Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 17, Issue 1
Diagnostic Value of Multimodal Ultrasound in Cytological Diagnosis of Indeterminate Thyroid Nodules
Ruijuan Huang1,2,3*, Shuzhen Cong1,2, Yanyan Liang3 and Zhenyi Lin32Department of Ultrasound, Guangdong Academy of Medical Sciences/Guangdong General Hospital, Guangzhou, Guangdong, P.R. China
3Department of Ultrasound, Guangdong Medical University, Yangjiang, P.R. China
Received: 06-Sep-2023, Manuscript No. BLM-23-22867; Editor assigned: 08-Sep-2023, Pre QC No. BLM-23-22867 (PQ); Reviewed: 22-Sep-2023, QC No. BLM-23-22867; Revised: 13-Jan-2025, Manuscript No. BLM-23-22867 (R); Published: 20-Jan-2025, DOI: 10.35248/0974-8369.25.17.767
Abstract
Background: This study aimed to investigate the discriminative diagnostic potential of multimodal Ultrasound (US) for thyroid nodules displaying Indeterminate Cytological (IC) characteristics.
Methods: A total of 61 thyroid nodules (Bethesda III/IV/V) that underwent both fine needle aspiration biopsy and surgical intervention were examined, comprising 37 malignant and 24 benign nodules. Distinctions in sonographic features between benign and malignant nodules were evaluated. Logistic regression analysis was conducted to establish prediction models utilizing conventional US, Superb Microvascular Imaging (SMI), Ultrasound Elastography (UE), and multimodal US. The assessment of sensitivity, specificity, and accuracy in identifying malignant thyroid nodules with IC was carried out for conventional US, SMI, UE, and multimodal US. Receiver Operating Characteristic (ROC) curves were generated, and the Area Under the Curve (AUC) values were computed and compared.
Results: The sensitivity, specificity, and accuracy rates were as follows: 62.05%, 64.86%, and 63.93% for conventional US; 70.83%, 72.97%, and 72.13% for SMI; 83.33%, 83.78%, and 83.61% for UE; and 91.67%, 91.89%, and 91.80% for multimodal US, respectively. The AUC values for evaluating benign and malignant thyroid nodules with IC were as follows: 63.7% for conventional US, 71.9% for SMI, 83.6% for UE, and 86.70% for multimodal US. Multimodal US exhibited the highest diagnostic efficacy in identifying malignant nodules.
Conclusions: Preliminary clinical findings indicate that conventional US, SMI, and UE hold diagnostic value in distinguishing thyroid nodules with IC. Multimodal US imaging enhances diagnostic efficacy for identifying malignant nodules and provides additional insights into the differentiation between benign and malignant nodules.
Keywords
Multimodal ultrasound; Ultrasound elastography; Superb microvascular imaging; Thyroid nodules
Abbreviations
US: Ultrasound; IC: Indeterminate Cytological; SMI: Superb microvascular imaging; UE: Ultrasound Elastography; ROC: Receiver Operating Characteristic; AUC: Area Under the Curve; FNAC: Fine Needle Aspiration Cytology; ACR: American College of Radiology; TI-RADS: Thyroid Imaging Reporting and Data System; CDFI: Color Doppler Flow Imaging
Introduction
Thyroid nodules are a prevalent and frequently encountered medical condition, with an occurrence rate reaching as high as 68% among the general population [1]. The primary diagnostic approach widely employed for assessing whether thyroid nodules are benign or malignant is Fine Needle Aspiration Cytology (FNAC). Nevertheless, owing to the presence of overlapping cytological characteristics and inherent limitations in cytological testing methods, approximately 25% of thyroid FNAC outcomes fail to provide a precise diagnosis for distinguishing between benign and malignant thyroid nodules. The Bethesda Reporting System for Thyroid Cell Pathology defines nodules with Indeterminate Cytological (IC) results as those of Bethesda category III (cellular atypical lesions or follicular lesions with unclear significance), Bethesda category IV (follicular tumors or suspected follicular tumors), and Bethesda category V (suspected malignant tumors) with a malignancy rate of 10%–30%. Differentiating between benign and malignant thyroid nodules with IC is a challenging topic in medical practice. Ultrasound (US) examination has the advantages of simple operation, high cost-effectiveness, and no radiation, making it the preferred method for screening and diagnosing thyroid nodules [2]. Although conventional US has a high clinical value, due to the diversity of thyroid nodule morphology and pathology, as well as the complexity of disease course, some benign and malignant nodules are difficult to distinguish. Based on this, multimodal US has emerged, which combines conventional US with new US imaging technologies, such as Ultrasound Elastography (UE) and Superb Microvascular Imaging (SMI). With the complementary effect of assessing different information, this method comprehensively evaluates thyroid nodules to improve the specificity, sensitivity, and accuracy of their diagnosis. The present study explored the application value of multimodal US in thyroid nodules with IC findings [3].
