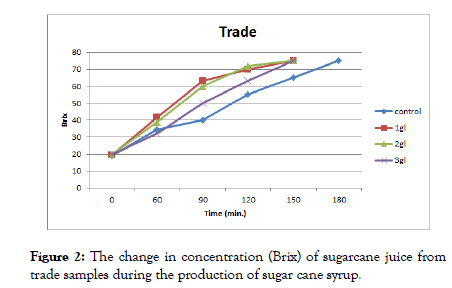
Pre-treatments of cane juice before the production of cane syrup (Figure 2) increased the rate of change in the concentration of sugarcane juice during the production of sugar cane syrup for the first treatments that were directly manufactured (commercial) greater than the control, where all treatments to which citric acid is added reached the required concentration to 75% before the control. Sucrose hydrolysis (commonly known as inversion) doubles the number of solute molecules in sugar solutions and, as a result, doubles the total activity of the solutes in the solution.
Figure 2: The change in concentration (Brix) of sugarcane juice from trade samples during the production of sugar cane syrup.
Due to the rapid rate of mass transfer during evaporation, the cooking process generally follows the boiling point elevation curve, which means that cooking to a certain temperature indicates specific water content as long as the boiling point elevation is known for the mixture of sweeteners. Thus, knowledge of the boiling point elevation curve for the sweetener mixture in a formulation is important for control of the manufacturing and specification of product characteristics (texture, shelf life, and so on) [23,24].
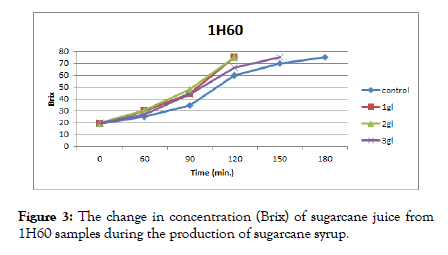
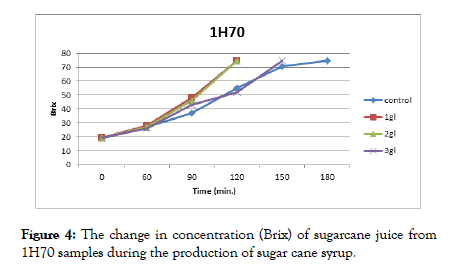
The results presented in Figures 3 and 4 show that heated sugarcane juice samples (1H60 and 1H70) with 1 g/l and 2 g/l citric acid reached the required concentration (75%) first after 120 min. followed by 3 g/l citric acid after 150 min, and control samples after 180 min.
Cooking sugars at very high temperatures (140°C and higher) causes the breakdown of monosaccharides, particularly fructose, which occurs readily at elevated temperatures, changes in the composition that can alter the boiling point. For one, inversion of sucrose into glucose and fructose occurs rapidly at elevated temperatures, introducing monosaccharides that change the boiling temperature [25].
Physicochemical characteristics
The physiochemical characteristics of syrup from different pretreated sugarcane juice and commercial syrup are presented in Table 1. A significant difference was noted for moisture, acidity, pH, ash, TSS, and OD420 at 5% level (p ≤ 0.05) in treatments.
Figure 3: The change in concentration (Brix) of sugarcane juice from 1H60 samples during the production of sugarcane syrup.
Figure 4: The change in concentration (Brix) of sugarcane juice from 1H70 samples during the production of sugar cane syrup.
