Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 4
Analytical Method Optimization and Determination of Sulfonamides, Chloramphenicol and Tetracyclines Drug Residues in Chicken Meat across Egypt
Mahmoud Abd Elkhabeer1*, Gouda A. Ramadan Gouda2, Lamia Ryad2 and Eglal R. Souaya32Agricultural Research Centre, Ministry of Agriculture and Land Reclamation, Central Laboratory of Residue Analysis of Pesticides and Heavy Metals in Foods, Egypt
3Ain Shams University, Khalifa El-Maamon Street, Cairo, 11566, Egypt
Received: 28-Mar-2020 Published: 15-Apr-2020, DOI: 10.35248/2157-7110.20.11.825
Abstract
This study was carried out to develop an accurate analytical method for the determination of 17 Sulfonamides (SAs), 4 Tetracyclines (TCs) and Chloramphenicol (CAP) residues to be used in a monitoring program for the analysis of studied veterinary drugs in chicken samples from different poultry farms of Egypt. Instrument linearity was established using a multi-level calibration curve from (1 to 100) μg/L for Sulfonamides and Tetracyclines and from (0.1 to 20) μg/L for Chloramphenicol; the correlation coefficient was ≥ 0.995 for all compounds. Methods linearity has been studied using different concentration levels which lie in between the calibration points. The method proved to be linear for all compounds from Limit of Quantitation (LOQ) to the highest level. The limit of quantitation was 10 μg/L for Sulfonamides and Tetracyclines and 0.2 μg/L for Chloramphenicol. Method accuracy was studied using various Certified Reference Materials (CRMs) and results were found to be valid within the acceptable limits. A total of 60 fresh samples from different poultry farms in Egypt were tested for the presence of studied compounds using liquid Chromatography-Mass Spectrometry (LC-MS/MS). Results showed that there were no positive samples detected except two samples contaminated with doxycycline but lower than the Maximum Residue Limits (MRLs) established by the European database (100 μg/kg). Hence, more monitoring and risk assessment studies required to cover new generations of veterinary drugs.
Keywords
Food of animal origin; LC-MS/MS; Method optimization; QuEChERS; Veterinary drugs
Introduction
The poultry industry is one of the main agricultural industries in Egypt and turn contributes to a larger part of the nation’s supply of animal protein. Across all income categories, poultry is considered to be very popular among Egyptian consumers due to its low cost when compared to red meat and fish. It also contributes 20% of the total daily per capita consumption which is about 30.3 grams/day [1,2]. Poultry also represents an income source for many poor families who practice traditional aviculture. About 90% of rural households and a great number of urban households rely on aviculture as a clean and cheap source for animal protein and as a contributor to income [1]. Poultry production differs from other animal production activities in several ways, broiler chicken production requires around 50-60 days whereas red meats production needs (9-12) months. Additionally, poultry requires about 3 kg of feed to produce 1 kg of meat when compared to 7 kg of feed needed to produce 1 kg of red meat [1]. More expansions are required day after day to meet these increasing needs of meat production and its related products [2].
Veterinary Drugs (VDs) are substances that can inhibit the growth of microorganisms. They are widely used in the prevention and treatment of infectious diseases to protect the health and welfare of humans and animals. Some of them are produced by microorganisms but most of them are now manufactured synthetically. They are generally administrated to food-producing animals for therapeutic purposes to treat infected animals, also as preventive epidemics or to promote animal growth. Administration of VDs through the feed, drinking water or by injection and there are also some practices involved such as the use of ‘ ‘ cocktails ’ ’ (mixtures of small amounts of several substances) [3].
Veterinary drugs are important to meet the challenges of providing adequate amounts of food for the growing world population where drugs improve the rate of weight gain, improve feed efficiency and prevent or treat diseases of animals [4,5]. However, the benefit of improved productivity from the use of VDs in food-producing animals is accompanied by the risk associated with VDs residues that remain in the tissues of treated animals [4,6] or residues in animal-derived products and this may pose a serious health hazard to consumers [7,8].