Materials and Methods
The current investigation took place at Guangdong General Hospital, spanning from January 2021 to April 2022. It involved the scrutiny of 174 thyroid nodules found in 155 consecutive patients who were initially classified as having Indeterminate Cytology (IC) following Fine-Needle Aspiration Cytology (FNAC) examination [4].
Out of these nodules, 78 found in 62 patients who didn't undergo surgical procedures at our hospital, one nodule identified in a single patient who didn't undergo pre-surgical multimodal Ultrasound (US) examination, and 34 nodules in 33 patients with unclear multimodal US images were excluded from the study (Figure 1). Consequently, the study encompassed 61 thyroid nodules situated within 59 patients. This group comprised 38 females and 21 males, ranging in age from 23 to 73 years, with an average age of (44.1 ± 11.1) years. All nodules were pathologically examined post-surgery (n=61).
There were 37 malignant nodules, including 32 papillary thyroid nodules, four follicular thyroid carcinomas, and one medullary thyroid nodule. In addition, 24 benign lesions were recorded,including 14 nodular goiter cases, eight thyroid adenomas, and two thyroid inflammatory lesions (Figure 1).
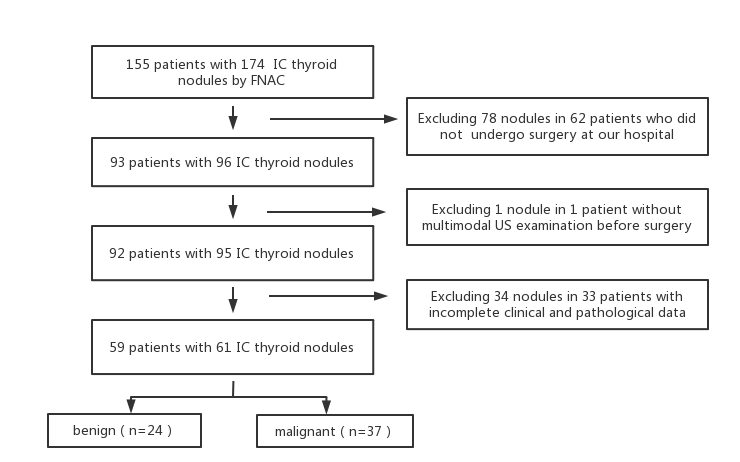
Figure 1: Inclusion criteria for the study.
All patients underwent examination using three imaging modes (conventional US, SMI, and UE) with a high-frequency transducer (L14-5, Aplio500) before their surgical procedures. Patients were positioned supine, with their heads tilted backward to fully expose their necks, and were instructed to hold their breath and avoid swallowing [5].
Initially, a conventional Ultrasound (US) assessment was performed to evaluate the ultrasound characteristics of the thyroid nodules. In this study, we employed the Thyroid Imaging Reporting and Data System (TI-RADS) classification system, established by the American College of Radiology (ACR). This system assesses five aspects of each nodule, including its composition, echogenicity, margin, shape, and calcification. A numerical score was assigned to each of these features, ultimately determining the TI-RADS classification for each nodule. All thyroid nodules were divided into five categories: 1, 2, 3, 4, and 5. Category 1–3 nodules are considered to be likely benign, and category 5 nodules are highly suspicious of being malignant [6].