| Moisture% | Acidity% | pH | TSS% | OD420 | Ash% | ||
|---|---|---|---|---|---|---|---|
| Trade | control | 23.67e | 0.25h | 6.68a | 75a | 0.424d | 2.12bcd |
| 1 g /l | 23.72cde | 0.51f | 5.08d | 75a | 0.344e | 1.84de | |
| 2 g/l | 23.77abcd | 0.81d | 4.61g | 75a | 0.218j | 2.13bcd | |
| 3 g/l | 23.70de | 1.40a | 4.38j | 75a | 0.191k | 2.10bcde | |
| 60H1 | control | 23.79abc | 0.19i | 6.55b | 75a | 0.465c | 1.99bcde |
| 1 g/l | 23.76abcd | 0.54f | 4.94e | 75a | 0.288f | 2.03bcde | |
| 2 g/l | 23.75abcde | 0.81d | 4.57h | 75a | 0.253h | 2.26abc | |
| 3 g/l | 23.79abc | 1.24c | 4.23k | 75a | 0.220j | 1.97cde | |
| 70H1 | control | 23.76abcd | 0.23hi | 6.16c | 75a | 0.503b | 1.78e |
| 1 g/l | 23.78abcd | 0.61e | 4.84f | 75a | 0.243i | 1.81de | |
| 2 g/l | 23.83ab | 0.82d | 4.46i | 75a | 0.279g | 2.04bcde | |
| 3 g/l | 23.82ab | 1.33b | 4.16l | 75a | 0.255h | 2.30ab | |
| Commercial | Com | 25.74a | 0.40g | 6.16c | 73.3b | 0.626 a | 2.53a |
| LSD 5% | 0.075 | 0.039 | 0.031 | 0.269 | 0.008 | 0.289 | |
Trade: Syrup produced immediately after addition citric acid to sugar cane juice
60H1: Syrup produced after addition citric acid and heat to 1 hour at 60°C
70H1: Syrup produced after addition citric acid and heat to 1 hour at 70°C
a-l: Values with the same letter in the same column are not statistically different (p<0.05)
Table 1: Physiochemical properties of syrup from different sugar cane juice pre-treatments and commercial syrup.
The moisture content: The moisture content of sugar cane syrup ranged from 23.67% in commercial samples (control) to 25.74% commercial samples. Total soluble solids of syrup from different pre-treated sugarcane juice 75% in all treatments highest than the commercial samples 73.33%.
Thus, for this attribute, different syrup from pre-treated sugarcane juice and commercial syrup were within 30% moisture content and 70% TSS. The Egyptian Organization for Standardization and Quality [26].
Acidity content: Acidity content as citric of syrup sugar cane in the control samples of all treatments varied from 0.19 for 60H1 to 0.25% for traditional treatment, this results lowest than the commercial samples 0.40%. However, sugarcane syrup acidity increased with the addition of citric acid to sugarcane juice used to produce syrup in all treatments ranged from 0.51% to 1.40% as citric acid.
The acidity value of cane syrup ranged between 0.44 and 0.97, with a mean of 0.70. Vicentini-Polette et al. and Barreto et al. found that in cane syrup, except for samples produced with citric acid or acidic lime, acidity values between 2.1% and 5.8% [27,28].
The pH value: The pH value of sugarcane syrup in the control samples of all treatments was the highest, ranged from 6.16 for 70H1 to 6.68 for traditional treatment while commercial samples 6.16. However, sugarcane syrup pH value decreased with the addition of citric acid to pre-treatment sugar cane juice used to produce syrup in all treatments ranged from 4.16 to 5.08. The superior level of pH was established according to pH (4.5 to 5.5) of the sugar cane juice [5].
Optical density (OD420): The commercial samples of sugarcane syrup attained a darker OD420 value (0.626), followed by control samples in 70H1 and 60H1 treatment (0.503 and 0.465). However OD420 value of citric acid pre-treated sugarcane juice used to produce syrup samples had the lowest value varied from 0.191 to 0.344 (as optical density at 420 nm). Generally, the color of food is a quality measurement and it means the acceptance of a product. These characteristics are obtained through hydrolyzed cane honey [29].
Ash content: Data showed that there are significant differences in the ash content of syrup samples (Table 1). The ash content of sugarcane syrup from different pre-treated sugar cane juice samples ranged from 1.78% to 2.30%, which is lower than that of commercial syrup (2.53%).
The maximum percentage of ash in sugar cane syrup is 3% as stated by the Egyptian Organization for Standardization and Quality [26]. The percentages of ash content in the studied syrup samples are lower than the maximum limit stated by the EOSQ.
Sugar content: The sugars content influences viscosity and product shelf life, and the greater the glucose and fructose content compared to sucrose, the lower will be the syrup crystallization. Additionally, the formation of the crystals is undesirable for the food industry because, in addition to hampering homogenization and standardization, it can cause damage to the equipment due to the accumulation of such crystals [27].
