Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 3
Effect of Different Processing Conditions on the Carotene Content of Different Vegetables
Somya Singh, Lalita Verma, Prachi Shukla, Aparna Agarwal* and Risha GuptaReceived: 28-Mar-2023, Manuscript No. JFPT-23-19714; Editor assigned: 01-Feb-2023, Pre QC No. JFPT-23-19714 (PQ); Reviewed: 15-Feb-2023, QC No. JFPT-23-19714; Revised: 22-Feb-2023, Manuscript No. JFPT-23-19714 (R); Published: 01-Mar-2023, DOI: 10.35248/2157-7110.23.14.991
Abstract
Benefits of β-Carotene are well known, most important being the pro vitamin A activity but experimental evidences are lacking regarding the effect of processing on β-Carotene. Processing is critical in determining the bioavailability of β-Carotene from foods and there is a perception that β-Carotene is destroyed by the heat process involved in cooking of vegetables. This study is intended to determine the stability of β-Carotene when selected vegetables are subjected to various processing techniques. Carrot and spinach were chosen for this study since they are the two most important sources. β-Carotene was quantified from vegetables by Reversed phased High Performance Liquid Chromatography (HPLC) System. The result indicated the control sample reading of carrot and spinach was 16833 μg/100 g and 19383 μg/100 g, the β-Carotene level increased substantially in all the processing conditions. Tray drying proved to be the best processing technique with highest percentage gain. Among the samples, Spinach was found to be the richest source of β-Carotene.
Keywords
β-Carotene; Vitamin A; Reversed Phased High Performance Liquid Chromatography (HPLC); Processing; Tray drying
Introduction
Carotenoids are pigments that can be found in higher plants, algae, fungus, bacteria, and animals like birds and crustaceans. Because plants and microorganisms can only synthesize carotenoids, their presence in animals is due to ingestion via food and accumulation in specific tissues, such as flamingo feathers, egg yolk, and invertebrate exoskeletons. They are found in subcellular organelles in plants i.e. Chloroplasts and chromoplasts Carotenoids are deposited in crystalline form (e.g., in carrot roots and tomato) or as oily droplets (e.g., in mango and paprika) in chloroplasts and serve as accessory pigments in photosynthesis, photo protective pigments, and membrane stabilizers, whereas in chromoplasts, they are deposited in crystalline form (e.g., in carrot roots and tomato) or as oily droplets (e.g., in mango and paprika) [1].
Carotenoids are an essential source of colors and vitamins, and researchers are interested in finding efficient ways to produce them. Both plants and microorganisms are utilized as raw resources in the technological process. However, chemically synthesized carotenoids created in the 1950s were the primary source of carotenoid colors for a long time.
Carotenoids are derived from the leaves, flowers, fruits, seeds, roots, and tubers of plants. They are present in vegetables like carrots, pumpkins, spinach, tomatoes, and fruits like watermelon and raspberries. Carotenoids are significant for their metabolism as provitamin A, with α-Carotene and β-Carotene being the most abundant in plant tissues and having the highest activity (β-Carotene has a biopotency of 100 % while α-Carotene has a biopotency of 53%). Human plasma contains oxygenated carotenoids (xanthophylls), which lack vitamin A activity in general, but their exact function, if any, is not defined.
β-Carotene is a carotenoid compound found in abundance in the human diet and, as a result, in all human tissues, including blood. It is also frequently utilized in medicine due to its potent bioactivity. Among the many roles of β-carotene in the human body, the most significant is provitamin a supply, affecting embryonic development, proper growth, and vision. β-Carotene is used as an orange-red color in many products in the food industry, including non-thermally treated non-alcoholic beverages with a tropical fruit taste, edible fats, cheese, pastry, and ice cream. It acts as a gene inhibitor, as well as having anticancer and antioxidant effects. Orange carrots are the most common source of β-Carotene have shown that when vegetables are subjected to various heat treatments before consuming, factors such as heat, light, chemical treatments, and oxygen exposure may have detrimental effect on several bioactive constituents [2]. Thermal processing affected trans–cis-isomerization of β-Carotene in carrot juice produced on a pilot plant and β-Carotene-containing preparations. While pasteurization and sterilization at 121°C. only resulted in minor isomerization, sterilization at 130°C. And blanching resulted in higher quantities of cis-isomer.
