Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 1
Characteristics of Physicochemistry , Microbiology and Antibacterial Activities from Fermentation of Viscera Fish Sauce
Martha Loana Wattimena1, Johanna Louretha Thenu2, Max Robinson Wenno1*, Dessyre M. Nandissa dan1 and Dwight Soukotta12Fishery Training and Extension Center, Ambon, Indonesia
Received: 03-Jan-2020 Published: 24-Jan-2020, DOI: 10.35248/2157-7110.20.11.818
Abstract
The fermentation process involved the hydrolysis of lactic acid bacteria, single amino acid and peptide were produced and were accounted for their antibacterial activity. This study aims to determine the physical and chemical characteristics, total lactic acid bacteria, total plate count, and antibacterial activity of tuna viscera sauce. The research results showed physicochemical characteristics including color (L* 8.3, a* 1.3 and b* 5.7), viscosity of 10.38 cP, pH 5.00, salt content of 13.21%, total acid 0.74% and TVBN 28.00 mgN/gr) Proximate analysis were also identified, resulting in moisture 62.87%, ash 1.37%, protein 23.18% and carbohydrate 0.42%. The total lactic acid bacteria and total plate count respectively were 2.3 log CFU/gr and 2.3 × 101 CFU/gr. The results of the antibacterial activity tested on three pathogenic bacteria, Vibrio pharahaemolyticus, Salmonella typhimurium, and Escherichia coli, showed inhibition with the presence of clear zones.
Keywords
Tuna fish sauce; Viscera; Fermentation; Physicochemistry; Microbiology; Peptides; Antibacterial activity
Introduction
Tuna is one of the favorite foods in Indonesia. It is one of the most hunted fish species in various countries because it has a high protein and fat content, and has a delicious taste with a high selling price. Tuna is generally used fresh or processed into frozen tuna loin and canned tuna. High demand for tuna products both in fresh and processed conditions caused an increase in waste or by-products in the form of head, skin, stomach contents, bones, fins, and red meat parts. From the fish production which is around 6.5 million tons per year, about (20-30)% are considered waste. This means that 2 million tons are wasted, which should be utilized. The process of preserving and processing fish generally results in altered waste viscera which is a source of fat, protein, and vitamins. According to Sabtecha et al. [1], the fisheries industry produces about 60% byproducts by weight and leaving only 40% by weight of products that can be consumed by humans. The waste can actually be utilized; one of the contents of the stomach (offal) can be made into fish sauce. This is done to increase the economic value of waste and reduce environmental pollution problems. Fish sauce is one of the processed food products through a fermentation process made from fish meal and fish by-products in the form of viscera. It has a distinctive taste and smell and long shelf life. Fish sauce can be made in three ways, namely by enzymatic, chemical and fermentation processes spontaneously. Usually, fish sauce is used as a condiment.
The names of fish sauce in several countries are different, for example, Bakasang (Indonesia), Yu-lu (China), Jeotkal (Korean), Nukazuke (Japan), Lanhouin (Benin, Togo, and Ghana), Momoni (Ghana), Feseekh (Egypt), Mahyaveh (Iranian), Garum (Italy), Plaa-som (Thailand) and Pekasam (Malaysia) [2-12]. However, the characteristics of fish sauce produced by Tuna have not been widely known, nor has there been information about physic-chemical characteristics. In addition, during the fermentation process lactic acid bacteria can hydrolyze proteins into peptides that have antibacterial activity [13,14]. This study aims to determine the physical and chemical characteristics, total lactic acid bacteria and antibacterial activity from fermented tuna viscera sauce.
Materials and Methods
Raw material
The raw material used in this study was tuna loin came from the Passo Village, Ambon City, Maluku-Indonesia. Samples are collected and inserted in a cool box containing ice and brought to the laboratory to be prepared and fermented to produce a sauce.
