Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Original Research Article - (2021) Volume 11, Issue 8
Benefits of Disposing Polyhydroxybutyrate-Co-Hydroxyvalerate (PHBV) Blends in Soil, as Alternative for Low Density Polyethylene
Elaine Cristina Bucioli1*, Adriano Uemura Faria1, Sandra Mara Martins-Franchetti1, Lusiane Malafatti Picca1 and Derlene Attili-Angelis1,22Division of Microbial Resources, CPQBA, State University of Campinas (Unicamp), Rua Alexandre Cazellato, 999, Paulínia, SP, 13148-218, Brazil
Received: 28-Jul-2021 Published: 27-Aug-2021, DOI: 10.35248/2252-5211.21.11.419
Abstract
The use of synthetic plastics generates environmental impacts due to their low biodegradability and inadequate disposal. One of the alternatives to minimize this problem is the use of biodegradable polymers and/or the production of blends with desired industrial and eco-friendly characteristics. The biodegradation of PHBV (Poly (Hydroxybutyrate-co-Hydroxyvalerate)), LDPE (Low Density Polyethylene) and LDPE / PHBV (70/30) blends in soil column was evaluated using Scanning Electron Microscopy (SEM), Fourier Transform Infrared (FTIR), and mass loss. Through SEM it was possible to observe micro morphological changes on the surface of the PHBV and the blends, in accordance with the mass loss variation. PHBV samples showed a reduction of 43.9 % and the blend had a reduction of 15.7 %, during their biodegradation process. FTIR analysis revealed that the crystallinity of the polymeric materials changed, suggesting the biodegradation of these films. Soil samples were characterized by determination of pH, organic matter (%), moisture (%), and CFU of the microbial community. The blend was susceptible to soil microbial activity, with significant changes in its micro morphology. The used 70/30 ratio (LDPE/ PHBV) showed susceptibility to soil microorganisms, favoring the increase of its microbial community. The use of polymeric blends also favors the reduction of the amount of polymers present in the environment because some of them are biodegradable.
Keywords
Biodegradation; Blend; Environment; FTIR analysis; LDPE
Introduction
Synthetic plastics have great economic importance since they are deeply inserted in the daily life of the present society. Due to their versatility, this material has replaced raw materials in countless artifacts, with benefits as practicality, product innovations and industrial process improvements [1].
The use of synthetic plastics in packaging and disposables causes socio-environmental impacts mainly due to their improper disposition, leading to a rapid accumulation and resilience in the environment. Paradoxically, the same properties that confer advantages to polymers (such as durability and strength) also become a challenge with respect to its deterioration and recycling in the environment [2,3].
Most of the studies on biodegradable polymers seek for materials with a shorter biodegradation time when compared to conventional ones. Some approaches may involve the production of polymers by microorganisms using renewable sources, the development of blends with synthetic and biodegradable polymers, or the addition of components that favor degradation process (metal salts, photosensitizers, plant fibers, etc.) [4-7].
Biodegradation refers to the physical and chemical changes caused by microbial action on a given material. The degradation of polymers is influenced by the properties of the material to be degraded and the microbiological and environmental conditions [8,6,9].
Low density polyethylene (LDPE) is widely used in films with high consumption and disposal for food packaging, agricultural and pharmaceutical products, wire and cable coatings, tubes and hoses [10]. In 2015 the world production of LDPE was 64 millions tons and its discard was 57 millions tons [11]. In 2017, the consumption of LDPE represented 11.4% of the Brazilian market among other polymers, occupying the fourth position of the segment [12] and, this same position was occupied in the previous year. Its economic importance and difficulty in being biodegradable clearly justifies the development of studies to propose alternatives to minimize the environmental impact caused by the accumulation of this plastic.
PHBV (poly (hydroxybutyrate-co-hydroxyvalerate)) is a natural, biodegradable, bacterial-synthesized copolymer with physical and mechanical properties similar to the most widely used thermoplastics, such as polyethylene and polypropylene [13]. Currently, this polymer has been applied in the medical field, e.g. in therapeutic applications, tissue manufacture and administration of medications [14].
Biopolymers have great potential to replace those coming from fossil sources, but their production still face technical limitations which makes the cost-benefit of industrial processes yet unattractive. Therefore, the study of blends intends to improve properties such as processability, thermal resistance, mechanical and rheological properties [14]. PHB (poly (hydroxybutyrate)) is insoluble in water and presents low permeability to oxygen, water and carbon dioxide [15]. While PE (polyethylene) has the properties of tenacity, high impact resistance, high flexibility and good processability [10].