Total Sugars (TS): The sugar contents of syrup from different sugarcane juice pre-treatments and commercial syrup are presented in Table 2. The Total Sugars (TS) content of the tested samples ranged from 74.67% to 74.83%, while the content of the commercial syrup sample was 74.76%.N
| TS% | RS% | Suc% | RS/TS | RS/Ash | Suc/TS | Suc/RS | ||
|---|---|---|---|---|---|---|---|---|
| Trade | control | 74.83a | 10.56h | 61.05a | 14.10h | 4.99f | 81.60a | 5.780b |
| 1 g/l | 74.78abc | 47.02f | 26.37c | 62.90f | 25.55cd | 35.30c | 0.561d | |
| 2 g/l | 74.73bcde | 51.66cd | 21.92ef | 69.10cd | 24.21d | 29.30ef | 0.425d | |
| 3 g/l | 74.8ab | 53.15c | 20.56f | 71.10c | 25.59cd | 27.50f | 0.387d | |
| 60H1 | control | 74.71cde | 11.34h | 60.21a | 15.20h | 5.67f | 80.60a | 5.475b |
| 1 g/l | 74.74bcde | 49.46e | 24.02d | 66.20e | 24.46cd | 32.10d | 0.488d | |
| 2 g/l | 74.75abcde | 53.16c | 20.51f | 71.10c | 23.64d | 27.40f | 0.387d | |
| 3 g/l | 74.71cde | 57.22b | 16.62g | 76.60b | 29.23ab | 22.20g | 0.291d | |
| 70H1 | control | 74.74bcde | 18.7g | 53.24b | 25.00g | 10.49e | 71.20b | 2.855c |
| 1 g/l | 74.72bcde | 50.16de | 23.34de | 67.10de | 27.72abc | 31.20de | 0.466d | |
| 2 g/l | 74.67e | 59.84a | 14.09h | 80.10a | 29.71a | 18.90h | 0.235d | |
| 3 g/l | 74.68de | 60.26a | 13.7h | 80.70a | 26.21bcd | 18.30h | 0.227d | |
| Commercial | Com | 74.76abcd | 9.38h | 62.11a | 12.54h | 3.72f | 83.08a | 6.68a |
| LSD | 0.075 | 2.085 | 1.992 | 0.028 | 3.409 | 0.027 | 0.712 | |
Trade: Syrup produced immediately after addition citric acid to sugar cane juice
60H1: Syrup produced after addition citric acid and heat to 1hour at 60°C
70H1: Syrup produced after addition citric acid and heat to 1hour at 70°C
a-h: Values with the same letter in the same column are not statistically different (p<0.05)
TS%: Total Sugar; RS%: Reducing Sugar; Suc%: Sucrose
Table 2: Sugar content of syrup from different pre-treated sugarcane juices and commercial syrup.
Reducing Sugar (RS): There are significant differences among the tested syrup samples of different sugarcane juice pretreatments and commercial syrup (Table 2) in the contents of Reducing Sugar (RS). The sugar content increased with pretreatment sugar cane juice before produced syrup, (control) samples commercial syrup had the lowest value 9.38% followed by control (Trade, 10.56; 60H1, 11.34 and 70H1, 18.70%).
However citric acid treatments had the height value ranged from 47.0% to 60.26%. For reducing sugars, the mean minimum value was, in percentage, 9.0, while the maximum was 61.5, and mean 38.0% [27].
Sucrose Content (Suc): The results are given in Table 2 show that the commercial syrup sample had the highest sucrose content at 62.11%. Juice processing before production of sugarcane syrup, due to decrease in sucrose content in all syrup samples, and it was less in the control samples (Trade, 61.05, 60H1, 60.21 and 70H1, 53.24%) respectively, and the decrease in sucrose content increased by increasing the amount of citric acid added to the juice and heat treatment of the juice before syrup produced, trade (1 g/l citric acid) samples had the highest sucrose content (26.37%) while, the lowest sucrose content was found in 70H1 treatment for 3 g/l citric acid samples (13.7%).
There is a great difference in the sucrose content of sugar cane syrup since sucrose hydrolysis is not achieved (by adding acid and increasing temperature or by enzymatic action in natural degradation), the values are naturally low and depend on various factors such as soil treatment, sugarcane, genotype, fertilization, and harvest time [30].
Sugars calculated parameters: From the obtained results it can be observed that the proportions and/or relationships between the main components of the treatment may directly affect its quality. Four calculated parameters were suggested, as shown in Table 2, to define new quality standards for treatment. Reducing sugar parameters were tested, namely the percent of reducing sugars divided by each of Total Sugar (TS) and ash content, the data showed that rates were increased with pre-treatment the juice before produced syrup, reducing sugars/total sugar rate ranged from (12.54 to 80.70)%, however reducing sugars/ash rate ranged from (3.72 to 29.71)%.