Methods
Fish sauce preparation: Viscera samples were washed and ± 1 cm was reduced. The sample was blended with a food processor and weighed ± 200 gr, then added the papain enzyme (Paya brand) with a concentration of 5% and 17.5% of salt. The samples are mixed until homogeneous and inserted into a bottle that covered with aluminum foil before and put in an incubator (Barnstead brand) for the fermentation process at 50°C for 6 days [15] modified.
Procedure analysis
Determination of physical characteristics (color and viscosity): Color test used a color reader (Minolta CR-10) to find out the values of L*, a*, and b* from the sample. Whereas, Viscosity used a viscometer (Elcometer 2300) [2].
Determination of chemical characteristics
pH and acidity: The pH value was analyzed using Senz pH digital tester (Trans instruments). The total acid analysis was used the titration method, by using 10 mL of suspension plus three drops of the phenolphthalein indicator, then titrating with 0.1 N NaOH solution [16].
Salinity: ± 1 g of the sample put into 250 mL Erlenmeyer, then added (25-50) mL AgNO3 0.1 N and 20 mL HNO3, simmer slowly using a hot plate in the acid chamber until all solids dissolved except (AgCl). A sample is cooled at room temperature, added 50 mL of halogen-free water, added ferric indicator, titrated with NH4CNS 0.1N until the solution is light brown permanent. The volume of 0.1 N NH4CNS used for the titration was noted [16].
Total of volatile bases-nitrogen: 5 g of the sample is weighed and blended with 15 mL of 5% TCA, then the solution was filtered to obtain clear filtrate used the filter paper. 1 mL of the boric acid solution is inserted into the Conway dish in the inner chamber then 1 mL of the analyzed sample is inserted into the outer chamber. The K2CO3 solution is inserted in the different outer chamber side, then tightly closed (on the edge of the cup previously smeared with vaseline). The blank is made with the same procedure, but as a filtrate, the sample is replaced with a 3% TCA. The cup was closed, shaken for 1 minute to be mixed and then incubated for 2 hours. After incubation, the boric acid solution in the inner chamber is titrated with 0.01 N HCl until it was pink.
Lactic acid bacteria (LAB): Determination of the total lactic acid bacteria applied the cup count method. The sample is weighed 10 g and put into a 90 mL diluent solution (0.85% sterile NaCl) then homogenized. 1 mL of the initial dilution is pipetted into a 9 mL diluent solution to the desired dilution level. Through the dilution level set, pipetted 1 mL of sample into a petri dish and poured it on ± 15 mL of MRSA (Man Rogosa Sharp Agar) liquid into the cup, shook it horizontally on the table, so the sample spread well, then chilled until it was dense. The cup was incubated upside down at 37°C for 48 hours. Colonies that grow on the cup were calculated and noted as the number of colonies per gram of sample [17].
Total plate count: Determination of the total bacteria applied to the cup count method. Weighed the agar plate count media and put it into Erlenmeyer which has been given with 250 ml of distilled water, then homogenized. The pH of the media was adjusted to pH 7.0 and then heated until the media dissolved and sterilized with an autoclave at a pressure of 15 psi at 121°C for 15 minutes. Prepare 0.9% NaCl diluent solution, each for 90 ml first level dilution, while for the second and third dilutions 9 ml NaCl 0.9% are each taken and then put into a Hush tube. 10 g of sample was weighed and put in 90 ml of sterile 0.9% NaCl to obtain a solution with a 10-1 dilution rate. From 10-1 dilution, pipette 1 ml into the second test tube and then homogenized so that a 10-2 dilution is obtained. From each dilution 1 ml is taken to transfer to a sterile petri dish which has been coded for each sample at a certain rate of dilution. Into all Petri dishes (15-20) ml PCA (Plate Count Agar) were poured. After pouring, the petri dish is shaken slowly then cooled until the solid media and put into the incubator for 24 hours at 35°C [18].