The results of the present study were in accordance with the above studies and statements, as it was seen that β-Carotene level increased substantially in all the processing conditions. Heating the food concentrates it and increases β-Carotene but heating and overcooking for very long time, say over 30 minutes or an hour, decreases β-carotene. A growing body of literature exists on the benefits of β-Carotene but experimental evidences are lacking on the effect of processing on β-Carotene. Thus, the objective of this study is to determine the gain or loss of β-carotene when subjected to different processing conditions such as, blanching, open pan boiling, microwave cooking, pressure cooking and tray drying using High Performance Liquid Chromatography (HPLC) system [3].
Materials and Methods
β-Carotene was quantified from vegetables by Reversed phased HPLC System (LC-10 A, Shimadzu, Kyoto, Japan) by the method of Khalil and Varananis (1996). HPLC is a rapid, efficient and sensitive technique for β-Carotene analysis. HPLC is distinguished from traditional liquid chromatography because operational pressures are significantly higher, up to 350 bars. For extraction, Tetra Hydro Furan (THF) was used in place of acetone for a highly efficient separation and analysis.
Sample collection
β-Carotene determination was carried out in two common vegetables, carrot and spinach, grown in agro ecological conditions of Delhi. These vegetables were purchased from nearby Reliance Fresh store. One kg of each carrot and spinach were purchased, and from that 12 samples of 100 gm were prepared (6 of carrot and 6 of spinach). The pre analyzed samples were washed with running tap water and rinsed with deionized water and kept in inert condition, at-4°C temperature [4]. The carrot samples were grated and Spinach samples were sorted and cut into small pieces and then the samples were mixed well.
Extraction of sample
Carotene was extracted from vegetables by reversed phased HPLC system by the methods. To determine the effect of processing, for both the vegetables, one sample of control was taken and five samples (10 g) of each were subjected to five processing conditions:
A-blanching (70ºC/5 minutes)
B-open pan boiling (15 minutes)
C-microwave cooking (5 minutes)
D-pressure cooking (10 minutes)
E-tray drying (60/5 hours)
Sample from each processing treatment was grinded by the use of mixer to convert it into pureed extract. This extract was then homogenized in 50 mL of THF and then 0.1% Butyrated Hydroxyl Toluene (BHT) was added as an anti-oxidant at the rate of 0.05 g into 50 ml of THF. The resulting extract was filtered through Buchnar’s funnel. The residue was washed twice with THF till it became colourless. About 20 g anhydrous sodium sulphate (a hygroscopic material) was added, and then removed through filtration. Volume of extract was reduced on rotary evaporator. The sample was further washed with 10 mL of THF and refiltered. Filtration was repeated thrice to confirm purity. The extract was transferred to 100 mL volumetric flask and the volume was made up to the mark with THF and water, so that the final extract contained 80% of THF.
Standard preparation of β-Carotene solution
A 100 ppm stock solution of standard β-Carotene was taken. From the 100 ppm stock solution, different concentrations of 10, 5, 3, 2 and 1 ppm standard β-Carotene were prepared with volume made up by THF. The standard solutions were run on HPLC system by isocratic elution. The concentrations of the standards were plotted against the peak area to obtain a standard curve (Figure 1)
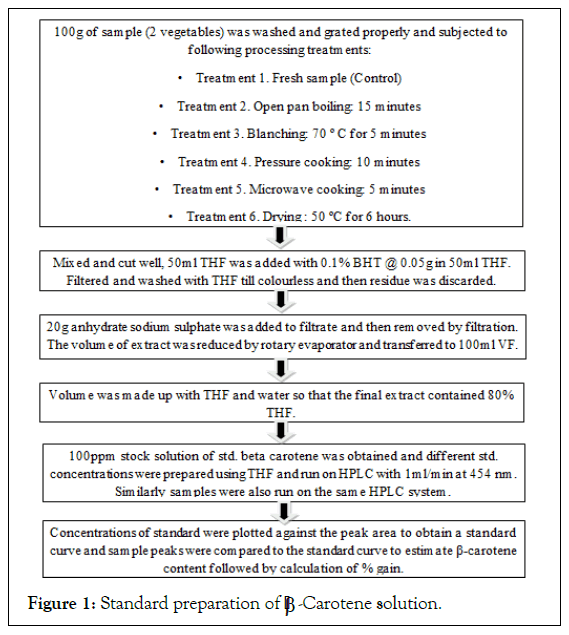
Figure 1: Standard preparation of β-Carotene solution.
