Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 14, Issue 3
Development, Validation of a Method for the Determination of Oxaluria by UV HPLC after Derivatisation with O-Phenyldiamine
Belkaid Nawal* and Ben Si Said HassanReceived: 26-Aug-2024, Manuscript No. BABCR-24-26793; Editor assigned: 29-Aug-2024, Pre QC No. BABCR-24-26793 (PQ); Reviewed: 12-Sep-2024, QC No. BABCR-24-26793; Revised: 11-Jun-2025, Manuscript No. BABCR-24-26793 (R); Published: 18-Jun-2025, DOI: 10.35248/2161-1009.25.14.587
Abstract
Introduction: Urinary oxalate is recognized as an important biomarker for the diagnosis and monitoring of hyperoxaluria responsible for oxalocalcic lithiasis.
Objective: The main objective of our study, carried out in the Biochemistry and Analytical Chemistry Laboratory of the Pharmacy Department at the Université Mouloud Mammeri Tizi-Ouzou (UMMTO), is to develop and validate a method for the determination of urinary oxalate by reverse-phase HPLC, to be applied routinely in the biochemistry laboratory for the benefit of patients.
Method: Determination of urinary oxalate by reverse-phase HPLC (C18) after derivatization with ophenylenediamine. Validation according to SFSTP 2006 protocol V2 followed by stability study and establishment of internal quality controls.
Results: The method was validated with analytical specificity within the quantification limit (12.5; 250) mg/L with coefficients of variation ≤ 6%, relative bias ≤ 7%, regression is linear with a linear coefficient of determination of 99.5%, satisfactory sample stability.
Conclusion: Our method is validated and can be used for routine oxaluria determination.
Keywords
Oxalate; SFSTP; HPLC; Hyperoxaluria
Introduction
Oxalic acid is an organic dicarboxylic acid, its chemical formula is C2H2O4 [1]. In solution, it forms salts called oxalates, the solubility depends on several chemical factors such as ionic strength and pH [2]. Under supersaturation conditions, three crystalline forms can be formed; monohydrate called Wewhellite, a dihydrate called Wedellite and rarely trihydrate called Caoxite [3].
Oxalate is mainly produced by the internal metabolism and comes in limited quantities from the diet [4]. In the liver, it is synthesized from glyoxalate; a substance generated during the intermediate metabolism of glycine, hydroxyproline and glycolate [1,5]. The transformation of glyoxalate into glycine is mainly carried out by the enzyme Alanine Glyoxalate Amino Transferase (AGT) present in the peroxisome of human hepatocytes. The process in which vitamin B6 acts as a cofactor [1]. Under normal conditions, only a portion of the glyoxalate is converted to oxalate by the enzyme Lactate Dehydrogenase (LDH) [5-7]. Circulating oxalate is freely filtered from the glomerulus, reabsorbed and secreted by the proximal tubuleand then eliminated by the kidneys in its unchanged form [4].
The presence of urinary crystals is not, except in special cases, pathological in itself. However, in a lithiasis subject, or in certain pathological contexts, crystalluria can therefore be a very useful indicator of lithogenic factors [8].
Hyperoxaluria is characterized by an abnormal increase in the level of oxalate in the urine, which can lead to the formation of oxalo-dependent kidney stones [1,9]. It can rarely causes by genetic mutations (primary hyperoxaluria: HOP) that affect oxalate metabolism, the most common are HOP type 1 characterized by a deficiency of the liver enzyme alanine glyoxylate aminotransferase, HOP type 2 resulting from dysfunction of the cytosolic enzyme glyoxylate/hydroxypyruvate reductase and HOP type 3 where the HOGA1 gene is mutated, thus altering the function of the enzyme 4-hydroxy-2- oxoglutarate aldolase responsible for the degradation of glyoxylate.