The combination of polymer blends may result in materials with distinguished usage properties as well as a more friendly destination in the environment. Blends appear as possible alternatives for minimizing the environmental impacts caused by synthetic polymers. It is relevant to investigate PHBV influence on LDPE biodegradation, which is quite resistant to microbial attack. This study aimed to investigate the biodegradation in soil of low-density polyethylene (LDPE) and polyhydroxybutyrate-co-valerate (PHBV) blend. For this, the ratio 70/30 LDPE/ PHBV was proposed, based on the hypothesis that PHBV may promote a more rapid LDPE biodegradation. The analyses took place in the soil environment because in Brazil it is the main destination of these type of residues.
Materials and Methods
Polymers
The polymers used in this study were:
PHBV – 18% of hydroxyvalerate; (Mw = 237.500)
LDPE - (density 0.922 g/cm3), melt flow rate 3.8 g/10 min. Blend: LDPE /PHBV in the mass ratio of 70/30.
The polymers were prepared in a Haake Polylab 900 Torque rheometer, at 170°C and 2.0 πrad.s-1 angular velocity (Department of Materials and Metallurgical Engineering, USP/SP), assuring an effective material dispersion. The 70/30 ratio was used due to low miscibility between these polymers.
The films of LDPE polymer and the LDPE /PHBV (70/30) blend were prepared in a hot press of, approximately, 0.25 g of each polymer at a temperature of 170°C and 89 kgf.cm-2 of pressure for 3 minutes and cooled down until it reached a 26 ±1°C room temperature. PHBV films were pressed at 71.3 kgf.cm-2 and at 170°C for 2.5 minutes. All the obtained films resulting in a 80 μm thick and were cut in dimensions of 3 cm × 3 cm.
The films that were not subjected to biotreatment were designated from original films, aiming to distinguish them from the films subjected to biotreatment.
Biodegradation
The soil used for the biodegradation tests was collected from a garden at the campus of UNESP, Rio Claro-SP, Brazil (22° 23’S and 47° 32’W), sifted in a 1.68 mm mesh.
The biodegradation columns were polypropylene containers containing 350 g soil (wet weight). The film was placed in the center of the column (Figure 1).

Figure 1: Soil column with drip system indicated by arrow.
The columns were stored in laboratory for up to 180 days at a temperature of 28 °C with the moisture controlled at 60% by a dripping system. Samples of LDPE and blend were removed from the columns after 60, 120 and 180 days. Samples of PHBV were removed after 10, 20 and 30 days.
The biotreated samples were subjected to the analyses described below for later comparison with the original films. The soil used in the columns was also analyzed before and after the film exposition period. The analyses performed were: pH, moisture and organic matter.
Characterization of the polymers
Visual changes: The visual characteristics of the films were recorded by a Sony ® digital camera Cyber-shot DSC H100 before and after the biodegradation period.
Mass measurement: The mass of each film was measured before and after the biotreatment process, by weighing in analytical balance JK-200 - Chyo, to determine the percentage of mass loss of these.
Scanning Electron Microscopy (SEM): The original and the biotreated polymeric films were analyzed in a LEO 435 VP scanning electron microscope, after being gold metalized in the SCD 050 Sputter Baltec metallizer. Furthermore, the alterations in the films surface were analyzed using the SEM.
Attenued total reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR): The analyses of FTIR of the samples were made in the films (before and after biotreatment), using the Prestige Infrared Spectrophotometer from Shimadzu, with a 4 cm-1 resolution and 16 scans for identifying the characteristic bands of the samples.
The spectral regions correspondent to the vibrations = 0 (carbonyl group) were solved by Lorentzian adjustment to obtain two bands: one correspondent to the carbonyl vibration of amorphous phase and, the other, crystalline [16,17], then, the index of crystalline and amorphous carbonyl were obtained and compared [16,18].
The spectra of PHBV films were normalized by the absorbance intensity of a band, considered as internal standard at 1380 cm-1, attributed to the symmetric deformation of CH3 groups [19].
The internal standard used to normalize the blend and LDPE spectra was the band intensity at 1469 cm-1, correspondent to the stretching of LDPE CH2 groups [20,21].
Characterization of the soil
The soil was characterized in relation to organic matter content, pH, moisture and quantification of microorganisms: before and after the biotreatment assays of the films.
Determination of Organic Matter: The determination of the organic matter (%) content was performed by the muffle combustion method [22], in which the soil was placed in porcelain crucibles and subjected to a temperature of 600 °C for 4 hrs, then, it was cooled in a desiccator.