Sucrose parameters were tested, namely the percentage of reducing sugars divided by each of Total Sugar (TS) and Reducing Sugar (RS). The date indicated that rates were decreased with pre-treatment the juice before the production of syrup, sucrose/total sugar rate ranged from (18.30 to 83.08)%, while sucrose/reducing sugars rate ranged from (0.23 to 6.68)%. Sucrose and reducing sugars affect the rate of sucrose crystallization [31]. This means that any of these ratios can be used to define sugaring which is one of the most important criteria of treacle quality. The ratio RS/TS deriving from the most important treacle components may be effectively used to define good quality treacle.
Color characteristics of syrup: The Hunter color parameters (L), (a) and (b) are widely used to describe color changes of food materials.
However, it is recommended to use the hue angle and Chroma as more practical measures of color. The color changes can also be expressed as a single numerical value ΔE. This value defines the magnitude of the total color difference. Preferred colors are those closest to the original color of samples [32,33]. The results of color parameters (L, a, b, ΔE, hue angle, and Chroma) for the different pre-treated sugar cane juices and commercial syrup are presented in Table 3.
| L | a | b | ΔE | Chroma | Huge | ||
|---|---|---|---|---|---|---|---|
| Trade | control | 37.45d | 33.20a | 46.20d | 33.00e | 56.90bcd | 51.25d |
| 1 g/l | 41.40c | 26.60b | 48.65cd | 34.38e | 55.51cd | 68.15c | |
| 2 g/l | 43.65b | 20.45c | 42.95e | 30.14f | 47.57f | 76.92ab | |
| 3 g/l | 47.40a | 24.90b | 49.20cd | 37.68cd | 55.14cd | 72.58abc | |
| 60H1 | control | 32.30e | 33.10a | 43.20e | 28.87f | 54.50de | 48.53de |
| 1 g/l | 37.90d | 32.65a | 48.70cd | 34.82e | 58.64abc | 54.84d | |
| 2 g/l | 43.90b | 27.90b | 52.30ab | 38.80c | 59.28ab | 68.86bc | |
| 3 g/l | 47.60a | 25.40b | 54.50a | 41.99ab | 60.13ab | 78.82a | |
| 70H1 | control | 27.60f | 33.50a | 39.15f | 24.80g | 51.53e | 42.95e |
| 1 g/l | 37.75d | 34.85a | 48.10cd | 35.35de | 59.40ab | 50.70de | |
| 2 g/l | 40.80c | 35.80a | 50.30bc | 38.77c | 61.74a | 51.61d | |
| 3 g/l | 48.10a | 27.90b | 55.00a | 43.24a | 61.67a | 72.43abc | |
| Commercial | Com | 21.05g | 19.85c | 28.55g | 0 | 34.99g | 55.32d |
| LSD | 0.998 | 3.292 | 2.768 | 2.546 | 3.395 | 7.645 | |
Trade: Syrup produced immediately after addition citric acid to sugar cane juice
60H1: Syrup produced after addition citric acid and heat to 1hour at 60°C
70H1: Syrup produced after addition citric acid and heat to 1hour at 70°C
a-g: Values with the same letter in the same column are not statistically different (p<0.05)
L: Lightness; a: Redness/greenness; b: Yellowness/blueness
Table 3: Color characteristics of syrup from different sugar cane juice pretreatments and commercial syrup.
The results showed that the color parameters L, a, b and Chroma values for commercial syrup are the lowest values respectively, while L value, b-values, ΔE values, Chroma values and Huge values for different syrups from pre-treatment sugar cane juice increased in majority samples with the addition of citric acid. It could also be seen that a-values decreased as a result of the addition of citric acid. This could be due to cane syrup color becomes higher the luminosity index (L*), the lighter the cane syrup. Colored compounds are formed during the process and they come from the thermal decomposition of sucrose and depleted sugars as well as the degradation of chlorophyll by acids [34]. No data previously published by other authors were found regarding sugarcane syrup color in the CIELAB scale [27].
Crystallization of syrup: The high-grade treacle must be free of noticeable sugar crystals precipitated out of the treacle mass [27].