The majority of hyperoxalurias are of dietary and metabolic origin (secondary hyperoxalurias). Several diseases of the digestive system increase intestinal absorption of oxalate or reduce its degradation. In certain metabolic diseases; such as diabetes and metabolic syndrome, insulin resistance is responsible for an increase of oxaluria; there is an increase in the level of glyoxylate (a product of glucose metabolism) which subsequently generates oxalate. Furthermore, inflammationinduced changes in intestinal oxalate transport which explain hyperoxaluria due to suppression of active intestinal secretion of oxalate or increased absorption of oxalate.
In addition, a diet rich in oxalate or a lack of hydration can also be the cause of hyperoxaluria.
The frequency of hyperoxaluria and stones oxalate dependent varies depending on the type of hyperoxaluria. Calcium oxalate stones are among the most common types. They represent 70-80% of all kidney stones.
Prevention is one of the most recommended therapies; based on the adoption of a diet low in oxalate and vit C and overhydration.
The measurement of oxalate in the urine is impotant in the diagnosis of hyperoxaluria and especially in the follow-up of patients. It also contributes to the monitoring of treatment effectiveness as well as the prevention of complications associated with hyperoxaluria. Many diverse methods for detecting and quantifying oxalate have been reported over the past. Urinary oxalate analyses are based on enzymatic assays, other analytical methods can also be used such as ion chromatography, Gas Chromatography (GC), and Liquid Chromatography with tandem Mass Spectrometry (LC-MS/MS); although LC-MS/MS has recently gained increasing interest due to its selectivity and high sensitivity, it is not widely adopted due to its high cost and complexity.
In view of the technical difficulties and the unavailability of the urinary oxalate assay in laboratories in our country, the diagnosis and monitoring of hyperoxaluria and kidney stones are more complex. As a result, it was imperative to develop an analytical method to make the measurement of this parameter more accessible to patients.
Therefore, High Performance Liquid Chromatography (HPLC) is of particular interest due to its low cost, ease of use and availability, our goal was to develop a robust and cost-effective HPLC urinary oxalate assay to validate it in order to use it routinely in laboratories.
Materials and Method
Reagents
Oxalic acid dihydrate (purified quality, MM=126.04 g/mol)). Ophenylenediamine (OPD, 99.5% purity). Hydrochloric acid (HCl, 35-38% with MM=36.46 g/mol) and sodium hydroxide (NaOH, MM=40 g/mol). Ammonium acetate (MM=77.08 g/ mol). Methanol (HPLC grade, MM=32.04 g/mol). The pure water was prepared by a water purification system.
Materials
- KERN electric scale
- Vortex IKA MS3
- MEMMERT oven
- Sigma 3 centrifuge
- SHIMADZU LC LC 20 HPLC device
- BOEKI pure water Purifier
Chromatographic conditions
High Performance Liquid Chromatography (HPLC) coupled with a SHIMADZULC 20 ultraviolet detector was used for quantification. HPLC analysis was performed on a shimadzushim-packgistc18 column (150 × 4.6 mm, particle size 5 μm). An isocratic elution of 15% methanol in water containing 0.17 M ammonium acetate was used as the mobile phase for chromatographic separation at a rate of 1 ml/min. The temperature of the column was maintained between 25°C and 29°C. The wavelength of the Ultraviolet (UV) detector has been set at 314 nm.
Analytical validation methodology
This study is based on the SFSTP guidelines published in 2003 and supplemented in 2006. These recommendations are based on the use of the accuracy profile, which takes into account both bias and standard deviation to assess precision. All data was acquired using agilentchemstation software. From the oxalate peak area recorded in the chromatogram and the calibration curve plotted by the peak surface as a function of the calibration concentration, the oxalate concentration in each sample was calculated.
Choice of validation protocol
Areas of application and concentration levels: Since the absence of the matrix effect has already been demonstrated by a prior specificity study (Pooled urine specimens were obtained to construct matrix-matched calibrators), the appropriate protocol is V2. The protocol proposes a minimum of 3 days (3 series), each day includes a calibration standard (SE) without a matrix with 2 replicates, a Validation Standard (SV) with 3 replicates and a blank with a matrix without dosed addition. The choice of concentration levels is established in such a way that the concentrations can cover the range of physiological and pathological values. A range is made by estimating that the overall range is 0.1 mmol/L to 2 mmol/L (12.5 mg/L to 250 mg/L).