Antibacterial activity: Before tested the antibacterial activity, the fermented viscera sauce of tuna was isolated by bioactive peptides using the Wang et al., [19] method with some modifications. About 100 g of the sauce is dissolved in distilled water with a ratio of 1:5 (b/v) then homogenized and inactivated at 90°C for 10 minutes. The next stage is centrifuged at 6000 rpm, then chilled at a temperature of 4°C for 20 minutes. Ethanol then added to supernatant with a ratio of 1: 1 (v/v) and dried. The isolated peptide is added with Tris-HCl buffer and stored at -20°C before use. Antibacterial activity test was tested by diffusion method and used paper discs 6 mm of diameter with Vibrio parahaemolyticus test bacteria, Salmonella typhimurium, and Eschericia coli. Paper discs were bioetifed peptide samples with concentrations of 500 μg/mL, then placed on NA media which had been inoculated with test bacteria. Incubation is stored at 37°C for 24 hours. Observations were made on the formation of inhibition zones around the paper disc.
Statistical Analysis
The test was in two replications, calculated by the average value and standard deviation. Data is analyzed descriptively.
Results and Discussion
The Tuna viscera sauce was analyzed for physical and chemical parameters (color, viscosity, pH, salinity, total acid, TVBN), total acid and antibacterial activity test.
Characteristics of tuna fish sauce
Physical characteristics: The physical characteristics of tuna fish sauce can be seen in Table 1.
| Physical characteristics | Value |
|---|---|
| Color (L*, a*, b*) | L* (8,3), a* (1,3), b*(5,7) |
| Viscosity (cP) | 10.38 ± 0.53 |
n=3, L* (0=black; 100=white), a*=red, b*=yellow
Table 1: Physical characteristics of the viscera of tuna fish.
The result from calculating the °Hue value from the L*, a*, b* value was 76.80 and based on the color table shows the fish sauce was reddish-yellow. The color of fish sauce is influenced by raw materials, pigmentation, additives and fermentation conditions [2,20]. Fish muscles contain sarcoplasmic protein like myoglobin. Generally, the pigment responsible for color is myoglobin. In addition, the additional ingredients also affect the color.
Chemical characteristics
Chemical characteristics (pH, salinity, total acid, and TVBN can be seen in Table 2).
| Components | Contents |
|---|---|
| pH | 5.00 ± 0.01 |
| Salinity (%) | 13.21 ± 0.11 |
| Acidity (%) | 0.74 ± 0.95 |
| TVBN (mgN/100 g) | 28.00 ± 0.07 |
n=3
Table 2: Chemical characteristics of the viscera of tuna fish.
In general, fermented products have an acidic pH. Some research results indicated that the pH value produced in fermented fish products is acidic pH, with values of 4.8-5.2; 4.90-6.23; 5.1-5.8 and 3.60-5.30 [13,21-23]. The research fish sauce had a pH of 5.0. The low pH value of fish sauce occurred due to the formation of acid compounds from the hydrolysis process. Jesebel et al. [24] reported fermentation of Dayok (fish sauce in Filipino) to produce a pH of 5.7-6.0 with a duration of 7 days fermentation and salt content of (10-25)%. Wenno et al. [2] reported that fermented products produced a pH of 4.66 with a fermentation period of 7-14 days.
Salt functions as a preservative where there is a reduction in water content in food through an osmotic process and also functions as a microbial selector during the fermentation process [25]. The higher the concentration of salt, the longer the storage capacity of fish sauce products would be. The salt content of the results of the study was 13.21%. Salt can increase the susceptible osmotic pressure which processes of exchange between salt particles and fish body fluids. Fish cell membranes are selectively permeable had the role of passing salt that is more concentrated outside the membrane so that the salt penetrates through the cell membrane. The water contained in cells will automatically be pushed out and almost all important solutes remain in the cell so that osmotic pressure is reached inside and outside the cell [26]. When compared with some research results including fish sauce in Taiwan amounting to (22.2-37.0)% [21] with a fermentation period of 6-12 months, the salt content of mahyaveh (traditional fish sauce in Iran) ranges from (8.19-17.1)% [10], whereas for fermented products such as bakasang has a salt content of 13.72% for 7-14 days of fermentation time [2].