HPLC Analysis of β-Carotene
HPLC system was equipped with Photodiode Array (PDA) detector (Serial Presence Detect-M 20A, Shimadzu). β-Carotene was separated on a C-18 column (25 cm–4.6 mm i.d., 5-μm particle size) with 1 mL/min flow rate. Two mobile phases were prepared. For the first one, Acetonitrile (ACN) and water were taken in the ratio of 9:1 and the second was composed of ethyl acetate. The pressure of the column was kept at 1800 psi-2000 psi. All the carotenoids were monitored at 450 nm with PDA detector (Shimadzu, Japan). β-Carotene was quantified from the peak area using the standard curve. The peak identity and λmax of β-Carotene was further confirmed by retention time and characteristic spectra. The β-Carotene peaks were integrated by the computer software to quantify the content of β-Carotene. The sample peaks were compared to the standards for estimation of the β-Carotene concentration.
Calculation
After obtaining the estimated concentration, β-Carotene was estimated in μg/100 g:
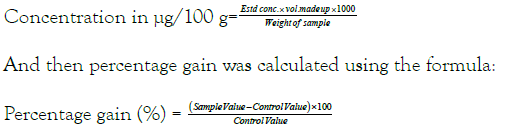
Results and Discussion
The present study was conducted to determine the stability of β-Carotene when vegetables, carrot and spinach, were subjected to different processing conditions. For both vegetables, one sample of control was taken and five samples (10 g) of each were subjected to five processing conditions: A-blanching (70ºC/5 minutes), B-open pan boiling (15 minutes), C-microwave cooking (5 minutes), D-pressure cooking (10 minutes) and E-tray drying (60ºC/5 hours). β-Carotene was quantified from vegetables by Reversed phased HPLC System with flow rate of 1 ml/min at 454 nm followed by calculation of percentage gain/loss.
The carotenoid content of the control sample were carrot and spinach were 16833 μg/100 g and 19383 μg/100 g, respectively. The β-Carotene level increased substantially in all the processing conditions. In carrots, percentage gain for A, B, C, D, E came out to be 91%, 1.5%, 4%, 51% and 1041%, respectively. In spinach, percentage gain was 52%, 84%, 100%, 55% and 1375%, respectively.