Limits of acceptability (λ) and probability of confidence (β): For dosing in a biological matrix and according to SFSTP requirements, λ is set to ± 15% and β=85%.
Solution preparation protocol
Choice of the diluent: For better solubility and stability of oxalate, purified water remains the most favorable diluent, in addition to its compatibility with the mobile phase and the chromatographic system in general. Five stock solutions were prepared in 100 ml vials, from which diluant solutions were obtained for the preparation of the standard and validation range.
Preparing stock solutions: In 100 ml volumetric flasks weighed with 100.8 mg oxalic acid, dissolve in a sufficient volume of the diluent (pure water). A total of five solutions will be prepared; two for calibration and three for validation.
Preparation of calibration and validation standards: From the stock solutions previously prepared at a concentration of 100.8 mg/100 ml (8 mmol/L), dilutions of 3/4, 2/4, 1/4 and 1/20 were performed to obtain the diluant solutions at concentrations of 6 mmol/L, 4 mmol/L, 2 mmol/L, 0.4 mmol/L respectively.
Preparation samples for stability study: Samples loaded with oxalate at a physiological level were prepared from urine controls. Intra-series stability was evaluated by launching the same sample at different times: 0 h, 1 h, 2 h, 4 h and 8 h. The stability of storage (2°C, 8°C, -20°C) was assessed on samples stored from day D0 to day D7.
Preparation of quality controls
Preparation of loaded controls: Preparation of two concentration levels; physiological L1 (0.2 mmol/L) and pathological L2 (1 mmol/L) concentrations by loading biochemistry urine controls.
Aliquoted in conical tubes and then frozen. Unloaded controls are aliquoted in conical tubes and then frozen. According to the SFBC (French Society of Clinical Biology), the appropriate protocol is to analyze 30 values (6 replicates for physiological concentrations and 6 replicates for pathological concentrations) in 5 days.
Each conical tube underwent the same protocol as the validation and calibration standards.
Sample preparation
Calibration standards: Prepared by mixing 250 μl of oxalate solution of each concentration level, 750 μl of pure water (diluent), 5 μl of pure HCl and 250 μl of OPD solution (0.46 M).
Validation standards: Prepared by mixing 750 μl of urine, 250 μl of oxalate solution of each concentration level, 5 μl of pure HCl and 250 μl of OPD solution.
Blank matrix: Prepared by mixing 750 μl of urine, 250 μl of pure water, 5 μl of pure HCl and 250 μl of OPD solution.
Pure water blank: Prepared by mixing 1 ml of pure water, 5 μl of pure HCl and 250 μl of OPD solution.
The tubes were sealed, vortexed for 1 minute and heated to 120°C for 30 minutes. The colour changes from yellow to dark brown. After cooling the solutions, 30 μl of 10M NaOH is added. The tubes are centrifuged for 10 min at 18000 rpm, the supernatants have been poured into vials for analysis.
Validation protocol
Specificity and matrix effect: The assessment of the specificity and absence of the matrix effect is carried out in two ways:
Comparison of chromatograms: The specificity assessment is made by comparing the chromatogram of a standard without a matrix and another with a matrix of the same concentration level as well as that of the unloaded urine and diluent. Statistical confirmation of specificity on both calibration and validation ranges.
Response function
In order to select the most appropriate calibration model that is capable of producing a sufficient proportion of future measurements that will fall within the limits of acceptability, we studied four functions relating the areas of the peaks to the introduced concentrations:
- 1st function: Right: y=ax+b
- 2nd function: Line passing through 0: y=ax
- 3rd function: Change of variables by the Logarithmic function: lny=f(lnx)
- 4th function: Change of variables by the square root: √y=f(√x)
Justness: Accuracy is expressed in terms of absolute bias, relative bias, and recovery rates for each concentration level of the validation standards.