Superb microvascular imaging
Next, SMI was routinely performed by the same ultrasonographer who carried out the conventional US. SMI has two modes: Color and monochrome. Because the latter has greater sensitivity, the monochrome mode was selected for the study. A double-screen function, which allowed simultaneous visualization of grayscale imaging and SMI, was used when vessels were located [7]. Blood flow distribution pattern in thyroid nodules was classified based on the semi-quantitative method proposed by Kim et al., as follows: Type I, there is no blood flow or a small amount of blood flow in the nodules and 1–2 point or short rod-shaped blood flow signals can be seen; type II, with ≥ 3 blood flow signals and mainly peripheral blood flow; type III, with ≥ 3 blood flow signals, with mainly central blood flow; and type IV, a mixed type with ≥ 2 blood flow signals present around and in the center of the nodule. Type III in SMI images suggested a benign nodule, while type I/II/IV suggested a malignant nodule.
Ultrasound elastography
UE was routinely performed by the same ultrasonographer who first performed the US and SMI examinations. The area of interest was set to be larger than the nodule to encompass the normal tissue surrounding it. A handheld probe was used to deliver small vibrations at the lesion site approximately 1–2 times/s so that the pressure curve on the instrument display screen appeared green. After obtaining a stable image, the longitudinal and transverse elastic section images were saved. The scoring criteria based on a 0–4-point scale proposed by Professor Ino of the University of Tsukuba in Japan (10) was utilized as follows: 0 points (the lesion area tissue appears redblue or blue-green-red), 1 point (the lesion area tissue and surrounding tissue appear as green with no significant color difference), 2 points (the lesion area tissue appears as a mixture of blue and green, and the proportion of green is more than 50%), 3 points (the tissue in the lesion area is mixed with bluegreen color, and the proportion of blue is more than 50%), and 4 points (the tissue in the lesion area shows an inconspicuous blue color difference). For UE images, a score of fewer than 3 points suggested a benign nodule, while a score of 3 points and higher indicated a malignant nodule [8].
To avoid interobserver variability, the above operations were independently completed by two ultrasonographers with 5 and 10 years of experience, respectively. In case of a disagreement, the opinions of experienced third-party ultrasonographers were obtained and decisions were discussed to reach the final diagnostic conclusion.
Fine needle aspiration cytology
All patients underwent FNAC with a high-frequency transducer (L13-5, HI VISION Ascendus) after US examination. The patients were maintained in a supine position with the head tilted backward to fully expose the neck. The patients were asked to hold their breath and avoid swallowing. The body surface position, puncture route, and puncture depth for the nodules were marked via US. After disinfecting the puncture point, a towel was spread over the surgical area and 2% lidocaine was used to anesthetize the puncture point. A 22G syringe was used under US guidance to puncture the target nodule. After performing negative pressure suction of cytological samples, they were fixed, stained, and sent to the pathology department for cytological examination [9]. According to the 2017 Bethesda reporting system, the diagnoses were as follows: (I) nondiagnostic or unsatisfactory; (II) benign; (III) atypical lesions of unknown significance or follicular lesions of unknown significance; (IV) follicular tumors or suspicious follicular tumors with unclear origin and atypical morphological characteristics; (V) suspected malignant tumor; and (VI) malignant tumors.
Statistical method
Statistical analyses were performed using the SPSS version 25.0 statistical package (IBM Corporation, Armonk, NY, USA). The Student’s t test was used to compare patient age between the malignant and benign nodule groups [10]. In addition, the χ2 test was used to analyze the differences in the distributions of conventional US, SMI, and UE features among the groups. To investigate whether the combination of the three methods facilitated the diagnoses of thyroid nodules with IC, their diagnostic value was compared using receiver operating characteristic curve (ROC) analysis. The Z test was used to compare the area under the ROC (Az). Statistical significance was defined as P<0.05 (twosided).
Results
Conventional US, SMI, and UE features associated with benign and malignant IC thyroid nodules.
In conventional US, malignant thyroid nodules with IC are manifested as those with solid, hypoechogenicity or marked echogenicity, an aspect ratio of >1, poorly defined, no calcification and TI-RADS classification 5 categories (all P<0.05; Figure 2). SMI examination showed that malignant thyroid nodules with IC were mainly characterized by type III blood flow patterns (P<0.05; Figure 3). UE examination showed that IC thyroid nodules were classified as those with scores of ≥ 3 points (P<0.01; Figure 4). These details are presented in Table 1.
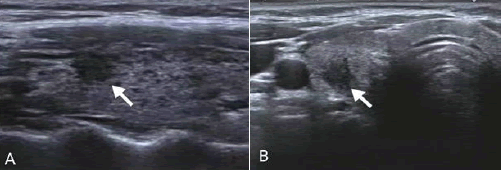
Figure 2: Images obtained from a 44-year-old female patient who underwent routine diagnosis of thyroid papillary carcinoma.