Determination of Soil pH: The method used for pH analysis consisted of the addition of 25.0 mL of CaCl2 solution (calcium chloride) at 0.01 mol.L-1 to 10.00 g of soil. This suspension was homogenized and the pH was determined by the potentiometric method [23].
Determination of soil gravimetric moisture: To determine the soil gravimetric moisture, the oven drying method was used [24]. 5.0 g of the sample was placed in a porcelain vessel and subjected to a temperature of 105 °C for 24 hours. After this period, the soil samples were cooled down in a desiccator (25 ± 1 °C) and the remaining mass was determined to constant weight.
Quantification of soil microorganisms: The colony forming units (CFU) of soil microorganisms were counted in Pour Plate with serial dilutions [25].
The culture medium used were Plate Count Agar for bacteria, Actinomycete Isolation Agar for actinobacteria and Potato Dextrose Agar with chloramphenicol (0,01% chloramphenicol) for fungi. Petri plates were used in duplicate for each dilution, and then incubated at 30 ± 1 °C for 24-48 hrs.
Results and Discussion
PHBV, LDPE and the (70/30) blend films were photographed before and after the biodegradation assay in soil column. The pictures of the original LDPE film showed smooth and homogeneous surfaces. Even after the analysis period, the films showed no perforations or any type of rupture, as can be seen in Figures 2a and 2b.

Figure 2: Visual analysis of LDPE film before (2a) and after the analysis period in soil column (2b).
Maroof et al. [26] studying bacteria with degrading potential for LDPE obtained photographs that allow correlated observations, indicating and justifying the difficulty of biodegradation for this polymer.
The PHBV subjected to microbial degradation in soil showed surface erosion in all its extension when compared with the original. After 30 days, modifications, such as opacity and spots could be noticed. Besides that, it was more brittle, with the presence of holes, including loss of parts of the film (Figures 3a and 3b).