Fidelity: Reliability is assessed for each concentration level, calculated by standard deviations and coefficients of variation that estimate repeatability and intermediate fidelity.
Accuracy: From the predicted concentrations, the relative accuracy with respect of the introduced concentration can be calculated.
Accuracy profile: After the calculation of the validation criteria, all the data obtained are collected to draw the accuracy profile that combines the relative bias, the two bounds of the tolerance interval and those of the acceptability interval, in a curve that gives the accuracy (%) as a function of the levels of concentrations introduced.
The two bounds of the tolerance interval and those of the acceptability range, in a curve that gives the accuracy (%) as a function of the levels of concentrations introduced.
Linearity: Linearity defined as the existence of proportionality between the results obtained and the predicted concentrations. Its calculation requires different parameters that allow us to draw linear regression lines of the predicted concentration as a function of the introduced concentration.
Limits of quantification: The limits of quantification are obtained from the accuracy profile by calculating the concentrations (high and low) at which the upper or lower limits of the tolerance interval go beyond the acceptability limits (± λ) at the chosen probability level (β).
Results
Retention time and resolution
The retention time obtained for oxalate is: 18.84 min.
Specificity
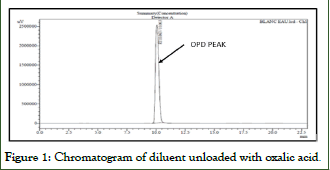
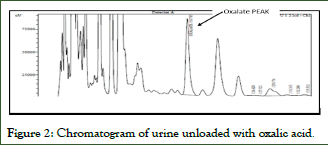
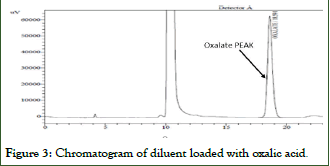
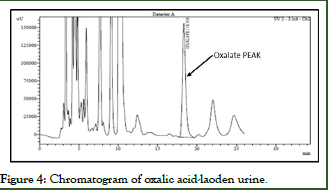
Since natural urine from healthy subjects was used, there is a peak of oxalate at its retention time, the area of this physiological peak is subtracted from the results of the samples by the use of unloaded urine blank (Figures 1-4).
Figure 1: Chromatogram of diluent unloaded with oxalic acid.
Figure 2: Chromatogram of urine unloaded with oxalic acid.
Figure 3: Chromatogram of diluent loaded with oxalic acid.
Figure 4: Chromatogram of oxalic acid-laoden urine.
Statistical confirmation of specificity on both calibration and validation ranges (Table 1).
| Comparison of the two slopes of the two regression lines | T calculated | 0,07 | Non-significant difference |
| T° (α; 26) | 2,06 | ||
| Condition | T calculated |
||
| Comparison of the y-coordinates at the origin of the two regression lines | T calculated | 0,18 | Non-significant difference |
| T° (α; 26) | 2,06 | ||
| Condition | T calculated |
Table 1: Comparison with student test of the two slopes and the two origin coordinates of oxalate in water and oxalate in urine.
Response function
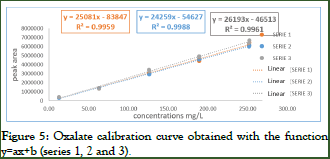
The curve chosen is that of the second series y=24259x–54627 with a coefficient of determination R2=0.9987 (Figure 5).
Figure 5: Oxalate calibration curve obtained with the function y=ax+b (series 1, 2 and 3).
Choosing the response function: The accuracy profiles obtained for Oxalate from the four models of response functions were compared. The best response function that is: y=ax+b and it will be chosen as the calibration model for our method. NB: To estimate the validation parameters, some intermediate calculations were made, namely the alignment of the responses and the inverse prediction using the chosen calibration curve.