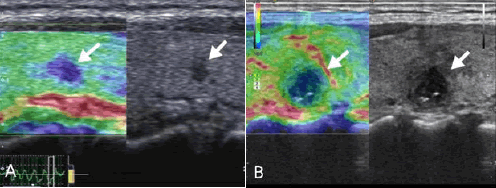
Figure 3: An Images obtained from a 48-year-old male patient who underwent routine diagnosis of thyroid papillary carcinoma.
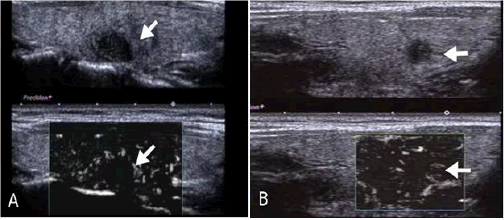
Figure 4: A Images obtained from a 38-year-old female patient who underwent routine diagnosis of thyroid papillary carcinoma.
| Variables | Classification | Pathological results | χ² | P-value | |
|---|---|---|---|---|---|
| Benign nodules (n=24) | Malignant nodules (n=37) | ||||
Components |
Mixed (n=1) |
1 (4.2) |
0 (0.0) |
- |
0.393f |
Solid (n=60) |
23 (95.8) |
37 (100.0) |
|||
Echogenicity |
Iso or hyperechogenicity (n=8) |
7 (29.2) |
1 (2.7) |
6.775 |
0.009 |
Marked or hypoechogenicity or hypoechogenicity (n=53) |
17 (70.8) |
36 (97.3) |
|||
Shape |
Wider than tall (n=35) |
18 (75.0) |
17 (45.9) |
5.025 |
0.023 |
Taller than wide (n=26) |
6 (25.0) |
20 (54.1) |
|||
| Margin | Well defined (n=18) |
12 (50.0) |
6 (16.2) |
7.988 |
0.005 |
Poorly defined (n=43) |
12 (50.0) |
31 (83.8) |
|||
Calcification |
No calcification (n=36) |
13 (54.2) |
23 (62.2) |
9.678
|
0.008
|
Macrocalcification (n=12) |
9 (37.5) |
3 (8.1) |
|||
Microcalcification (n=13) |
2 (8.3) |
11 (29.7) |
|||
TI-RADS classification |
3 (n=5) |
4 (16.7) |
1 (2.7) |
-
|
0.012f
|
4 (n=20) |
11 (45.8) |
9 (24.3) |
|||
5 (n=33) |
9 (37.5) |
27 (73.0) |
|||
SMI |
I (n=7) |
5 (20.8) |
2 (5.4) |
-
|
<0.001f
|
II (n=11) |
4 (16.7) |
7 (18.9) |
|||
III (n=34) |
7 (29.2) |
27 (73.0) |
|||
IV (n=9) |
8 (33.3) |
1 (2.7) |
|||
UE |
0–2 score (n=26) |
20 (83.3) |
6 (16.2) |
26.814 |
<0.001 |
3≥ score (n=35) |
4 (16.7) |
31 (83.8) |
|
||
Note: f represents the use of Fisher's exact test. |
|||||
Table 1: Features of conventional US, SMI, and UE associated with benign and malignant IC thyroid nodules.
Diagnostic efficacy of conventional US, SMI, UE, and multimodal US
The sensitivity of multimodal US diagnosis was not significantly different from that of conventional US, SMI, and UE alone (P>0.05), but the specificity, accuracy, and AUC of multimodal US in IC thyroid nodules were higher than those of conventional US, SMI, and UE alone (P<0.05; Table 2 and Figure 5).