Drug residues in foods are of a major public health concern because they may cause severe health hazards, causing allergic reactions, carcinogenicity, and promotion of the spread of bacterial resistance to antibiotics used in human medicines. Their improper use, non-respect of withdrawal periods, and cross-contamination can lead to the presence of residues in food of animal origin. These residues may include the non-altered parent compound as well as metabolites and/or conjugates and may have direct toxic effects on consumers, e.g. allergic reactions in hypersensitive individuals. Moreover, indirect problems in clinical treatment may be caused by the induction of resistant strains of bacteria (development of bacterial resistance) [7].
Harmful consequences of drug residues in food have created an important need for monitoring the food. So many sensitive and more specific methods were optimized and validated for qualitative and quantitative determination of different veterinary drug residues in poultry meat samples such as Thin Layer Chromatography (TLC), ELISA, Four Plate Test (FPT), High-Performance Liquid Chromatography (HPLC), Liquid Chromatography (LC), Liquid Chromatography-Mass Spectrometry (LC-MS/MS) [9,10]. There are also qualitative or semi qualitative methods by Microbiological assays, based on a specific reaction between a susceptible organism (generally bacteria) and the veterinary drug present in the sample. These tests can detect any antibiotic or metabolite with antibacterial activity [11,12].
The objective of this study is to develop and optimize a highly sensitive analytical method for the determination of various veterinary drugs such as sulfacetamide, sulfadiazine, sulfamerazine, sulfamethazine, sulfamethoxazole, sulfapyridine, sulfathiazole, sulfachloropyradazine, sulfadimethoxine, sulfadoxine, sulfisoxazole, sulfaquinoxaline, sulfamethizole, sulfamoxole, sulfaguanidine, sulfamonomethoxine, sulfamethoxypyridazine, trimethoprim, chloramphenicol, tetracycline, chlortetracycline, oxytetracycline and doxycycline in chicken which also has been used in a monitoring program for the analysis of studied VD residues in chicken samples from different poultry farms in Egypt to ensure the safety of chicken for human.
Materials and Methods
Sampling
In 2018, a total of 60 samples of chicken were randomly collected from three different farmers namely Benha, Kafr- Elsheikh and El-Gharbia (20 samples from each farm). Samples were weighed about 1kg each and labeled by the following information (name, date, serial number and location) and transported immediately to the respective laboratory. Samples were ground as a whole to homogenize and then divided into two equal portions, one for testing purpose and the other was kept in the deep freezer as a reference sample for any future requirements.
Tested veterinary drugs
In this study, a total of Twenty-two veterinary drugs (22) were tested which belongs to three different categories: Sulfonamides (17), Tetracyclines (4) and Amphenicol (1) as shown in Table (1). Above mentioned drugs were selected based on their wide commercial use in Egypt for controlling of chicken. Tetracyclines and Sulfonamides are well known to be used as growth promoters [13,14]. Due to their low cost, low toxicity and broad spectrum of activity against bacterial disease, Sulfonamides (SA) and Tetracyclines (TC) are used widely for effective chemotherapeutic agents in the treatment of various infectious diseases. Chloramphenicol (CAP) has been categorized as Carcinogenic in humans by the International Agency for Research on Cancer (IARC), classified as group 2A [15,16]. Due to its high efficiency, a broad spectrum of activity, prompt availability and low cost it can still be used with proper authorization [17-19] (Table 1).