Accuracy
All relative biases are less than 7% (Table 2).
| Concentration levels | 12.5 mg/L | 62.5 mg/L | 125 mg/L | 187.5 mg/L | 250 mg/L |
| Theoretical average concentration (mg/L) | 1,25,975 | 6,29,825 | 12,59,675 | 188,95 | 25,19,325 |
| Average concentration found(mg/L) | 1,34,325 | 59,515 | 124,54 | 19,54,225 | 25,03,575 |
| Absolute bias (mg/L) | 0,334 | -1,387 | -0,57 | 2,589 | -0,63 |
| Relative bias (%) | 6,63 | -5,51 | -1,13 | 3,43 | -0,63 |
| Recovery rate (%) | 106,63 | 94,49 | 98,87 | 103,43 | 99,37 |
Table 2: Accuracy calculated for each concentration level of oxalate.
Fidelity
All coefficients of variance are less than 6% (Table 3).
| Concentration levels | 12.5 mg/L | 62.5 mg/L | 125 mg/L | 187.5 mg/L | 250 mg/L |
| Theoretical average concentration (mg/L) | 1,25,975 | 6,29,825 | 12,59,675 | 188,95 | 25,19,325 |
| Average concentration found (mg/L) | 1,34,325 | 59,515 | 124,54 | 19,54,225 | 25,03,575 |
| Repeatability CV (%) | 2,44 | 5,054 | 5,975 | 5,07 | 1,645 |
| CV Intermediate loyalty (%) | 2,44 | 5,054 | 5,975 | 5,07 | 1,645 |
Table 3: Reliability calculated for each concentration level of oxalate.
Accuracy
From the predicted concentrations, the relative accuracy with respect to the introduced concentration can be calculated (Table 4).
| Levels | Rehearsals | The series | ||||||||
| 1 | 2 | 3 | ||||||||
| Introduced concentration mg/L | Predicted concentration mg/L | Relative accuracy (%) | Introduced concentration mg/L | Predicted concentration mg/L | Relative accuracy (%) | Introduced concentration mg/L | Predicted concentration mg/L | Relative accuracy (%) | ||
| 12.5 mg/L | 1 | 12,6 | 13,35 | -5,74 | 12,6 | 13,775 | -8,68 | 12,6 | 13,5 | -6,6 |
| 2 | 12,6 | 13 | -3,02 | 12,6 | 13,325 | -5,41 | 12,6 | 13,9 | -9,29 | |
| 3 | 12,6 | 13,475 | -6,59 | 12,6 | 13,575 | -7,2 | 12,6 | 13 | -3 | |
| 62.5 mg/L | 1 | 62,975 | 62,875 | 0,14 | 62,95 | 61,225 | 2,79 | 63,025 | 59,675 | 5,6 |
| 2 | 63 | 55,6 | 13,29 | 63 | 56,275 | 11,92 | 63 | 63,2 | -0,32 | |
| 3 | 62,975 | 60,325 | 4,38 | 62,975 | 58,6 | 7,48 | 63 | 57,85 | 8,92 | |
| 125.5 mg/L | 1 | 1,25,925 | 1,17,475 | 7,2 | 125,9 | 1,26,075 | -0,15 | 126,05 | 130,45 | -3,37 |
| 2 | 126 | 112,1 | 12,38 | 1,25,975 | 123,85 | 1,71 | 1,25,975 | 122,6 | 2,75 | |
| 3 | 125,95 | 1,21,225 | 3,89 | 125,95 | 144,25 | -12,69 | 1,26,025 | 1,22,825 | 2,59 | |
| 187.5 mg/L | 1 | 188,9 | 193,85 | -2,56 | 1,88,825 | 1,96,675 | -3,99 | 189,05 | 1,75,625 | 7,65 |
| 2 | 1,88,975 | 1,96,075 | -3,62 | 1,88,975 | 198,95 | -5,02 | 1,88,975 | 190,8 | -0,96 | |
| 3 | 1,88,925 | 1,90,375 | -0,76 | 1,88,925 | 2,20,725 | -14,41 | 1,89,025 | 1,95,725 | -3,43 | |
| 250 mg/L | 1 | 251,85 | 235,7 | 6,85 | 2,51,775 | 259,45 | -2,96 | 2,52,075 | 253,9 | -0,72 |
| 2 | 2,51,975 | 244,5 | 3,05 | 251,95 | 2,55,425 | -1,36 | 251,95 | 2,51,575 | 0,15 | |
| 3 | 251,9 | 2,47,475 | 1,79 | 251,9 | 2,57,375 | -2,13 | 2,52,025 | 2,47,825 | 1,7 | |
Table 4: Results of the calculation of relative accuracy for oxalate.