The results for the five processing conditions have been summed up in Tables-1 and 2 and Figures 2 and 3, respectively
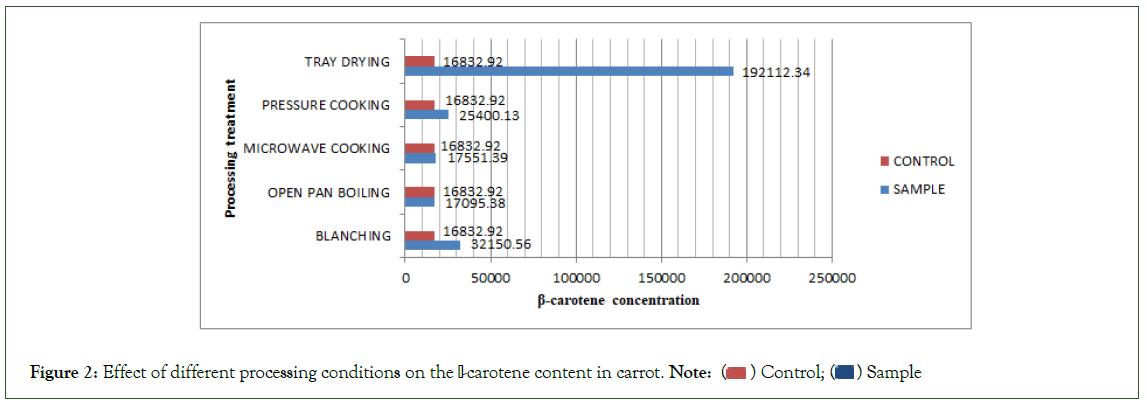
Figure 2: Effect of different processing conditions on the β-carotene content in carrot. Note: ( ) Control; (
) Control; (  ) Sample
) Sample
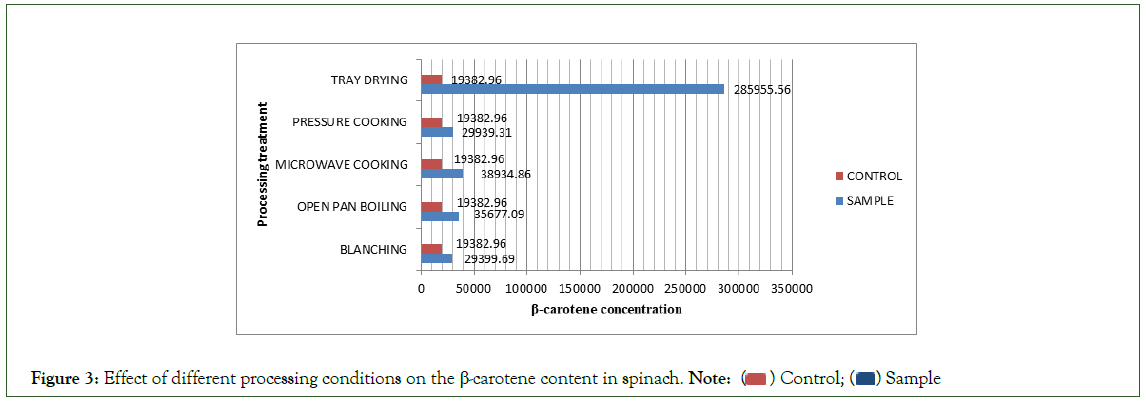
Figure 3: Effect of different processing conditions on the β-carotene content in spinach. Note: ( ) Control; (
) Control; (  ) Sample
) Sample
| S.NO. | Sample | Estd Conc | Vol. Make Up (ml) | Weight (G) | Final Conc. (µg/100 g) | Percentage gain (%) |
|---|---|---|---|---|---|---|
| 1 | Carrot Control | 1.863 | 50 | 5.5338 | 16832.92 | - |
| 2 | Carrot Blanched | 3.309 | 50 | 5.1461 | 32150.56 | 91% |
| 3 | Carrot Open Pan Boiled | 1.712 | 50 | 5.0072 | 17095.38 | 1.50% |
| 4 | Carrot Microwave Cooked | 1.759 | 50 | 5.011 | 17551.39 | 4% |
| 5 | Carrot Pressure Cooked | 2.663 | 50 | 5.2421 | 25400.13 | 51% |
| 6 | Carrot Tray Dryed | 19.441 | 50 | 5.0598 | 192112.34 | 1041% |
Table 1: Final concentration and percentage gain for control and processed samples of carrot.
| S.NO. | SAMPLE | ESTD CONC | VOL MAKE UP (ml) | WEIGHT (g) | FINAL CONC. (µg/100 g) | PERCENTAGE GAIN (%) |
|---|---|---|---|---|---|---|
| 1 | SPINACH CONTROL | 2.317 | 50 | 5.9769 | 19383.96 | - |
| 2 | SPINACH BLANCHED | 3.152 | 50 | 5.3606 | 29399.69 | 51% |
| 3 | SPINACH OPEN PAN BOILED | 3.768 | 50 | 5.2807 | 35677.09 | 84% |
| 4 | SPINACH MICROWAVE COOKED | 4.037 | 50 | 5.1843 | 38934.86 | 100% |
| 5 | SPINACH PRESSURE COOKED | 3.098 | 50 | 5.1738 | 29939.31 | 54% |
| 6 | SPINACH TRAY DRIED | 23.704 | 50 | 5.1447 | 285955.56 | 1375% |
Table 2: Final concentration and percentage gain for control and processed sample of spinach.