During the fermentation process, there was a change caused by changes in the Total Titrated Acid (TTA). The amount of TTA is a parameter that affects the durability of the sauce, because of the organic acid content that will make the sauce last longer. Increased TAT is strongly related to the number of lactic acid bacteria during the fermentation process. The longer the fermentation time changes in the total amount of lactic acid increases. Lactic acid growth is supported by the availability of food or nutrients in the media during the fermentation process. During the fermentation process, chemical changes occur causing changes in Titrated Total Acid (TTA). The total titrated acid from the results of the study was quite low at 5.12%. Hwanhelm et al., [23] also reported plasom fermentation (fish sauce in Thailand) with the addition of 100 g of salt and 8 days of fermentation at room temperature resulting in a total acidity of (0.3-0.9)%. The role of lactic acid bacteria is to produce organic acids such as lactic acid, acetic acid, and other secondary metabolites. In addition, the lactic acid produced can create organic acid flavor.
TVBN values indicated the process of protein hydrolysis by the activity of enzymes and bacteria that produce amine compounds which can reduce the value of product quality. The TVBN value permitted for fishery products is <35 mg N%. The longer the fermentation time is usually the detected level of TVBN will also be higher, this is caused by the accumulation of microorganism metabolites including TVBN in these food products that indicated decay [27]. The level of TVBN research results was 28.00 mgN/100 gr. During fermentation, microorganism macromolecules are reshaped and produce volatile base compounds or Total Volatile Base (TVB). High salt content will be a preservative and will inhibit the growth of microorganisms, one of which is decomposing microorganisms which will produce alkaline compounds or TVB. When compared with the results of Jesebel et al., [24] on dayok fermentation (fish sauce in the Philippines) obtained TVB levels of 76.41 mgN/100 gr by adding 25% salt concentration and 7 days fermentation time in room temperature. Jiang et al., [3] also reported fermentation of yu-lu (fish sauce in China) with 40% salt addition and 30 days fermentation time resulting in a TVB level of 50 mgN/100 gr.
Proximate analysis
The Proximate analysis of viscera tuna fish sauce can be seen in Table 3.
| Components | Contents (%) |
|---|---|
| Moisture | 62.87 ± 0.02 |
| Ash | 12.16 ± 0.03 |
| Protein | 23.18 ± 0.02 |
| Fat | 1.37 ± 0.05 |
| Carbohydrate (by different) | 0.42 |
n=3
Table 3: Proximate analysis of viscera tuna fish sauce.
From Table 3, it appears that protein and ash levels are higher than fat and carbohydrate content, whereas moisture content in very high amounts. Fish sauce is a semi-solid product or pasta that has a high moisture content. The moisture content of tuna viscera sauce comes from viscera fish and the number of dissolved solids from hydrolysis [28]. The added salt can attract a certain amount of moisture in the fish sauce which can reduce the moisture content. The high ash content is thought to originate from salt added as a flavoring and to extend the product's shelf life. Besides being sourced from salt, minerals are also thought to be derived from the samples used. The average protein is 23.18%. The protein content is sourced from the viscera of fish and the hydrolysis process during fermentation also produces a number of short-chain proteins. Carbohydrates in fisheries products are generally very small and mostly in the form of glycogen in the form of glucose, fructose, sucrose and some monosaccharides and disaccharides [2].
Total plate count (TPC): The results of the TPC analysis of the tuna viscera sauce sample were 2.3 101 CFU/g. This value is quite low, presumably due to the condition of the medium that can inhibit the growth of bacteria where the added salt and enzymes can inactivate decomposing bacteria and pathogens in the product.