Accuracy profile
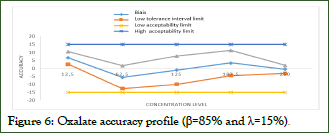
After the calculation of the validation criteria, all the data obtained are collected to plot the accuracy profile that combines the relative bias, the two bounds of the tolerance interval and those of the acceptability interval, in a curve that gives the total error (%) as a function of the concentration levels introduced (Figure 6).
Figure 6: Oxalate accuracy profile (β=85% and λ=15%).
Linearity
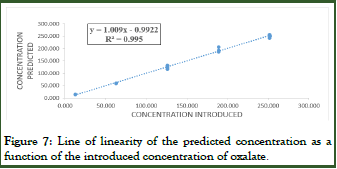
The linearity of the predicted concentration is statistically evaluated by comparing the slope and intercept with 0 (Table 5 and Figure 7).
Figure 7: Line of linearity of the predicted concentration as a function of the introduced concentration of oxalate.
| Slope comparison with 1 | T calculated | 0,46 | Non-significant difference |
| T°(α; 13) | 2,16 | ||
| Condition | T calculated | ||
| Comparing the y-intercept with 0 | T calculated | 0,33 | Non-significant difference |
| T°(α; 13) | 2,16 | ||
| Condition | T calculated |
Table 5: Comparison of slope and northing at origin with 0.
Total error and total error profile
The maximum total error is estimated to be 10.561% (Table 6).
| Level (mg/L) | 1 (12.5 mg/L) | 2 (62.5 mg/L) | 3 (125 mg/L) | 4 (187.5 mg/L) | 5 (250 mg/L) |
| Average theoretical concentrations (mg/L) | 12,60 | 62,98 | 125,97 | 188,95 | 251,93 |
| Relative bias (%) | 6,627 | -5,507 | -1,132 | 3,425 | -0,625 |
| Relative bias in absolute value | 6,627 | 5,507 | 1,132 | 3,425 | 0,625 |
| Intermediate coefficient of variation of fidelity (%) | 2,440 | 5,054 | 5,975 | 5,070 | 1,645 |
| Total error (%) | 9,067 | 10,561 | 7,107 | 8,495 | 2,270 |
Table 6: Calculation of total error for each oxalate concentration level.
Limits of quantification
From the accuracy profile of oxalate, we can see that there is no point of intersection between the tolerance limits and the acceptability limits, so we can consider the upper and lower limits of quantification to be the highest and lowest concentrations studied respectively (12.5 mg/L and 250 mg/L).
Stability
Intra-series stability: At different ranges intra-series stability value shows in Table 7.
| Intra-series stability | ||||
| Vial | Peak area | Blank area | Concentration mg/L | Concentration (mmol)/L |
| T0 H | 1030129 | 913573 | 44,72 | 0,35 |
| T1 H | 975944 | 859388 | 42,48 | 0,34 |
| T2H | 912658 | 796102 | 39,87 | 0,32 |
| T4 H | 956472 | 839916 | 41,68 | 0,33 |
| T8 H | 931158 | 814602 | 40,64 | 0,32 |
| Blank sample | 116556 | |||
| ΔC T1-T0 | -5,3% | -5,9% | ||
| ΔC T2-T0 | -11,4% | -12,9% | ||
| ΔC T4-T0 | -7,2% | -8,1% | ||
| ΔC T8-T0 | -9,6% | -10,8% | ||
Table 7: Intra-series stability values.