| No. | Common name | Class | Pharmacology | Activity |
|---|---|---|---|---|
| 1 | Sulfacetamide | Sulfonamide | Interferes with bacterial growth by inhibiting bacterial folic acid synthesis through competitive antagonism of PABA | Has a broad-spectrum activity in treating many diseases caused by gram-negative and gram-positive bacteria |
| 2 | Sulfadiazine | |||
| 3 | Sulfamerazine | |||
| 4 | Sulfamethazine | |||
| 5 | Sulfamethoxazole | |||
| 6 | Sulfapyridine | |||
| 7 | Sulfathiazole | |||
| 8 | Sulfachloropyridazine | |||
| 9 | Sulfadimethoxine | |||
| 10 | Sulfadoxine | |||
| 11 | Sulfisoxazole | |||
| 12 | Sulfaquinoxaline | |||
| 13 | Sulfamethizole | |||
| 14 | Sulfamoxole | |||
| 15 | Sulfaguanidine | |||
| 16 | Sulfamonomethoxine | |||
| 17 | Sulfamethoxypyridazine | |||
| 18 | Chloramphenicol | Amphenicol | Bacteriostatic effect where they inhibit protein biosynthesis in bacterial cells after binding to the 30s ribosomal sub particle | Has a broad-spectrum activity in treating many diseases caused by gram-negative and gram-positive bacteria |
| 19 | Tetracycline | Tetracyclines | Bacteriostatic effect where they inhibit protein biosynthesis in bacterial cells after binding to the 30s ribosomal sub particle | Has a broad-spectrum activity in treating many diseases caused by gram-negative and gram-positive bacteria |
| 20 | Chlortetracycline | |||
| 21 | Oxytetracycline | |||
| 22 | Doxycycline |
Table 1: The common name, chemical group, pharmacology and recommendation of each group against bacteria.
Sample preparation
Sample preparation for SAs and CAP were analyzed using the following extraction method: Fresh or thawed samples were ground with the homogenizer. About 2 g (2.0 g ± 0.04 g) of homogenized samples were weighed in 50 ml Extraction tubes and dissolved with 8 ml De-Ionized Water (DIW). Samples should be mixed well and sonicated for 15 min at an ultrasound bath, then diluted with 10 ml, 0.1% acetic acid in acetonitrile, vortexed for 1 min, then sonicated with ultrasound bath for 15 min. QUECHERS kits added and tubes were shaken gently, centrifuged for 10 min at 10,000 rpm. 2nd centrifuge tube was prepared to transfer the supernatant from the 1st tube to the 2nd Extraction tube. Double extraction was carried out by transferring another 10 ml of 0.1% acetic acid in acetonitrile to the1st Extraction tube, vortexed and centrifuged for 10 min at 10,000 rpm. The supernatant was again transferred to the 2nd Extraction tube. Extracts were cleaned-up using PSA for the dispersive solid-phase extraction step, Interference removal was done by transferring 7 ml of extractant from 2nd tube to SPD tube then vortexed and centrifuged at speed 10,000 rpm for 10 min, 4 ml extractant was transferred to glass tubes for evaporation at 40°C. The dry residues were dissolved in 800 μl solution containing 10% methanol and 0.1% formic acid, finally filtered through 0.20 μm disposable syringe filter. 1 ml was transferred to HPLC vials and test samples were injected along with calibration standards using SCIEX 6500 Q trap LCMS/ MS.
Sample preparation for TCs: Samples were ground with a homogenizer. About 2 g (2.0 g± 0.04 g) of homogenized samples were weighed in 50 ml Extraction tubes and diluted with 10 ml (70% methanol in DIW at 4 pH), vortexed for 1 min and then sonicated with ultrasound bath for 15 min. Samples were shaken gently and then centrifuged for 10 min with 10,000 rpm. The supernatant was transferred to the SPD tube to purify and clean up the samples from matrices after that SPD tubes were vortexed and centrifuged for 10 min with 10,000 rpm. Samples were filtered through 0.20 μm disposable syringe filter and 1 ml of sample was transferred to HPLC vials. Samples were injected along with calibration standards using SCIEX 6500 Q trap LCMS/ MS [20].
Chemicals and reagents
Analytical standards such as Sulfacetamide (SMD), Sulfadiazine (SDZ), Sulfamerazine (SMZ), Sulfamethazine (SMT), Sulfamethoxazole (SMX), Sulfapyridine (SPD), Sulfathiazole (STZ), Sulfachloropyradazine (SCP), Sulfadimethoxine (SDM), Sulfadoxine (SD), Sulfisoxazole (SSX), Sulfaquinoxaline (SQX), Sulfamethizole (SMT), Sulfamoxole (SM), Sulfaguanidine (SG), Sulfamonomethoxine (SMM), Sulfamethoxypyradazine (SMP), Trimethoprim, Chloramphenicol (CAP), Tetracycline (TC), Chlortetracycline (CTC), Oxytetracycline (OTC) and Doxycycline (DC) were obtained from Sigma-Aldrich respectively and stored at room temperature as mentioned in the certificate.