Lactic acid bacteria (LAB): Total lactic acid bacteria during the 6-day fermentation process at 50°C is 2.3 log CFU/g. Traditional fish sauce in Iran (mahyaveh) produces a total average of lactic acid bacteria ranging from (3.22-4.80) log CFU/g [10]. BAL is the dominant microorganism in some fish fermentation products. Halophilic lactic acid bacteria are the dominant bacteria found in the final stages of fermented fish sauce. The genus Leuconostoc and Lactibacillus are found in plaara and plaa-som, fermented fish sauce in Thailand. Lactic acid production from BAL is used in preservation because acidic conditions in the fermentation process can suppress harmful bacteria. Some strains of lactic acid bacteria are known to have proteolytic activity including Lactobacillus plantarumi, L. pentosus, L. sakei, L. farciminis, Staphylococcus xylosus, P. acidilactici, P. pentosaceus are lactic acid bacteria that have been known to have proteolytic activity capable of degrading fish protein [17,29-33]. Some bacterial stains from Weisella cibaria, W. confusa Nh 02, and W. paramesentroides Dfr 8 are reported to produce antibacterial substances in the form of bacteriocins [12,34,35].
Antibacterial activity test: The antibacterial activity of fermented viscera of tuna fish on three pathogenic bacteria, Vibrio pharahaemolyticus, Salmonella typhimurium, and Escherichia coli, showed inhibitory activity with the presence of a clear zone around the paper disc. The diameter of the clear zone as shown in Table 4 and Figure 1.
| Treatment | Test Bacteria | ||
|---|---|---|---|
| V. pharahaemolyticus | S. typimurium | E. coli | |
| Positive control (Tetracycline) | 13.8 mm | 24.5 mm | 43.4 mm |
| Negative control (Without treatment) | - | - | - |
| Bioactive peptide crude | 8.6 mm | 4.3 mm | 2.8 mm |
Table 4: Inhibited zone diameter.
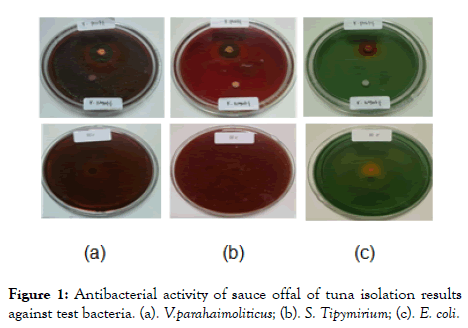
Figure 1: Antibacterial activity of sauce offal of tuna isolation results against test bacteria. (a). V.parahaimoliticus; (b). S. Tipymirium; (c). E. coli.
During the fermentation process, lactic acid bacteria hydrolyzed proteins into peptides that had an antibacterial function. The higher the number of lactic acid bacteria in fermented products, the higher the protein content. This is due to the fact that most of the constituent material of lactic acid bacteria is protein. Generally, short-chain peptides consisting of 2-3 amino acids have activities as antibacterial biopeptides, this is evidenced by the presence of clear zones around bacterial colonies. Of the three pathogenic bacteria used in this study, Vibrio prahaimoloticus, Salmonella typhymirium, and Escherichia coli, it was seen that bioactive peptide isolated could inhibit the three test bacteria. The results of this study have not been maximized because the antibacterial activity test still used crude peptide, so it needs to be purified to obtain maximum inhibitory activity. Peptides derived from protein breakdown will undergo changes in physicochemical characters compared to their natural proteins. The degradation of proteins into nutrient peptides will increase the bioavailability of amino acids compared to proteins or free amino acids.
Conclusion
The fermentation process had an effect on physical (viscosity and color), chemical properties (pH, salt content, total acid, and TVBN), also on the content of lactic acid bacteria and the total plate count. In addition, the fermentation process hydrolyzes proteins into short-chain peptides that have the potential to be antibacterial.
REFERENCES
- Sabtecha B, Jayapriya J, Tamilselvi A. Extraction and characterization of proteolytic enzymes from fish visceral waste: potential applications as destainer and dehairing agent. Int J Chem Tech Res. 2014;6:4504-4510.