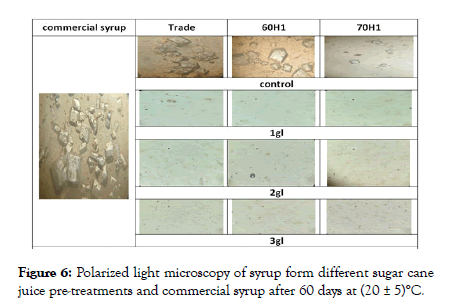
The crystallization of syrup results in Table 4 was confirmed with a light microscope. Figures 5 and 6 show a sequence of images obtained by a light microscope for sucrose crystallization. The crystals appeared as a bright area surrounded by a dark back. No crystallization was observed in the syrup from different sugarcane juice pre-treatments and commercial syrup in zero time, little small size crystals can be seen in the trade, and 60H1 (control) samples and more in commercial syrup after storage samples ten days at room temperature (20 ± 5)°C.
| 0 | 10 | 30 | 60 | ||
|---|---|---|---|---|---|
| Trade | control | - | + | ++ | +++ |
| 1 g/l | - | - | - | - | |
| 2 g/l | - | - | - | - | |
| 3 g/l | - | - | - | - | |
| 60H1 | control | - | + | + | ++ |
| 1 g/l | - | - | - | - | |
| 2 g/l | - | - | - | - | |
| 3 g/l | - | - | - | - | |
| 70H1 | control | - | - | - | + |
| 1 g/l | - | - | - | - | |
| 2 g/l | - | - | - | - | |
| 3 g/l | - | - | - | - | |
| Commercial | Com | - | ++ | +++ | ++++ |
Trade: Syrup produced immediately after addition citric acid to sugar cane juice
60H1: Syrup produced after addition citric acid and heat to 1hour at 60°C
70H1: Syrup produced after addition citric acid and heat to 1hour at 70°C
(-): Non crystallization; Crystallization : (+) Low; (++) Medium; (+++) High; (++++) Full
Table 4: Crystallization of syrup from different pre-treated sugar cane juices and commercial syrup.
Figure 5: Polarized light microscopy of syrup form different sugar cane juice pre-treatments and commercial syrup after 10 days at (20 ± 5)°C.
Crystals grew (Figure 6) until they became large and contact with each other in trade, and 60H1 (control) samples, commercial syrup completely crystallized after 60 days, while little small size crystals can be seen in 70H1 (control) after 60 days. The crystallization in syrup from different sugar cane juice pre-treatments was more inhibited by the addition of citric acid and as the amount of citric acid increased (Table 3), no crystallization observed in all syrup from different sugarcane juice pre-treated with citric acid after 60 day days of storage at room temperature (20 ± 5)°C.
Figure 6: Polarized light microscopy of syrup form different sugar cane juice pre-treatments and commercial syrup after 60 days at (20 ± 5)°C.
An understanding of the principles that control the prevention of the formation of the crystalline sugar system phase is quite important in developing satisfactory food products. Although the presence of many of the other compounds, including other sugars, is known to impede sucrose crystallization [5,35].
High-quality sugarcane syrup should be free of colloidal sedimentation and crystallization, or if present, limited to a few small crystals [11].
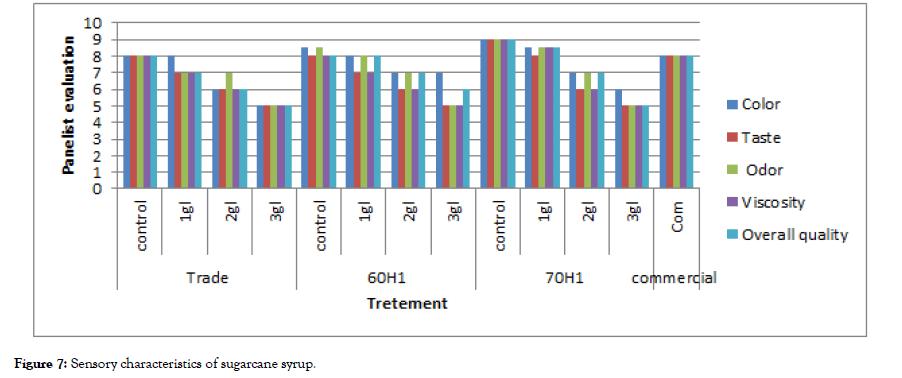
Sensory characteristics: Sensorial evaluation for color, texture, taste, odour, and overall acceptability of the produced syrup from different sugar cane juice pre-treatments and commercial syrup samples were done to determine consumer acceptability and the results are shown in Figure 7. It can be seen that the 70H1 (control) recorded the highest sensory quality in terms of color, viscosity, taste, odor, and overall acceptability followed by 70H1 (1 g/l). The addition of citric acid at a concentration greater than 1 g/l to sugarcane juice resulted in sugarcane syrup with lower sensory quality. Also, it can be seen that the quality attributes of syrup from different sugarcane juice pre-treatments are comparable to those of the commercial syrup samples.