| Methods | Pathological results | Sensitivity | Specificity (%) | Accuracy (%) | AUC | ||
|---|---|---|---|---|---|---|---|
| Benign nodules (n=24) | Malignant nodules (n=37) | ||||||
Conventional US |
Benign |
15 |
13 |
62.05 |
64.86 |
63.93 |
0.637 |
Malignant |
9 |
24 |
|||||
SMI |
Benign |
17 |
10 |
70.83 |
72.97 |
72.13 |
0.719 |
Malignant |
7 |
27 |
|||||
UE |
Benign |
20 |
6 |
83.33 |
83.78 |
83.61 |
0.836 |
Malignant |
4 |
31 |
|||||
Multimodal US |
Benign |
22 |
3 |
91.67 |
91.89 |
91.8 |
0.867 |
Malignant |
2 |
34 |
|||||
Test |
|
|
|
6.84 |
9.25 |
16.08 |
-0.579 |
P |
|
|
|
>0.05 |
<0.05 |
<0.01 |
<0.01 |
Table 2: Diagnostic efficacy of conventional US, SMI, UE, and multimodal US.
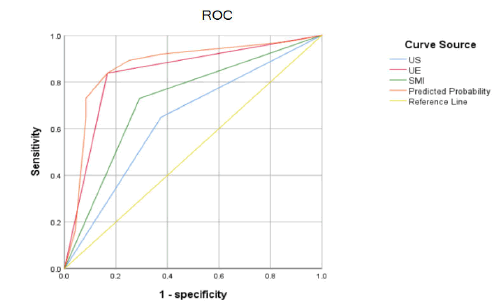
Figure 5: ROC curves for conventional US, SMI, UE, and multimodal US for diagnosis of benign and malignant thyroid nodules with IC.
Discussion
Thyroid nodules are common and can present as benign or malignant. Clinical treatment of thyroid nodules typically takes a surgical approach, and thyroid nodules of different nature have significantly different prognoses due to the different surgical options and the extent of resection. For thyroid nodules with high morbidity, the challenge for clinicians is to properly select malignant thyroid nodules to develop the next treatment strategy. FNAC is the most frequently used diagnostic method for evaluating benign and malignant thyroid nodules. However, approximately 25% of diagnostic results for FNAC show IC features. American Thyroid Association guidelines on thyroid diagnosis recommend a repeat FNAC after three months for these nodules [11]. However, in clinical practical work, patients lack awareness of thyroid nodules and are stressful. In order to obtain a definite diagnosis, most cases do not undergo a repeat FNAC after three months and unilateral lesion lobectomy or molecular marker testing is selected instead. Unilateral lobectomy causes excessive treatment for patients with benign nodules, and the high cost of molecular marker testing imposes a significant economic burden on patients, limiting its wide use in clinical practice. Therefore, differentiating benign and malignant IC thyroid nodules is challenging, and determining how to accurately evaluate them is of paramount importance in guiding clinical treatment and management.
Conventional US examination has the advantages of simple operation, high cost effectiveness, and no radiation, making it the preferred method for screening and diagnosing thyroid nodules. High-frequency US can not only detect thyroid nodules larger than 2–3 mm in diameter, but also help to clearly observe the location, shape, size, number, echogenicity, margin, calcification, Color Doppler Flow Imaging (CDFI) results, and many other nodule features. The American Thyroid Association defines sonographic features associated with malignancy of thyroid nodules as hypoechogenicity, irregular borders, aspect ratio of >1, and presence of microcalcifications [12]. The ACR further revised the TI-RADS based on the breast imaging reporting and data system, adopted a more standard and standardized scoring metric, and proposed to quantitatively score thyroid nodules according to five aspects: components, echogenicity, shape, Margin, and calcification type, thereby evaluating the degree of malignancy risk. TI-RADS class 5 was used as a diagnostic cut-off value in the present study based on prior research. The diagnostic performance of conventional US in identifying IC nodules yielded a sensitivity of 62.05%, specificity of 64.86%, and an overall accuracy of 63.93%. In comparison to previous studies, our findings indicated lower diagnostic accuracy than what has been reported for conventional US in distinguishing between benign and malignant thyroid nodules [13]. This discrepancy may be attributed to the specific nature of the nodules examined in our study.
Traditionally, conventional US have been employed for the evaluation of all types of thyroid nodules. However, the nodules under investigation in our study were categorized as Indeterminate Cytological (IC). These nodules often present with less distinct benign or malignant characteristics, rendering it challenging to make a definitive diagnosis solely through conventional US. Consequently, there is a pressing need to establish a more effective diagnostic method for accurately discerning nodules of this particular type.