Carrot
The β-Carotene content of control sample of carrot was 16832.82 μg/100 g. The β-Carotene of carrot was in fair agreement with the result reported in which β-Carotene was 14000 μg/100 g. The β-Carotene content increased significantly in all the five processing conditions. In tray drying, the percentage gain was maximum 1375%. This was due to absolute removal of water and concentration of the sample thus the powder form being the richest β-Carotene concentrate. In blanching, the content increased to 91%. Blanching being a mild heat treatment proved to the best cooking method followed by pressure cooking, 51%. High temperature treatments such as Open pan boiling and Microwave cooking had relatively very low increase of 1.5% and 4%, respectively. This was because carrot does not have moisture content as high as spinach so high temperature after certain time leads to losses in β-Carotene have reported that boiling for one hour of carrots resulted in approximately 50% loss. In this study, boiling (15 minutes) and microwave cooking (5 minutes) were both done for short intervals thus no decrease was seen but the increase was quite small. Hence, we can say high temperatures after a short while leads to losses thus cooking should to be optimized to prevent losses. Another study suggests that carrot that contain maximum amount of β-Carotene (11210 μg/100 g) [5]. It appeared that their sample of carrot may have higher moisture content as compared to those analyzed in the present study and the variation in tomato content of β-Carotene may be due to the use of immature samples, because content of β-Carotene drops by 77% during the ripening process [6].
Spinach
The control sample reading of Spinach was 19382.96 μg/100 g. This was different than the content reported by 9000 μg/100 g. This was maybe due to variation in variety, area, season, soil, environmental conditions, etc., [7-15]. In spinach also, all the five processing conditions showed substantial increase with tray drying showing the maximum gain of 1375%. In Spinach, in a slight contrast to carrot, treatments that required very high temperatures such as microwave cooking and open pan boiling had a greater increase of 100% and 84%, respectively as compared to blanching, 52% and pressure cooking 55%. The reason for this contrast was maybe that spinach has a very high moisture content of about 95% so since the moisture content was high the concentration was greater in the case of spinach at very high temperatures and thus the high percentage gains. From another data it was evident that dark green vegetables contained more β-Carotene as compared to other vegetables e.g. spinach contained 9940 μg/100 g, followed by mint, kulfa, lettuce and lady finger that were all dark green in appearance., who analyzed 24 green vegetables for different micro nutrients contents including β-Carotene [16-25]. They reported that β-Carotene content in green vegetables ranges from 80 μg-9204 μg/100 gm the above results were supported by Bioavailability of Carotene is Lower in raw than in Processed Carrots and Spinach in Women.
Heat treatment of vegetables promotes conversion of the Trans isomer of β-Carotene to cis forms, which is biologically inactive. As a point of practical application, the thermal processing of spinach and carrot did not negate the enhanced bioavailability of β-Carotene, relative to raw vegetables. In fact, it was seen, carotene loss was minimal with moderate cooking, and in many cases, β-Carotene become more bio available after cooking, probably because heat processing liberates them from cell matrices. Another reason for the retention of carotenoids may be the inactivation or reduction in the activity of peroxidase and lipoxygenase that are involved in carotenoid destruction [25-34]. Thus, we can say, heating foods that contain β-Carotene doesn't destroy the β-Carotene. In fact, it makes it more available by breaking down the walls of the plant cells that contain β-Carotene. This is why, high percentage gains were noted in all the five processing conditions.
Conclusion
There is a perception that β-Carotene is destroyed by the heat process involved in cooking of vegetables. In fact, carotenoid loss is minimal with moderate cooking, and in many cases, β-Carotene become more bio available after cooking, probably because heat processing liberates them from cell matrices. Another reason for the retention of carotenoids may be the inactivation or reduction in the activity of peroxidase and lipoxygenase that are involved in carotenoid destruction. As a point of practical application, the thermal processing of spinach and carrot did not negate the enhanced bioavailability of β-Carotene, relative to raw vegetables Thus, we can say, heating foods that contain -carotene doesn't destroy the β-carotene. In fact, it makes it more available by breaking down the walls of the plant cells that contain β-Carotene.