Figure 3: Visual analysis of PHBV film before (3a) and after the analysis period in soil column (3b).
In the original blend film, it was possible to identify a certain heterogeneity caused by the immiscibility of PE with PHBV, and distinct translucency areas (spots). After 180 days of biodegradation, the films presented spots and opacity. In addition, they also presented peeling, evidencing the beginning of the biodegradation process (Figures 4a and 4b), fact that corroborates with the results found by other authors [18,27].

Figure 4: Visual analysis of the blend film before (4a) and after the biodegradation process in soil column (4b).
About the mass measurement, results showed that 30 day-old PHBV films presented a significant mass loss (around 44%). In the work of Gonçalves, Martins-Franchetti and Chinaglia [8] PHVB films buried in the soil were completely degraded after 30 days. The consumption of hydroxyvalerate occurs intensely at the beginning of the process, increasing the fraction of hydroxybutyrate residues, which is more crystalline and resistant to biodegradation [28,4,9]. On the other hand, LDPE films presented a much lower mass loss compared to that found in PHBV (1.5% after 180 days).
Concerning the LDPE/ PHBV blend, 15.7% of its mass was lost after 180 days, less than that observed in the biodegradable copolymer. The biodegradation of a blend depends on the compatibility and miscibility of the present polymers, since microorganisms initiate biodegradation in the PHBV fraction but not in the interphase of the blend [2,7].
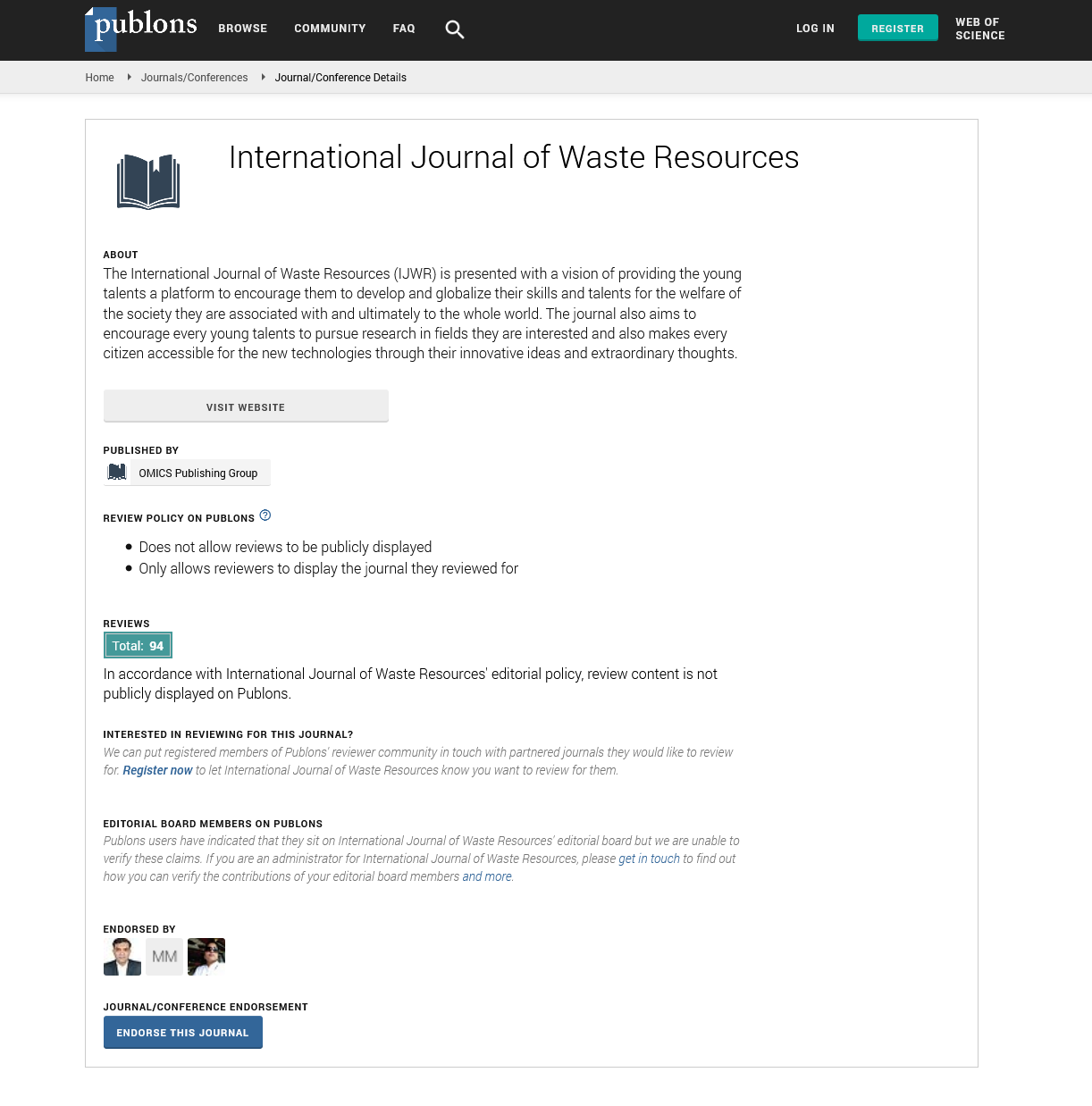
SEM analyses showed modification on the surface of PHBV films, including holes and peelings (Figures 5a and 5b), after 30 days. This evidenced that the degradation of the material occurred, first, by the erosion of the surface, caused by the microbial action in the film and, then, it deepened, entering into the other layers of the material, this fact was also observed by Sundar Dey, et al [29].
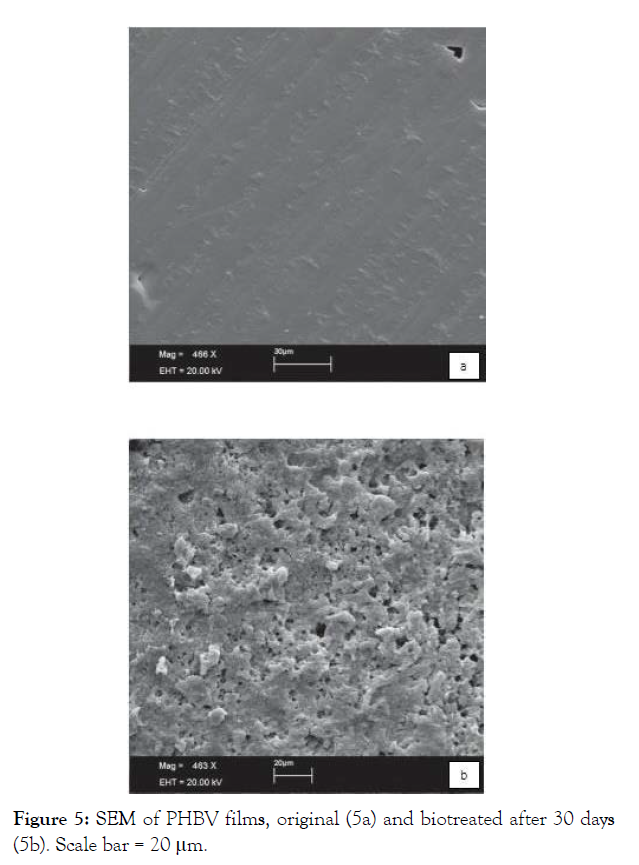
Figure 5: SEM of PHBV films, original (5a) and biotreated after 30 days (5b). Scale bar = 20 μm.