SMI is currently the latest imaging technique for blood flow in the world. It uses an adaptive signal processing algorithm with intelligent filtering techniques to eliminate flow signals from tissue motion artifacts, preserves low-velocity flow signals, and detect microvessels (>0.1 mm in diameter) with low flow rates (minimum of 0.8 cm/s), which overcomes the limitations of CDFI and power Doppler flow imaging techniques [14]. Good diagnostic values were demonstrated when SMI was used to evaluate IC nodules in the present study. In terms of diagnosing benign and malignant IC nodules, type III was used as a diagnostic cut-off value based on previous studies. The sensitivity, specificity, and accuracy of SMI were 70.83%, 72.97%, and 72.13%, respectively. These results are comparable to those from a study by Li et al. [15], who have reported a specificity of 79%. The morphology of microfluidic perfusion and vascularity varies between benign and malignant nodules and may be related to the development of nodular metaplasia. Benign thyroid tumors often grow expansively, and the tumor tissue continues to grow and expand in size, resulting in direct extrusion of blood vessels surrounding the tissue and small blood vessels surrounding the thyroid nodule that produce blood flow signals. Malignant tumors often grow via infiltration and destroy their own internal blood vessels, leading to partial ischemia, necrosis, fibrosis, and microcalcification within the tumor. The simultaneous release of proangiogenic factors by malignant tumor cells also stimulates the convergent formation of new blood vessels from distant sites into the tumor.
UE is a more typical method of power-assisted elastography. With the aid of autocorrelation analysis, comparing the relative stiffness values between lesioned and surrounding tissue can help to identify benign and malignant focal thyroid lesions and compensate for the lack of conventional US. To some extent, the hardness of thyroid nodules can reflect the histopathological condition. The interior of the benign nodule is embedded with many follicular and gliotic components and has a softer texture, whereas the stroma of the malignant nodule is rich in fibers and blood vessels and contains gritty calcifications with a stiffer texture [16]. The present study evaluated IC nodules using UE and a score of 3 was used as a diagnostic cut-off value based on the previous studies. Nodules were classified as malignant when the nodule elastic score was ≥ 3. Nodules with a score of <3 were classified as benign. The present study results confirmed that the sensitivity, specificity, and accuracy of UE for the IC nodules were 83.33%, 83.78%, and 83.61%, respectively. These results are consistent with those from a study by Zhao et al. [17], who have reported a specificity of 83.19%. The present study results showed that UE has a higher specificity and accuracy than conventional US and SMI in diagnosing IC nodules, and the difference between the two methods was statistically significant (P<0.05). UE had a lower false positive rate compared to that of SMI. A possible reason for this is that flow signals detected by monochrome SMI appear hyperechoic, while calcification or fibrosis within the nodules also appears hyperechoic on SMI, leading to difficulty in nodule differentiation and being prone to missed diagnosis or misdiagnosis. However, four benign nodules with an elasticity score of 4 were misdiagnosed as malignant in the present study. This may have occurred for multiple reasons [18,19]. For example the study encompassed various nodules exhibiting either peripheral circular calcifications or internal semi-arc calcifications. In cases where calcifications were present, they tended to elevate the elasticity score. Conversely, certain nodules displayed a mixed echo pattern, comprising hypoechoic and slight echo patterns as well as irregular forms. These characteristics are often associated with long-term nodule fibrosis, which also contributes to higher elasticity scores. Consequently, Ultrasound Elastography (UE) exhibits certain constraints when it comes to distinguishing between benign and malignant intrathyroidal nodules.
Furthermore, it's worth noting that conventional Ultrasound (US), shear wave imaging (SMI), and UE each possess distinct advantages and drawbacks in the context of diagnosing benign and malignant intrathyroidal nodules.