The salient findings and conclusions emerging from this study are as follows
Tray drying emerged as the best processing condition with a huge percentage gain of 1375% in spinach and 1041% in carrot. Blanching and pressure cooking proved to better cooking conditions in carrot while very high temperature treatments like open pan boiling and microwave cooking proved to be better in spinach owing it to it’s very high moisture content. In carrot, the percentage gain for blanching and pressure cooking was 91% and 51%, respectively but for microwave cooking and open pan boiling was 4% and 1.5%. In contrast, spinach readings for blanching and pressure cooking were 51% and 54%, respectively but for microwave cooking and open pan boiling were very high, 100% and 84%.It was noted that β-Carotene increased to high amounts for temperatures for short time. If subjected to continuous high temperature treatment for long intervals, losses may occur up to 50%. The widely held belief that fresh produce is nutritionally superior to commercially processed produce is not valid with regard to β-carotene because the bioavailability of β- carotene was enhanced as a result of food processing.
References
- Marx M, Stuparic M, Schieber A, Carle R. Effects of thermal processing on trans–cis-isomerization of β-carotene in carrot juices and carotene-containing preparations. Food Chem. 2003; 83(4):609-617.
- Mamatha BS, Arunkumar R, Baskaran V. Effect of processing on major carotenoid levels in corn (Zea mays) and selected vegetables: Bioavailability of lutein and zeaxanthin from processed corn in mice. Food Bioprocess Technol. 2012;5(2):1355-1363.
- Khalil IA, Varananis FR. Carotenoid extraction and analysis by reversed phase HPLC system. Sarhad J Agric. 1996; 105(67):15-21.
- Lozano-Alejo N, Carrillo GV, Pixley K, Palacios-Rojas N. Physical properties and carotenoid content of maize kernels and its nixtamalized snacks. Innov Food Sci Emerg Technol. 2007; 8(3):385-389.
- Rego ER, Finger FL, Casali VW, Cardoso AA. Inheritance of fruit color and pigment changes in a yellow tomato (Lycopersicon esculentum Mill.) mutant. Genet Mol Biol. 1999;22(1):101-104.
- Agte VV, Tarwadi KV, Mengale S, Chiplonkar SA. Potential of traditionally cooked green leafy vegetables as natural sources for supplementation of eight micronutrients in vegetarian diets. J Food Compost Anal. 2000; 13(6):885-891.
- Rock CL, Lovalvo JL, Emenhiser C, Ruffin MT, Flatt SW, Schwartz SJ. Bioavailability of β-carotene is lower in raw than in processed carrots and spinach in women. J Nutr. 1998; 128(5):913-916.
- Rodriguez-Amaya DB. Carotenoids and food preparation: the retention of provitamin A carotenoids in prepared, processed and stored foods. Arlington, VA: John Snow Incorporated/OMNI Project. 1997.
- Anjum F, Khan BA, Noreen N, Masood T, Faisal S. Effect of boiling and storage on beta-carotene content of different vegetables. J life soc sci. 2008;6(1):63-7.
- Baloch AK, Buckle KA, Edwards RA. Effect of processing variables on the quality of dehydrated carrot: II. Leaching losses and stability of carrot during dehydration and storage. Int J Food Sci Technol. 1977;12(3):295-307.
- Clevidence B, Paetau IN, Smith JC. Bioavailability of carotenoids from vegetables. HortScience. 2000; 35(4):585-587.
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50(10):3010-3014.
- Gimeno E, Castellote AI, Lamuela RM, Raventós M, Boronat MC. Rapid high-performance liquid chromatographic method for the simultaneous determination of retinol, α-tocopherol and beta-carotene in human plasma and low-density lipoproteins. J Chromatogr. 2001;758(2):315-352.
[Crossref]
- Hackett MM, Lee JH, Francis D, Schwartz SJ. Thermal stability and isomerization of lycopene in tomato oleoresins from different varieties. J Food Sci. 2004;69(7):536-41.