Holes and evidence of erosion pointed by SEM of LDPE films after 180 days of biotreatment, were attributed to the colonization of the material surface (Figures 6a and 6b).
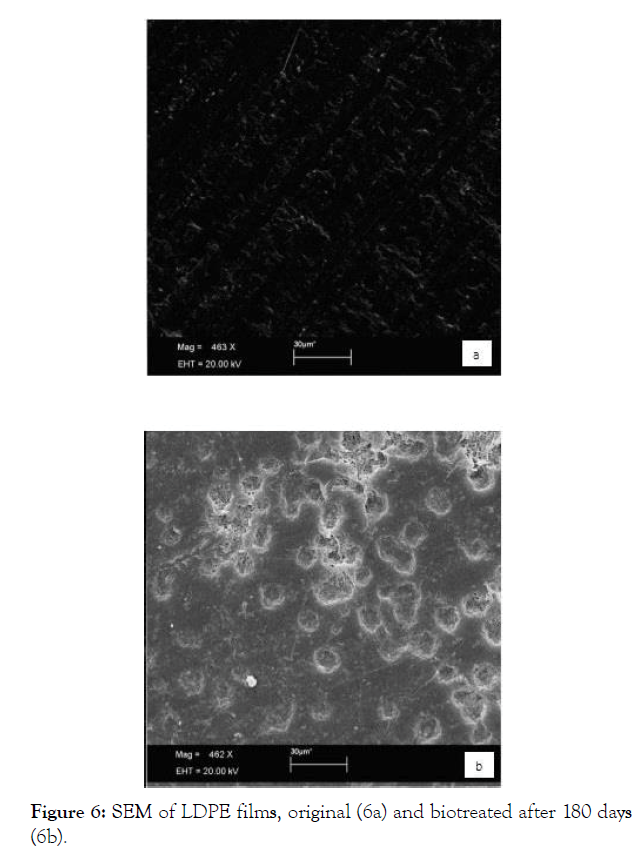
Figure 6: SEM of LDPE films, original (6a) and biotreated after 180 days (6b).
Biofilm formation is observed, what is an important step for the biodegradation process, indicating that the microbial activity can be responsible for peelings, fractures and erosions seen in the micrographs of the biotreated blend (Figures 7a and 7b).
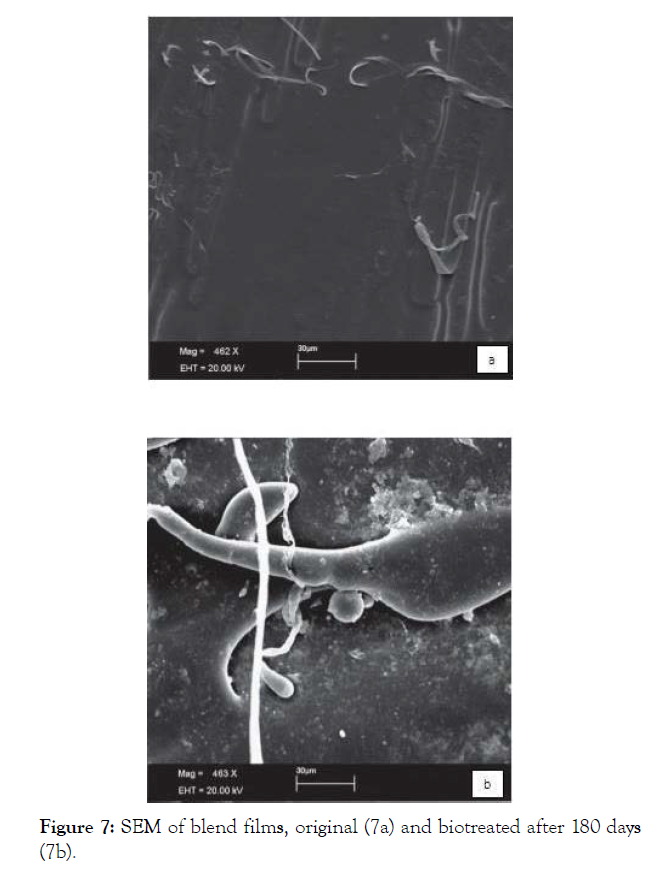
Figure 7: SEM of blend films, original (7a) and biotreated after 180 days (7b).