- Wenno MR, Suprayitno E, Hardoko H. The physicochemical characteristics and Angiotensin Converting Enzyme (ACE) inhibitory activity of skipjack tuna (Katsuwonus pelamis) “bakasang”. J Teknologi. 2016;78.
- Jiang JJ, Zeng QX, Zhu ZW, Zhang LY. Chemical and sensory changes associated Yu-lu fermentation process-A traditional Chinese fish sauce. Food Chem. 2007;104:1629-1634.
- Ezzat MA, Zare D, Karim R, Ghazali HM. Trans-and cis-urocanic acid, biogenic amine and amino acid contents in ikan pekasam (fermented fish) produced from Javanese carp (Puntius gonionotus) and black tilapia (Oreochromis mossambicus). Food Chem. 2015;172:893-899.
- Mah JH, Han HK, Oh YJ, Kim MG, Hwang HJ. Biogenic amines in Jeotkals, Korean salted and fermented fish products. Food Chem. 2002;79:239-243.
- Kuda T, Izawa Y, Ishii S, Takahashi H, Torido Y, Kimura B. Suppressive effect of Tetragenococcus halophilus, isolated from fish-nukazuke, on histamine accumulation in salted and fermented fish. Food Chem. 2012;130:569-574.
- Anihouvi VB, Sakyi-Dawson E, Ayernor GS, Hounhouigan JD. Microbiological changes in naturally fermented cassava fish (Pseudotolithus sp.) for lanhouin production. Int J Food Microbiol. 2007;116:287-291.
- Sanni AI, Asiedu M, Ayernor GS. Microflora and chemical composition of momoni, a Ghanaian fermented fish condiment. J Food Compos Anal. 2002;15:577-583.
- Rabie M, Simon-Sarkadi L, Siliha H, El-seedy S, El Badawy AA. Changes in free amino acids and biogenic amines of Egyptian salted-fermented fish (Feseekh) during ripening and storage. Food Chem. 2009;115:635-638.
- Zarei M, Najafzadeh H, Eskandari MH, Pashmforoush M, Enayati A, Gharibi D, et al. Chemical and microbial properties of mahyaveh, a traditional Iranian fish sauce. Food Control. 2012;23:511-514.
- Smriga M, Mizukoshi T, Iwahata D, Eto S, Miyano H, Kimura T, et al. Amino acids and minerals in ancient remnants of fish sauce (garum) sampled in the “Garum Shop” of Pompeii, Italy. J Food Compos Anal. 2010;23:442-446.
- Kopermsub P, Yunchalard S. Identification of lactic acid bacteria associated with the production of plaa-som, a traditional fermented fish product of Thailand. Int J Food Microbiol. 2010;138:200-204.
- Desniar M. Characterization of lactic acid bacteria isolated from an Indonesian fermented fish (bekasam) and their antimicrobial activity against pathogenic bacteria. Emir J Food Agric. 2013:489-494.
- Harris LJ, Daeschel MA, Stiles ME, Klaenhammer TR. Antimicrobial activity of Lactic Acid Bacteria against Listeria monocytogenes. J Food Prot.1989;52:384-387.
- Simanjorang E, Kurniawati N, Hasan Z. The influence of use of papain enzymes with different concentration on chemical characteristics of tapes. J Marine Fisheries.2012;3:209-220.
- El-Demerdash FM, Yousef MI, El-Naga NA. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57-63.
- Yin LJ, Pan CL, Jiang ST. Effect of lactic acid bacterial fermentation on the characteristics of minced mackerel. J Food Sci. 2002;67:786-792.
- Fardiaz S. Food microbiology analysis. 2004
- Wang J, Hu J, Cui J, Bai X, Du Y, Miyaguchi Y, et al. Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the antihypertensive effect of hydrolysate in spontaneously hypertensive rats. Food Chem. 2008;111:302-308.