Storage stability between +2°C to +8°C and -20°C: The maximum concentration loss rate of oxalate for refrigerated samples is estimated to be 23%. The maximum oxalate concentration loss rate for frozen samples is estimated to be 26% (Table 8).
| Stability of storage | |||||||
| Level 1 | D0 | D1 | Day 2 | Day 3 | D5 | D7 | |
| Concentration in mmol/L | +2°C to +8°C | 0,37 | 0,29 | 0,37 | 0,29 | / | / |
| -20°C | 0,37 | 0,28 | / | 0,29 | 0,29 | 0,29 | |
| ΔC Refrigerated | / | -23% | -2,00% | -22% | / | / | |
| ΔC Frozen | / | -26% | / | -22% | -22% | -23% | |
Table 8: Storage stability between +2°C to +8°C and -20°C.
Internal quality control values shows in Table 9.
| Interval | ||||
| Minimum value | Maximum value | Target | Standard deviation | |
| Level 1 (mmol/L) | 0,164756025 | 0,334889826 | 0,24982 | 0,0425335 |
| Level 2 (mmol/L) | 0,711197812 | 1,213642435 | 0,96242 | 0,1256112 |
Table 9: Internal quality control values.
Reference intervals
The adult reference intervals of wake-up urine to creatinine ratio was determined in subjects aged 18 years to 80 years (n=40 with 3 repetitions).
Discussion
Specificity
After confirming the retention times of oxalate by injecting their standards separately, we moved on to the study of specificity by two methods:
Comparison of chromatograms: According to the results obtained, the specificity of the method is confirmed by:
- The absence of peaks in the retention times of Oxalate on the chromatogram obtained from the solutions of the unloaded diluent (water).
- The retention times corresponding to Oxalate obtained on the standard chromatograms without a matrix and with a matrix of the same concentration level are comparable.
- Unloaded urine shows a peak in the Oxalate retention time corresponding to the physiological amount present in healthy subjects.
Comparison of the two slopes a1 and a2, and the two originally ordinates b1 and b2: From Table 5, it is concluded that:
- The two slopes are not significantly different at the risk α=5% considered, hence the absence of the matrix effect after subtracting the quantity naturally present in the matrix.
- There is no significant difference between the original ordinates at risk α=s5% considered, thus explaining the absence of systematic error.
It can be deduced that with a risk of 5%, the method does not have a systematic error, so it is statistically specific. These results confirm those obtained by the study of chromatograms.
Justness
The agreement between the value of the predicted mean concentration, obtained from the three validation series, and the value of the mean of the introduced concentrations considered to be the reference value is quite close for the concentration levels included in the quantification interval (12.5; 250) mg/L, taking into account that the relative biases of the latter are well below 7%, confirming the accuracy of our method.
Fidelity
Since the coefficients of variation of the repeatability and intermediate fidelity of the concentration levels included in the (12.5; 250) mg/L range are less than 6% for oxalate, the degree of dispersion of the predicted concentrations obtained is quite narrow for all levels, so the method is accurate.
Accuracy profile
Referring to the results obtained from the accuracy profiles in Figure 3 for oxalate, it is considered that the method used in this work is valid over the assay interval where the accuracy profile is within the acceptance limits, i.e., its range of validity is within the concentration range (12.5; 250) mg/L thus including normal and pathological values relevant to the diagnosis of diseases related to urinary oxalate disturbances (physiological values less than 0.3 mmol/L. Taking into account the acceptability limit λ=± 15% and the confidence probability β=85%.
This means that it can be guaranteed that the method is capable of producing acceptable future results with an 85% probability.
Linearity
Linearity was assessed from five concentration levels (12.5, 62.5, 125, 187.5, 250 mg/L) with three independent replicates for each concentration level.
The linear regression line obtained for oxalate is in the form y=1.009x+0.9922, with a coefficient of determination R2 of 0.99504 thus meaning that the total variability of the predicted concentration is explained at 99.5% by the variability of the introduced concentration of the sample.