Concerning Fourier Transform Infrared Spectroscopy, the studies indicate that the structure, composition and hydrophilicity present in polymers are factors that do influence biodegradation. Crystalline phase and the size of crystallites play an important role in determining the rate of biodegradation. It was demonstrated that amorphous regions are more susceptible to microbial action than crystalline ones [9,21].
Tables 1 and 2 demonstrate the carbonyl indices between the amorphous and the crystalline phases present in the polymers.
| Carbonyl Index | ||||
|---|---|---|---|---|
| Original | Biotreated | |||
| 10 days | 20 days | 30 days | ||
| Amorphous (A1750/1380) | 0,41 | 0,40 | 0,42 | 0,52 |
| Crystalline (A1710/1380) | 0,74 | 0,70 | 0,81 | 0,61* |
| + 0,05 | * band displacement (1697 cm-1) | |||
Table 1: Carbonyl index of original and biotreated PHBV films.
| Carbonyl Index | ||||
|---|---|---|---|---|
| Original | Biotreated | |||
| 60 days | 120 days | 180 days | ||
| Amorphous (A1745/1469) | 0,71 | 0,43 | 0,43 | 0,45 |
| Crystalline (A1722/1469) | 0,52 | 0,47 | 0,48 | 0,53 |
| ± 0,05 | ||||
Table 2: Carbonyl index of original and biotreated blend films.
In the PHBV film, after 20 days of biotreatment, the carbonyl index of the amorphous phase increased when compared to the original one. The increase of the amorphous fraction during the biodegradation of PHBV is related to the increase in the amount of free 3HV (3-hydroxyvalerate) by the hydrolysis of the ester bonds. This indicates an exo-cleavage promoted by the enzyme depolymerase [30].
In the blend, the corresponding index to the crystalline phase carbonyls (1722 cm-1) did not change significantly. This fact can be explained by the consumption of the amorphous phase component of PHBV fraction of the blend, since it is more susceptible to the action of the depolymerases [30,31].
According to Sudhakar et al. [20] and Esmaeili et al. 2014 the carbonyl index initially increases due to abiotic factors; however, prolonged exposure to microorganisms leads to a decrease in the carbonyl index due to biodegradation. The decrement trend of the carbonyl groups (which can be obtained by calculating the carbonyl index) indicates the utilization of PE by the microorganisms.
Tables 3 and 4 show LDPE and blend analyses, with an evaluation from 0 to 180 days. The percentages of organic matter content showed differences after the experiment, mainly in the last period, reflecting the microbial consumption of the organic matter. At day 180, the soil had 2.5% less organic matter than in day 0 where LDPE films were buried, against 1.9% in the soil samples with the blends.
| Biotreatment | Organic matter (%) | |
|---|---|---|
| (days) | LDPE soil | Blend soil |
| 0 | 68,0 | 68,0 |
| 60 | 69,7 | 69,3 |
| 120 | 56,7 | 62 |
| 180 | 65,5 | 66,1 |
| +0,20% | ||
Table 3: Percentages of organic matter in soil, during the respective biotreatment periods.
| Biotreatment | CFU. mL-1 | |||||
|---|---|---|---|---|---|---|
| (days) | Bacteria | Actinobacteria | Fungi | |||
| LDPE soil | Blend soil | LDPE soil | Blend soil | LDPE soil | Blend soil | |
| 0 | 2,3×106 | 2,3×106 | 2,6×106 | 2,6×106 | 6,1×103 | 6,1×103 |
| 60 | 3,8×105 | 7,3×105 | 3,1×105 | 2,1×105 | 2,5 × 102 | 1,5 × 102 |
| 120 | 4,3×105 | 6,7×105 | 5,0×105 | 4,3×105 | 3,9 × 103 | 4,9 × 103 |
| 180 | 6,8×105 | 1,2×106 | 4,5×105 | 6,3×106 | 4,2×103 | 4,4×103 |
Table 4: Values of the CFU counts of bacteria, actinobacteria and fungi in the soils used in degradation columns, with respect to the period of the biodegradation process.
Since pH may both favor or inhibit microbial action, measurements were systematically taken during this study. Luo and Netravali [13] identified that the biodegradation process of PHBV in soil occurred at a pH of 7.5. Weng et al. [31] evaluated PHBV degradation (3% of HV) in an organic-matter-rich soil and the pH values obtained during 12 weeks of the process ranged from 6.4 to 8.8.
Although still acidic, the pH of soil samples with PHBV tended to increase in a period of 30 days (data not shown) with a range from 6.0 to 6.4. However, a pH decrease (from 6.5 to 5.6) was reported in the soil samples of the treatments with LDPE films and the blends, over 180 days. Even so, the variation found in the values of pH were considered insufficient to explain possible differences among the UFC number of bacteria, actinobacteria and fungi, because in general an acid condition was prevalent.