Water which was used throughout the analysis obtained from Millipore, Milli-Q, Gulf scientific corporation. Solvents such as Methanol (purity>99.9%), Acetonitrile (purity>99.9%), Acetic acid (purity 99%) and Formic acid (purity 98%) were LC/MS grade and obtained from Sigma Aldrich. Solution of 10% methanol and 0.1% formic acid was prepared by transferring 100 ml methanol and 1 ml formic acid to a 1L volumetric flask, the volume then made up to 1000 ml with water and mixed well. A solution of 50% methanol was prepared by transferring 50 ml of methanol to a 100 ml volumetric flask, the volume then made up to 100 ml with millipore water and mixed well. Extraction solvent for SAs and CAP, 1% acetic acid in acetonitrile was prepared by transferring 10 ml acetic acid to a 1L volumetric flask; volume then made up to 1000 ml with acetonitrile and mixed well.
Extraction solvent for TCs (70% methanol in DIW at pH=4) was prepared by transferring 700 ml of methanol to a 1 L volumetric flask, volume made up to 1000 ml with DIW, then adjust the pH to 4 by adding required acetic acid and mixed well.
Mobile phase composed of the following components: Component A-0.1% formic acid in water (prepared by transferring 1 ml formic acid to a 1 L volumetric flask and filled to volume with milli-pore water and mixed well). Component B-0.1% formic acid in methanol (prepared by transferring 1 ml of formic acid to a 1 L volumetric flask and filled to volume with methanol and mixed well). The mobile phase was degassed with an ultrasonic bath to remove air bubbles.
Stock solutions for SAs, CAP and TCs (1000 mg/L) were prepared individually by weighing 0.01 g to a 10 ml volumetric flask, dissolved with 5 ml of (50% methanol) and made up to 10 ml and mixed well. Flasks should be sonicated for 30 min to be well dissolved and stored at -20°C.
Three different mixed Working solutions for SAs, CAP and TCs (100 mg/L) were prepared by diluting 1 ml of each stock solution to a 10 ml volumetric flask with (50% methanol), mixed well and stored at -20°C.
Three different mixed Working solutions for SAs, CAP and TCs (10 mg/L) were prepared by diluting 1 ml of mixed working solutions for SAs, CAP and TCs (100 mg/L) to a 10 ml volumetric flask with (50% methanol), mixed well and stored at -20°C.
Three different Intermediate mixtures of SAs, CAP and TCs standard solution (1 mg/L) were prepared by diluting 1 ml of 10 mg/L mixed working solution to a 10 ml volumetric flask with (50% methanol), mixed well and stored at -20°C.
Multi-level calibration levels are used for quantitation of SAs and TCs which was prepared by serial dilution of six levels (1, 5, 10, 25, 50 and 100) μg/L and (0.1, 0.5, 1, 5, 10 and 20) μg/L for CAP respectively.
Apparatus and software
Chromatographic analysis was performed using Highperformance liquid chromatography Agilent 1260 infinity series equipped with an analytical HPLC column.
Separation, Identification, and Quantification of compounds were done by 6500 Q trap liquid chromatography-mass spectrometry with SCIEX (Q trap LC-MS/MS), equipped with Ion Source, Turbo V Ion drive and data station with ANALYST software and MULTIQUANT for quantitation.