Multimodal US imaging, combined use of two or more US techniques, and differential diagnosis of benign and malignant thyroid nodules made by integrating the diagnostic advantages of each technique have shown that multimodal US evaluation of the nature of thyroid nodules has a high diagnostic value [20]. Reviewing the nodules misdiagnosed by conventional US and UE in the present study, two false positive nodules that were corrected by multimodal US were identified. One of the false positive nodules diagnosed using conventional US showed solid hypoechogenicity, aspect ratio of >1, macrocalcification, TIRADS score of 8 and 5 categories, and UE score of 3, whereas SMI showed a type II nodule with a perinodular pattern and a predicted probability of <0.5 on multimodal US. Comprehensive analysis diagnosed this nodule as benign, with the final pathological result of nodular goiter with adenomatous hyperplasia. After reviewing the nodules misdiagnosed by SMI, it was determined that a total of five nodules were corrected by multimodal US. One of the false positive nodules showed an SMI type III and was diagnosed as malignant, while conventional US represented it as a solid hypoechoic with aspect ratio of <1, no calcification, ACR TI-RADS category of 4, and UE score of 2. The diagnosis was benign using multimodal US imaging, with the final pathologic result of nodular goiter. The above results may be due to the following reasons. First, with regard to the combination of the three methods, SMI helped to compensate for the disadvantages of UE in cases of calcification and depth. For some smaller malignant lesions, the blood distribution pattern of SMI may show a type I or II pattern, leading to a false negative result. For some nodules with fibrous calcification, SMI may show a hyperechoic nodule, which is similar to the blood flow signal results, leading to a false positive diagnosis. On the other hand, the UE imaging pattern was able to obtain information on the nodule “hardness” through an elasticity score and achieve accurate diagnoses. The elasticity score was affected if the location was too deep or too close to the trachea, or if the nodules presented with peripheral annular calcifications.
In summary, a single US imaging technique often cannot synthesize all of the information about the characteristics of IC thyroid nodules, leading to a one-sided diagnosis. However, the combined application of multimodality US, such as conventional US, SMI, and UE, which comprehensively analyzes different information, may improve the accuracy of malignant diagnosis of IC nodules and help clinicians to stratify and manage their malignancy risk.
There were also some limitations in the present study. On the one hand, the number of nodules we studied was small. On the other hand, the assessments of SMI and UE were not grouped according to nodular size. In future studies, we plan to collect more data, classify nodules according to size, and evaluate the diagnostic performances of SMI and UE within the different groups of nodules.
Conclusion
For thyroid nodules with IC findings that are difficult to define as benign and malignant, multimodal US can provide multidirectional and multi-angle diagnostic information, which is beneficial for a more accurate diagnosis. This method is characterized by its high diagnostic efficacy and will be important for clinical decision-making and disease treatment and management in the future.
Ethics approval and consent to Participate
The research protocol was approved by the Guangdong General Hospital, Guangdong Academy of Medical Sciences Research Ethics Committee (approval letter No.GDREC2018202H (R1)). All participants gave informed consent. We confirmed that the study was carried out in accordance with relevant guidelines and regulations of Helsinki Declaration.
Consent for Publication
This article is distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Availability of Data and Materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Competing Interests
The authors declare that there is no conflict of interest.
Funding
The financial support from the National Natural Science Foundation of China (Project number 8190070543) and Guangzhou Municipal Science and Technology Bureau (Project number 202002030235).
Authors’ Contributions
RH: Planning study design, conducting study, data collection and analysis, and writing and final approval of the manuscript. SC: data collection and analysis, data interpretation and review/ approval, manuscript revision and editing, and final approval of the manuscript. YL: data analysis, writing and final approval of the manuscript. ZL: planning study design, conducting study, data analysis, and writing and final approval of the manuscript.
Acknowledgment
We acknowledge the financial support from the National Natural Science Foundation of China (Project number 8190070543) and Guangzhou Municipal Science and Technology Bureau (Project number 202002030235). In addition, we would like to thank all staff members in the Department of Ultrasound of Guangdong Provincial People’s Hospital and Department of Ultrasound of The Affiliated Yangjiang People’s Hospital of Guangdong Medical University.
References
- Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39(8):699-706.
[Crossref] [Google Scholar] [PubMed]
- Valderrabano P, McIver B. Evaluation and management of indeterminate thyroid nodules: The revolution of risk stratification beyond cytological diagnosis. Cancer Control. 2017;24(5).
[Crossref] [Google Scholar] [PubMed]
- Trimboli P. Advancements in Ultrasound and Ultrasound-Based Risk Stratification Systems for the Assessment of Thyroid Nodule. Cancers. 2022;14(7):1668.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Wei W, Guo R. Ultrasonic elastography and conventional ultrasound in the diagnosis of thyroid micro-nodules. Pak J Med Sci. 2019;35(6):1526.
[Crossref] [Google Scholar] [PubMed]
- Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14(5):587-595.