During the experiment period, water was added by a dripping system to maintain moisture in the biodegradation columns. Studies have shown that maintaining the water content between 40 and 50% favored the biodegradation of polymers [13]. The average soil moisture during the experiments was 48.7%, as reported in other studies.
Table 4 presents the CFU count values for bacteria, actinobacteria and fungi, respectively, considering the periods of the biodegradable processes.
Microbial activity is influenced by several variables such as temperature, moisture, nutrient availability and pH, which can directly affect metabolism, permeability and adsorption.
The decrease in organic matter content indicates its consumption by microorganisms present in the soil. CFU indicates that there was a decrease in the biomass of microorganisms due to the period of adaptation to the polymers used. After 180 days, growth recovery was observed. Fungi increased CFU in 120 days of treatment, showing faster recovery than bacteria and actinobacteria, where recovery was in 180 days.
According to Gonçalves and Martins-Franchetti [27] working with blends of polypropilene (PP/PHBV) and PE/PHBV changes in the soil microbial community after biodegradation were dependent on the type of polymer. The bacterial population in the soil containing decreased and the fungal population increased after 180 days of biodegradation.
In this work, the soil used in the biodegradation of the blend presented a larger increase of bacteria and actinobacteria than the soil used in the biodegradation of LDPE. The consumption of organic matter and the largest increase of microorganism in the soil of the blend demonstrates that in addition to biodegradation, the disposal of PHBV can contribute to the increase of soil microbiota.
It is important to point out that some works use physical chemical alternatives such as photooxidation and chemical additives [26, 32] to the developed polymers, in an attempt to facilitate the degradation process, this purpose presents promising results with the use of a PE/PHVB (70/30) blend without the implementation of chemical additives or techniques that can make polymer production costly.
Conclusions
The purpose of this study was to evaluate the biodegradation in soil of the blend (LDPE / PHBV) 70/30, aiming to reduce LDPE residues in the environment. Even LDPE not undergoing biodegradation, the use of the blend is advantageous because part of it is biodegraded, thus reducing the number of polymers dispersed in the environment. The blend may be an alternative for the replacement of PE, as part of it breaks down because the present PHBV is accessible to soil microorganisms. In addition to the biodegradability of part of the blend, the study demonstrated that it contributes to the increase of soil microbiota, which may favor fertilization and increase of nutrient cycle.
Acknowledgments
Department of Biochemistry and Microbiology, UNESP – RC and CNPQ.
REFERENCES
- Faria AU. Biodegradação da blenda PP/PHB e homopolímeros por microrganismos de rio poluído e efluente bruto de refinaria de petróleo. Polímeros. 2010.
- Rani-Borges B, Faria AU, De Campos A, Gonçalves SPC, Martins-Franchetti SM. Biodegradation of additive PHBV/PP-co-PE films buried in soil. Polímeros. Polímeros. 2016;26:161-167.
- Vivi VK, Martins-Franchetti SM, Attili-Angelis D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC16021) activity. Folia microbial. 2018;64(1):1-7.
- Shah A, Hasan F, Hameed A, Ahmed S. Biological degradation of plastics: A comprehensive review. Biotechnol. 2008;26(3):246-265.
- Singh S, Mohanty AK, Sugie T, Takai Y, Hamada H. Renewable resource based biocomposites from natural fiber and polyhydroxybutyrate-co-valerate (PHBV) bioplastic. Compos Part A Appl Sci Manuf. 2008;39(5):875-886.
- Nowak B, Pajak J, Labuzek S, Rymarz G, Talik E. Microorganisms participating in the biodegradation of modified polyethylene films in diferente soils under laboratory conditions. Int Biodeter Biodegr. 2011; 65(6):757-767.
- Passos TM, Marconato JC, Martins-Franchetti SM. Biodegradation of films of low density polyethylene (LDPE), poly(hydroxibutyrate-co-valerate) (PHBV), and LDPE/PHBV (70/30) blend with Paecilomyces variotii. Polímeros. 2015;25:29-34.
- Gonçalves SPC, Martins-Franchetti SM, Chinaglia DL. Biodegradation of the films of PP, PHBV and its blend in soil. J Polym Environ. 2009;17(4):280-285.
- Costa CZ, De Albuquerque M de CC, Brum MC, De Castro AM. Degradação microbiológica e enzimática de polímeros: Uma revisão. Quím. Nova. 2014;38:259-67.