Figure 7:Sensory characteristics of sugarcane syrup.
Correlations and principle component analysis correlations
Table 5 shows the correlation among TSS, pH, acidity, total sugar, sucrose, reducing sugar, color, L* and B* value of the different syrups. A strong positive correlation was found between reducing sugar and acidity (r=0.791) as well as color parameter B* and L* (r=0.749 and r=0.825, p<0.01), respectively. On the other hand, a strong negative correlation was found between reducing sugar and pH (r=-0.978, p<0.01) and color (r=-0.903, p<0.01). In opposite situation, we found a strong positive correlation between sucrose and pH (r=0.978, p<0.01) and color (r=0.903, p<0.01), while a strong positive correlation with and acidity (r=-0.791) as well as color parameter B* and L* (r=-0.749 and r=-0.825, p<0.01), respectively.
| Acidity | Ash | pH | TSS | Moisture | TS | RS | Sucrose | OD420 | L | A | B | ΔE | Chroma | Huge | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acidity | 1 | 0.176 | -0.873** | 0.217 | -0.199 | -0.19 | 0.791** | -0.791** | -0.754** | 0.799** | -0.349* | 0.614** | 0.687** | 0.363* | 0.752** |
| Ash | 0.176 | 1 | -0.008 | -0.492** | 0.541** | 0.056 | -0.095 | 0.095 | 0.182 | -0.087 | -0.465** | -0.227 | 0.410** | -0.373* | 0.231 |
| pH | -0.873** | -0.008 | 1 | -0.331* | 0.303 | 0.320* | -0.978** | 0.978** | 0.867** | -0.799** | 0.214 | -0.678** | -0.599** | -0.466** | -0.681** |
| TSS | 0.217 | -0.492** | -0.331* | 1 | -0.984** | -0.026 | 0.463** | -0.462** | -0.651** | 0.661** | 0.454** | 0.749** | -0.233 | 0.790** | 0.169 |
| Moisture | -0.199 | 0.541** | 0.303 | -0.984** | 1 | -0.02 | -0.436** | 0.435** | 0.651** | -0.660** | -0.466** | -0.740** | 0.25 | -0.788** | -0.145 |
| TS | -0.19 | 0.056 | 0.320* | -0.026 | -0.02 | 1 | -0.326* | 0.328* | 0.159 | -0.078 | -0.217 | -0.154 | -0.187 | -0.217 | 0.109 |
| RS | 0.791** | -0.095 | -0.978** | 0.463** | -0.436** | -0.326* | 1 | -1.00** | -0.903** | 0.825** | -0.068 | 0.749** | 0.520** | 0.580** | 0.604** |
| Sucrose | -0.791** | 0.095 | 0.978** | -0.462** | 0.435** | 0.328* | -1.00** | 1 | 0.903** | -0.825** | 0.067 | -0.749** | -0.520** | -0.580** | -0.603** |
| OD420 | -0.754** | 0.182 | 0.867** | -0.651** | 0.651** | 0.159 | -0.903** | 0.903** | 1 | -0.922** | 0.005 | -0.813** | -0.358* | -0.658** | -0.606** |
| L | 0.799** | -0.087 | -0.799** | 0.661** | -0.660** | -0.078 | 0.825** | -0.825** | -0.922** | 1 | -0.1 | 0.879** | 0.436** | 0.675** | 0.734** |
| A | -0.349* | -0.465** | 0.214 | 0.454** | -0.466** | -0.217 | -0.068 | 0.067 | 0.005 | -0.1 | 1 | 0.284 | -0.221 | 0.626** | -0.718** |
| B | 0.614** | -0.227 | -0.678** | 0.749** | -0.740** | -0.154 | 0.749** | -0.749** | -0.813** | 0.879** | 0.284 | 1 | 0.449** | 0.925** | 0.453** |
| ΔE | 0.687** | 0.410** | -0.599** | -0.233 | 0.25 | -0.187 | 0.520** | -0.520** | -0.358* | 0.436** | -0.221 | 0.449** | 1 | 0.275 | 0.468** |
| Chroma | 0.363* | -0.373* | -0.466** | 0.790** | -0.788** | -0.217 | 0.580** | -0.580** | -0.658** | 0.675** | 0.626** | 0.925** | 0.275 | 1 | 0.085 |
| Huge | 0.752** | 0.231 | -0.681** | 0.169 | -0.145 | 0.109 | 0.604** | -0.603** | -0.606** | 0.734** | -0.718** | 0.453** | 0.468** | 0.085 | 1 |
**: Correlation is significant at the 0.01 level (2-tailed)
*: Correlation is significant at the 0.05 level (2-tailed)
Table 5: Quality attributes correlation of syrup form different sugar cane juice pre-treatments and commercial syrup.