The coefficient of linear determination of the relationship between the introduced concentration and the predicted concentration is very acceptable. Although it tells us very little about the quality of the regression, its value can guide us, especially since it is very close to 1.
The statistical study of linearity for oxalate, illustrated in Table 5, confirmed that the slope is statistically no different from 1 and that the intercept is statistically comparable with 0, which proves the validity of the regression and linearity.
Total error
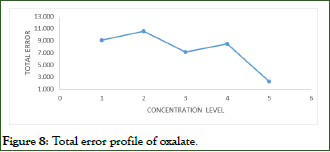
Based on the total error profiles elucidated in Figure 8, it can be concluded that all oxalate concentration levels are included within the validity interval, as they have acceptable relative total errors that do not exceed the predefined acceptability limits (15%) with a maximum error of 10.561 for oxalate, observed for the second concentration level (62.5 mg/L).
Figure 8: Total error profile of oxalate.
Stability
Intra-series stability is acceptable (-5.3% -11.4%). For preservation, the concentration of oxalate can be reduced by up to 26%. It is recommended that samples be acidified prior to storage for better stability.
Quality control
The range obtained from the minimum and maximum values is for level 1 (0.16, 0.33) and for level 2 (0.71, 1.21).
Reference intervals
The adult reference intervals of wake-up urine to creatinine ratio was: The mean oxalate concentration was 59,99 mmol/mol Cr, with a range of 9,17-100,80 mmol/mol Cr.
Conclusion
We have successfully developed and validated an R-HPLC-UV method (C18, 15 cm, λ=314 nm), the technique developed has been shown to be simple, specific, linear, faithful, and accurate within the tolerance interval (12.5; 250) mg/L; for the determination of oxaluria. Because of its reliability and costeffectiveness, it is a screening and monitoring tool that will be routinely applied in the biochemistry laboratory for the benefit of patients.
References
- Bouzidi H, Majdoub A, Daudon M, Najjar MF. Primary hyperoxaluria: A review. Nephrol Ther. 2016;12(6):431-436.
[Crossref] [Google Scholar] [PubMed]
- Daudon M, Traxer O, Lechevallier E, Saussine C. La lithogenèse. Prog Urol. 2008;18(12):815–827.
[Crossref] [Google Scholar] [PubMed]
- Evan AP, Coe FL, Lingeman JE, Shao Y, Sommer AJ, Bledsoe SB, et al. Mechanism of formation of human calcium oxalate renal stones on Randall's plaque. Anat Rec (Hoboken). 2007;290(10):1315–1323.
[Crossref] [Google Scholar] [PubMed]
- Efe O, Verma A, Waikar SS. Urinary oxalate as a potential mediator of kidney disease in diabetes mellitus and obesity. Curr Opin Nephrol Hypertens. 2019;28(4):316–320.
[Crossref] [Google Scholar] [PubMed]
- Lorenzo V, Torres A, Salido E. Primary hyperoxaluria. Nefrologia. 2014;34(3):398–412.
- Amoroso A, Pirulli D, Florian F, Puzzer D, Boniotto M, Crovella S, et al. AGXT gene mutations and their influence on clinical heterogeneity of type 1 primary hyperoxaluria. J Am Soc Nephrol. 2001;12(10):2072–2079.
[Crossref] [Google Scholar] [PubMed]
- Marengo SR, Romani AM. Oxalate in renal stone disease: the terminal metabolite that just won't go away. Nat Clin Pract Nephrol. 2008;4(7):368–377.
[Crossref] [Google Scholar] [PubMed]
- Jouvet P. Crystalluria. Hôpital des Enfants, Paris: KI; 1998.
- Daudon M, Jungers P, Traxer O. Lithiase urinaire. Paris: Lavoisier; 2012.
Citation: Nawal B, Hassan BSS (2025) Development, Validation of a Method for the Determination of Oxaluria by UV-HPLC after Derivatisation with O-Phenyldiamine. Biochem Anal Biochem. 14:587.
Copyright: © 2025 Nawal B, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.