- Coutinho FMB, Mello IL, Maria LCS. Polietileno: principais tipos, propriedades e aplicações. Polímeros, 2003;13:01-13.
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782.
- Abiplast (2018) Perfil 2018
- Luo S, Netravali AN. A study of physical and mechanical properties of poly (hydroxybutyrate-co-hydroxyvalerate) during composting. Polym Degrad Stab. 2003.
- Brito GF, Agrawal P, Araújo EM, Melo TJA. Biopolímeros, Polímeros Biodegradáveis e Polímeros Verdes. REMAP. 2011.
- Machado MLC, Pereira NC, Miranda L F, Terence MC. Study of mechanical and thermal properties of the polymer Poly-3-hydroxybutyrate (PHB) and PHB/wood flour composites. Polímeros. 2010.
- Fei B, Chen C, Wu H, Peng S, Wang X, Dong L. Quantitative FTIR study of PHBV/bisphenol A blends. Eur. Polym. J. 2003.
- Kansiz M, Domínguez-Vidal A, Mcnaughton D, Lendl B. Fourier-transform infrared (FTIR) spectroscopy for monitoring and determining the degree of crystallization of polyhydroxyalkanoates (PHAs). Anal. Bioanal. Chem. 2007.
- Faria AU, Martins-Franchetti SM. Biodegradação de filmes de polipropileno (PP), Poli(3-hidroxibutirato) (PHB) e blenda de PP/PHB por microrganismos das águas do Rio Atibaia. Polímeros. 2010.
- Bloembergen S, Holden DA, Hamer GK, Bluhm TL, Marchessault RH. Studies of composition and crystallinity of bacterial poly (B-hydroxybutyrate-co-Bhydroxyvalerate). Macromolecules. 1986.
- Sudhakar M, Double M, Murthy PS, Venkatesan R. Marine microbe-mediated biodegradation of low- and high-density polyethylenes. Int Biodeter Biodegr. 2008.
- Ojha N, Pradan N, Singh S, Barla A, Shrivastava A, Khatua P, et al. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 2017.
- Pereira MG, Valladares GS, Anjos LHCD, Benites VDM, Espíndula Jr A, Ebeling AG. Organic carbon determination in histosols and soil horizons with high organic matter content from Brazil. Sci. Agric. 2006.
- Claessen MEC, Barreto WO, De Paula JL, Duarte PM. Manual de métodos de análise do solo. Embrapa. 1997.
- Dobriyal P, Qureshi A, Badola R, Hussain SA. A review of the methods available for estimating soil moisture and its implications for water resource management. J. hydrol. 2012.
- Pelczar MJ, Reid RD, Chan ECS. Microbiologia: Conceitos e Aplicações. Makron Books, São Paulo. 1997.
- Maroof L, Khan I, Yoo SH, Kim S, Park H, Ahmad B, et al. Identification and characterization of low density polyethylene-degrading bacteria isolated from soils of waste disposal sites. Environmental Engineering Research. 2021.
- Gonçalves SPC, Martins-Franchetti SM. Biodegradation of the films of PP and PE blended with PHBV in soil samples. Adv Polym Tech. 2014.
- Choi GG, Kim HW, Rhee YH. Enzymatic and non-enzymatic degradation of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolyesters produced by Alcaligenes sp. MT-16. J. Microbiol. 2004.
- Sundar Dey A, Bose H, Mohapatra B, Sar P. Biodegradation of Unpretreated Low-Density Polyethylene (LDPE) by Stenotrophomonas sp. And Achromobacter sp., Isolated From Waste Dumpsite and Drilling Fluid. Frontiers in Microbiology. 2020.
- Shah A, Hasan F, Hameed A, Ahmed S. Degradation of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by a newly isolated Actinomadura sp. AF-555, from soil. Int Biodeterior Biodegradation. 2010.
- Weng Y, Wang X, Wang Y. Biodegradation behavior of PHAs with different chemical structures under controlled composting conditions. Polym Test. 2011.
- Esmaeili A, Pourbabaee AA, Alikhani HA, Shabani F, Esmaeili E. Biodegradation of Low-Density Polyethylene (LDPE) by Mixed Culture of Lysinibacillus xylanilyticus and Aspergillus niger in Soil. PLoS ONE. 2013.
Citation: Bucioli EC, Faria AU, Martins-Franchetti AM, Picca LM, Attili-Angelis D (2021) Benefits of Disposing Polyhydroxybutyrate-Co- Hydroxyvalerate (PHBV) Blends in Soil, as Alternative for Low Density Polyethylene. Int J Waste Resour 11: 419.
Copyright: © 2021 Bucioli EC, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.