The ‘ L ’ value had a significant strong correlation with TSS (r=0.661, p<0.01), acidity (r=0.799, p<0.01), reducing sugar (r=0.825, p<0.01) and B* value (r=0.879, p<0.01) but had a significant negative correlation with pH (r=-0.799, p<0.01), and sucrose (r=-0.825, p<0.01) and color (r=-0.922, p<0.01). The results obtained in this study agree with Prati and Moretti, and Moreno et al. [5,34].
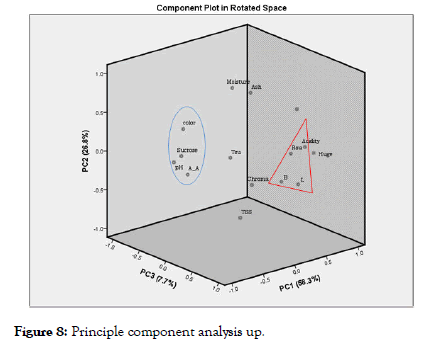
To have a better understanding between the correlation among the physiochemical parameters of syrup form different sugar cane juice pre-treatments and commercial syrup, the statistical technique Principal Component Analysis [36,37] was employed. In this study, the three Principal Components (PC1, PC2, and PC3) explain 92.8% of the total variability (Figure 8), where PC1, PC2, and PC3 explain (58.3, 26.8, and 7.7)% of the variability's, respectively. Each PC identifies the variables more strongly related to the juice's quality parameters and how they contribute to explaining the total variability [37,38]. PC1+PC2 contains 84% of the total variability of the set of original data regarding physicochemical parameters. The graph shows that compositional factors are positively correlated with compositional factor B*, L*, acidity, and reducing sugar. In order of significance, the attributes that were more related to this component were B (0.79), L (0.80), acidity (0.89), and reducing sugar (0.94). These are the main parameters that express the physicochemical quality of products. PC2 was positively correlated with sucrose (0.93), pH (0.96) and color (0.82).
Figure 8: Principle component analysis up.
Conclusion
Based on the obtained results, it could be concluded that pretreatments of the sugarcane juice by the addition of citric acid and heating improved the quality of the produced sugarcane syrup and reduced the crystallization during storage. The addition of 1 g/l citric acid to the juice combined with heating at 70°C for 1 h obtaining high-quality free crystals syrup during marketing and storage by preheating treatment and addition of citric acid.
REFERENCES
- Sampaio MR, Tomasini D, Cardoso LV, Caldas SS, Primel EG. Determination of pesticide residues in sugarcane honey by QuEChERS, and liquid chromatography. J Braz Chem Soc. 2012;23:197-205.
- Belé JS, Polette CMV, Antonini SRC, Spoto MH, Belini VL, Bernardi MRV. Microbiological and microscopic analysis of sugarcane syrup. J Agric Sci. 2019;11.
- CAPMAS. Central Agency for Public Mobilization and Statistics. Annual bulletin of income estimates from the agricultural sector. 2019.
- Hartel RW. Crystallization in foods. Aspen Publishers. 2001.
- Moreno C, Suárez CE, David W, Torres Q, Aguas IM. Cane honey: process, quality and harmlessness. Int J Eng Res. 2016;5:589-593.
- Shrivastav P, Verma A, Walia R, Parveen R, Singh A. Jaggery: a revolution in the field of natural sweeteners. Eur J Pharma Med Res. 2016;3:198-202.
- Singh J, Solomon S, Kumar D. Manufacturing jaggery, a product of sugarcane, as health food. Agrotechnol. 2013;11.
- Pancoast HM, Junk W. Handbook of sugars. 2nd Edition. AVI Publishing. 1980.
- Hartel RW, Hofberger R, von Elbe JH. Confectionery science and technology. Springer International Publishing. 2018.
- Abbas H, El Hoda TN. Effect of harvesting at different maturity stages on the yield, yield components, and syrup characteristics of sweet sorghum. Egyptian J Appl Sci. 2000;15:103-118.
- Broadhead DM, Zummo N. Sugarcane culture and syrup production. ARS-United States Department of Agriculture, Agricultural Research Service (USA). 1988.