- Riebroy S, Benjakul S, Visessanguan W, Tanaka M. Physical properties and microstructure of commercial Som-fug, a fermented fish sausage. Eur Food Res Technol. 2005;220:520-525.
- Tsai YH, Lin CY, Chien LT, Lee TM, Wei CI, Hwang DF. Histamine contents of fermented fish products in Taiwan and isolation of histamine-forming bacteria. Food Chem. 2006;98:64-70.
- Park JN, Fukumoto Y, Fujita E, Tanaka T, Washio T, Otsuka S, et al. Chemical composition of fish sauces produced in Southeast and East Asian countries. J Food Compos Anal. 2001;14:113-125.
- Hwanhlem N, Buradaleng S, Wattanachant S, Benjakul S, Tani A, Maneerat S. Isolation and screening of lactic acid bacteria from Thai traditional fermented fish (Plasom) and production of Plasom from selected strains. Food Control. 2011;22:401-407.
- Besas JR, Dizon EI. Influence of salt concentration on histamine formation in fermented tuna viscera (Dayok). Food Nutr Sci. 2012;3:201-206.
- Tariq AS, Swastawati F, Surti T. Effect of differences in salt concentration on the puffed fish (Rastrelliger neglectus) on the content of glutamic acid which gives a savory taste (umami). J Fisheries Product Process Biotechnol. 2014;3:104-111.
- Moelyanto J. Canning fish, handling fresh fish, preservation and processing of fishery product (Jakarta: PT. Penebar Swadaya). 1982. Self-Publisher Issuers.
- Dissaraphong S, Benjakul S, Visessanguan W, Kishimura H. The influence of storage conditions of tuna viscera before fermentation on the chemical, physical and microbiological changes in fish sauce during fermentation. Bioresour Technol. 2006;97:2032-2040.
- Harikedua SD. Correlation between sensory flavor profile of “Bakasang” with its physicochemical and microbiological quality. 2009. Thesis. Bogor Agriculture Institute.
- Mauriello GAC. Proteolytic activity of Staphylococcus xylosus strain on pork myofiblillar and sarcoplasmic protein and use of the selected strain in the production of “Naple-Type” salami. J Appl Microbiol. 2002;93:482-490.
- Aktaş N, Aksu MI, Kaya M. Changes in myofibrillar proteins during processing of pastirma (Turkish dry meat product) produced with commercial starter cultures. Food Chem. 2005;90:649-654.
- Basso Al, Picariello G, Coppola R, Tremonte P, Musso Ss, Luccia Ad. Proteolytic activity of Lactobacillus sakei, Lactobacillus farciminis and Lactobacillus plantarumi on sarcoplasmic proteins of pork lean. J Food Biochem. 2004;28:195-212.
- Rai KP, Shrestha AK, Xia W. Proteolytic effect of starter cultures on dry fermented Chinese-style sausage. J Food Sci Technol Nepal. 2008;4;64-69.
- Wikandari PR, Suparmo S, Marsono Y, Rahayu ES. Potential of milkfish formation (Chanos chanos) as a source of Angiotensin I converting enzyme inhibitors. Biota: Scientific J Life Sci. 2011;16:145-152.
- Srionnual S, Yanagida F, Lin LH, Hsiao KN, Chen YS. Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Appl Environ Microbiol. 2007;73:2247-2250.
- Pal A, Ramana KV. Purification and characterization of bacteriocin from Weissella paramesenteroides DFR-8, an isolate from cucumber (Cucumis sativus). J Food Biochem. 2010;34:932-948.
Citation: Wattimena ML, Thenu JL, Wenno MR, Nandissa dan DM, Soukotta D (2020) Characteristics of Physicochemistry, Microbiology and Antibacterial Activities from Fermentation of Viscera Fish Sauce. J Food Process Technol 11:818. doi: 10.35248/2157-7110.20.11.818.
Copyright: © 2020 Wattimena ML, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.