- Huck KL, Popp H. Scherz, GK Bonn. Development and evaluation of a new method for rapoid carotenoid determination in vegetables. J Chromatogr Sci. 2000; 43(3):21-65.
- Kala A, PRAKASH J. Nutritional composition and sensory profile of microwave and conventionally cooked vegetables. J Food Sci. 2004; 15(1):1745-4506.
- Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001; 385(1):28-40.
- Lightstone A. Eye health and sight loss: the UK Vision Strategy: the UK Vision Strategy. Journal of Family Health Care. 2008 Oct 1;18(5):162-165.
- Livny O, Reifen R, Levy I, Madar Z, Faulks R. β-carotene bioavailability from differently processed carrot meals in human ileostomy volunteers. Eur J Nutr. 2003;42(1):338-345.
- Nguyen M, Francis D, Schwartz S. Thermal isomerisation susceptibility of carotenoids in different tomato varieties. Journal of the Science of Food and Agriculture. 2001; 81(9):910-917.
- Nishino H, Tokuda H, Satomi Y, Masuda M, Onozuka M. Cancer prevention by carotenoids. Pure Appl Chem. 1999; 71(12):2273-2278.
- Rajyalakshmi P, Venkatalaxmi K, Venkatalakshmamma K, Jyothsna Y, Suneetha V. Total carotenoid and beta-carotene contents of forest green leafy vegetables consumed by tribals of south India. Plant Foods Hum Nutr. 2001;56(4):225-238.
- Sanjuan N, Benedito J, Clemente G, Mulet A. The influence of blanching pretreatments on the quality of dehydrated broccoli stems. Food Sci Technol Int. 2000; 6(3):227-234.
- Stahl W, Schwarz W, Sundquist AR, Sies H. Cis-trans isomers of lycopene and β-carotene in human serum and tissues. Arch Biochem Biophys. 1992; 294(1):173-177. [Crossref]
- Van Jaarsveld PJ, Faber M, Tanumihardjo SA, Nestel P. β-Carotene-rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am J Clin Nutr. 2005; 81(5):1080-1087.
- You CS, Parker RS, Goodman KJ, Swanson JE, Corso TN. Evidence of cis-trans isomerization of 9-cis-beta-carotene during absorption in humans. Am J Clin Nutr. 1996; 64(2):177-183.
- During A, Smith MK, Piper JB, Smith JC. β-Carotene 15, 15′-Dioxygenase activity in human tissues and cells: evidence of an iron dependency. J Nutr Biochem. 2001;12(11):640-647.
- Holden JM, Eldridge AL, Beecher GR, Buzzard IM, Bhagwat S. Carotenoid content of US foods: an update of the database. J Food Compos Anal. 1999; 12(3):169-196.
- Guo WH, Tu CY, Hu CH. Cis-trans isomerizations of β-carotene and lycopene: A theoretical study. The J Phys Chem B. 2008; 112(38):12158-12167.
- Kuki M, Koyama Y, Nagae H. Triplet-sensitized and thermal isomerization of all-trans, 7-cis, 9-cis, 13-cis and 15-cis isomers of. Beta.-carotene: configurationally dependence of the quantum yield of isomerization via the T1 state. J Phys Chem. 1991; 95(19):7171-7180.
- Vásquez-Caicedo AL, Schilling S, Carle R, Neidhart S. Effects of thermal processing and fruit matrix on beta-carotene stability and enzyme inactivation during transformation of mangoes into purée and nectar. Food Chem. 2007;102 (1):1172–1186.
[Crossref]
- Tang YC, Chen BH. Pigment change of freeze-dried carotenoid powder during storage. Food Chem. 2000; 69(1):11-17.
- Chen BH, Huang JH. Degradation and isomerization of chlorophyll a and β-carotene as affected by various heating and illumination treatments. Food Chem. 1998; 62(3):299-307.
- Breitenbach J, Sandmann G. ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta. 2005; 220(1):785-793.
Citation: Singh S, Verma L, Shukla P, Agarwal A, Gupta R (2023) Effect of Different Processing Conditions on the Carotene Content of Different Vegetables. J Food Process Technol.14:991.
Copyright: © 2023 Singh S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