- AOAC. Official methods of analysis, 17th ed. Association of Official Analytical Chemists International, Maryland. 2000.
- Adekunte AO, Tiwari BK, Cullen PJ, Scannell AG, O’donnell CP. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010;122:500-507.
- White JW Jr. Honey. Adv Food Res. 1978;24:287-374
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350-356.
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426-428.
- Francis FJ. Colorimetry of foods. In: physical properties of foods. In: Peleg M and Edwards BB (Eds). Westport AVI Publishing. 1983:105-123.
- Laos AK, Kirs BE, Kikkas CA, Paalme DT. Crystallization of the supersaturated sucrose solutions in the presence of fructose, glucose and corn syrup. In Proceedings Eur Congress Chem Eng. 2007:16-20.
- Larmond E. Laboratory Methods for Sensory Evaluation of Food. Ottawa: Agriculture Canada, Research Branch. 1977.
- Snedecor GW, Cochran WG. Statistical methods applied to experiments in agriculture and biology. 5th ed. Ames, Iowa: Iowa State University Press. 1956.
- Eggleston G. Positive aspects of cane sugar and sugar cane derived products in food and nutrition. J Agric Food Chem. 2018;66:4007-4012.
- Chungu AS, Kimambo CZM, Bali TAL. Assessment of village level sugar processing technology in Tanzania. Tanzania: Mkuki na Nyota Publishers. 2001.
- Mageean MP, Kristott JU, Jones SA. Physical properties of sugars and their solutions. British Food Manufacturing Industries Research Association. 1991.
- Peacock S, Starzak M. The effect of sucrose inversion on boiling point elevation. Zuckerind. 1995;120:1051-1059.
- Hartel RW, Ergun R, Vogel S. Phase/state transitions of confectionery sweeteners: Thermodynamic and kinetic aspects. Compr Rev Food Sci Food Saf. 2011;10:17-32.
- EOSQ. Egyptian Organization for Standardization and Quality. 2006.
- Polette CMV, Belé JS, Borges MT, Spoto MH, Bernardi MRV. Physicochemical and sensorial characterization of commercial sugarcane syrups. Agric Sci J. 2019;42:808-816.
- Barreto PP, Bettani SR, Borges MT, Bernardi MRV. Physical-chemical and sensory evaluation of different molasses. Brazilian J Agric. 2015;90:217-228.
- Guerra MJ, Mujica MV. Physical and chemical properties of granulated cane sugar "panelas". Food Sci Technol. 2010;30:250-257.
- Ferweez H. Chemical and technological studies on the sugar crops syrup (Treacie). M.Sc. Thesis, Fac Agric Minia Univ. 1997.
- Baikow VE. Manufacturing and refining of raw cane sugar. Elsevier Scientific publishing company. 1982.
- Albanese D, Russo L, Cinquanta L, Brasiello A, Di Matteo M. Physical and chemical changes in minimally processed green asparagus during cold-storage. Food Chem. 2007;101:274-280..
- Shih MC, Kuo CC, Chiang W. Effects of drying and extrusion on colour, chemical composition, antioxidant activities and mitogenic response of spleen lymphocytes of sweet potatoes. Food Chem. 2009;117:114-121.
- Prati P, Moretti RH. Study of clarification process of sugar cane juice for consumption. Food Sci Technol. 2010;30:776-783.
- Bhandari BR, Hartel RW. Co‐crystallization of sucrose at high concentration in the presence of glucose and fructose. J Food Sci. 2002;67:1797-1802.
- Serrano ABC, Amodio ML, Cornacchia R, Rinaldi R, Colelli G. Suitability of five different potato cultivars (Solanum tuberosum L.) to be processed as fresh-cut products. Postharvest Biol Technol. 2009;53:138-144.
- Kubzdela ER, Czapski J, Czaczyk K, Marecik RB. The effect of pre‐treatment and modified atmosphere packaging on contents of phenolic compounds and sensory and microbiological quality of shredded celeriac. J Sci Food Agric. 2014;94:1140-1148.
- Chand K, Shahi NC, Lohani UC, Garg SK. Effect of storage conditions on keeping qualities of jaggery. Sugar Tech. 2011;13:81-85.
Citation: Abdel-Aleem WM (2020) Effect of Sugarcane Juice Pre-treatment on the Quality and Crystallization of Sugarcane Syrup (Treacle). J Food Process Technol 11:834. doi: 10.35248/2157-7110.20.11.834
Copyright: © 2020 Abdel-Aleem WM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.