[Crossref] [Google Scholar] [PubMed]
- Hasegawa J, Suzuki N. SMI for imaging of placental infarction. Placenta. 2016;47:96-98.
[Crossref] [Google Scholar] [PubMed]
- Kim DW, Jung SJ, Eom JW, Kang T. Color Doppler features of solid, round, isoechoic thyroid nodules without malignant sonographic features: A prospective cytopathological study. Thyroid. 2013;23(4):472-476.
[Crossref] [Google Scholar] [PubMed]
- Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Comparison between ultrasonic elastogram and histologic findings in breast diseases. InSeventh Congress of Asian Federation of societies for ultrasound in Medicine and Biology (AFSUMB 2004) 2004.
- Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Eng J Med. 2012;367(8):705-715.
[Crossref] [Google Scholar] [PubMed]
- R HB, K AE, C BK, M DG, J MS, E NY, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid: official journal of the American Thyroid Association. 2016;26(1).
- Shuzhen C. Comparison analysis between conventional ultrasonography and ultrasound elastography of thyroid nodules. Eur J Radiol. 2012;81(8):1806-1811.
[Crossref] [Google Scholar] [PubMed]
- Bonacchi G, Becciolini M, Seghieri M. Superb microvascular imaging: A potential tool in the detection of FNH. J Ultrasound. 2017;20:179-80.
[Crossref] [Google Scholar] [PubMed]
- Jiang L, Zhang D, Chen YN, Yu XJ, Pan MF, Lian L. The value of conventional ultrasound combined with superb microvascular imaging and color Doppler flow imaging in the diagnosis of thyroid malignant nodules: a systematic review and meta-analysis. Front Endocrinol. 2023;14:1182259.
[Crossref] [Google Scholar] [PubMed]
- Li H, Xue J, Zhang Y, Miao J, Jing L, Kang C. Diagnostic efficacy of a combination of the Chinese thyroid imaging reporting and data system and shear wave elastography in detecting category 4a and 4b thyroid nodules. Front Endocrinol. 2023;14:1161424.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Jing H, Han X, Shao H, Sun YX, Wang QC, Cheng W. Shear wave elastography combined with the thyroid imaging reporting and data system for malignancy risk stratification in thyroid nodules. Oncotarget. 2017;8(26):43406.
[Crossref] [Google Scholar] [PubMed]
- Zhang WB, Deng WF, Mao L, He BL, Liu H, Chen J, et al. Comparison of diagnostic value of SWE, FNA and BRAF gene detection in ACR TI-RADS 4 and 5 thyroid nodules. Clin Hemorheol Microcirc. 2022;81(1):13-21.
[Crossref] [Google Scholar] [PubMed]
- Yoon JH, Kim EK, Kwak JY, Park VY, Moon HJ. Application of various additional imaging techniques for thyroid ultrasound: Direct comparison of combined various elastography and Doppler parameters to gray-scale ultrasound in differential diagnosis of thyroid nodules. Ultrasound Med Biol. 2018;44(8):1679-1686.
[Crossref] [Google Scholar] [PubMed]
- Pei S, Cong S, Zhang B, Liang C, Zhang L, Liu J, et al. Diagnostic value of multimodal ultrasound imaging in differentiating benign and malignant TI-RADS category 4 nodules. Int J Clin Oncol. 2019;24:632-639.
[Crossref] [Google Scholar] [PubMed]
- Han Z, Huang Y, Wang H, Chu Z. Multimodal ultrasound imaging: A method to improve the accuracy of diagnosing thyroid TI‐RADS 4 nodules. J Clin Ultrasound. 2022;50(9):1345-1352.
[Crossref] [Google Scholar] [PubMed]
- Huang ST, Zhang B, Yin HL, Li B, Liao JT, Wang YB. Incremental diagnostic value of shear wave elastography combined with contrast-enhanced ultrasound in TI-RADS category 4a and 4b nodules. J Med Ultrason. 2020;47:453-462.
[Crossref] [Google Scholar] [PubMed]
Citation: Huang R, Cong S, Liang Y, Lin Z (2025) Diagnostic Value of Multimodal Ultrasound in Cytological Diagnosis of Indeterminate Thyroid Nodules. Bio Med. 17:767.
Copyright: © 2025 Huang R, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