The analytical balance used was Mettler Toledo, weighing from 0.0001 g up to 210 g, refrigerator with temperature up to -20°C, Sonicator, Turbo-vap and N2 gas evaporator, pure N2 gas cylinder, Vortex mixer, model VM1000 dig system lab. Instruments Inc., Centrifuge, Beckman Coulter, Allerga 64 R (50 ml tube carriers and adaptors for 15 ml tubes with speed up to 10,000 rpm, Centrifuge tubes used are polypropylene, disposable 15 ml, and 50 ml, Syringe filters used was PVDF 0.20 μm, Agilent, Pipettes transfer 5 ml and 10 ml disposable polyethylene, Beakers (50 ml, 250 ml), Certified grade A, Volumetric flasks (5 ml, 10 ml, 1 L), Certified grade A, QUECHERS extract tubes, EN method, Agilent, USA. QUECHERS dispersive Solid-Phase Dispersion EMR-Lipids (dSPE) 15 ml, Lipids, Agilent, USA. Glass tubes bottom typeround 12 mm × 100 mm, Variable volume micropipettes (range of 20 μl-200 μl and 100 μl-1000 μl) used was Transferpette, Germany with their specific tips.
Instrumental conditions
UPLC conditions: Separation was performed on column C18, Phenomenex (synergi 2.5 μm fusion-RP 100A, 100 × 4.60 mm, 2.5 μm) for SAs and CAP. Kinetics Biphenyl 100A, 50 × 1.70 mm was used for TCs. The injection volume was 10 μl. A gradient elution program was used as mentioned in Table 2 for SAs, different gradient programs for TCs as mentioned in Table 3. But the gradient program for CAP has been reduced to be 8 min as shown in Table 4.
| Time | Flow rate | A | B |
|---|---|---|---|
| 0 | 500 | 98 | 2 |
| 0.6 | 500 | 98 | 2 |
| 7.5 | 500 | 20 | 80 |
| 8 | 500 | 1 | 99 |
| 10.9 | 500 | 1 | 99 |
| 11 | 500 | 98 | 2 |
| 20 | 500 | 98 | 2 |
Table 2: LC. Pump gradient program for Sulfonamides (SAs).
| Time | Flow rate | A% | B% |
|---|---|---|---|
| 0 | 300 | 98 | 2 |
| 0.6 | 300 | 98 | 2 |
| 18 | 300 | 1 | 99 |
| 19.9 | 300 | 1 | 99 |
| 20 | 300 | 98 | 2 |
| 29 | 300 | 98 | 2 |
Table 3: LC. Pump gradient program for TCs.
| Time | Flow rate | A% | B% |
|---|---|---|---|
| 0 | 500 | 90 | 10 |
| 0.6 | 500 | 90 | 10 |
| 2.5 | 500 | 5 | 95 |
| 6 | 500 | 5 | 95 |
| 6.1 | 500 | 90 | 10 |
| 8 | 500 | 90 | 10 |
Table 4: LC. Pump gradient program for CAP.
Mass analyzer conditions: The ESI source was used in the positive mode for SAs and TCs compounds and negative mode for CAP, nitrogen nebulizer, curtain, and other gas settings were optimized according to recommendations made by the manufacturer. Source parameters mentioned in Table 5. Optimization for mass analyzer has been done by injecting 100 μg/L of individual veterinary drugs into the MS instrument. The Selective Reactions Monitoring mode (SRM) used in which one SRM for quantification and the other for confirmation as shown in Table 6 and Figures 1-5.
| Source parameters | Setting for SAs, CAP, and TCs |
|---|---|
| Curtain gas (CUR) | 35 |
| Collision gas (CAD) | High |
| Ion spray voltage (IS) | 4500 |
| Temperature | 450°C |
| Ion source gas 1 (GS1) | 40 |
| Ion source gas 2 (GS2) | 60 |
Table 5: Q trap 6500 ion source, Turbo spray Ion Drive parameters.
| Parameter | Q1 mass | Q3 mass | Time (m.sec) | DP | CE | CXP |
|---|---|---|---|---|---|---|
| Sulfacetamide 1 | 215.1 | 156.1 | 20 | 75 | 10 | 15 |
| Sulfacetamide 2 | 215.1 | 108 | 20 | 75 | 10 | 25 |
| Sulfadiazine 1 | 251.1 | 156 | 20 | 95 | 10 | 20 |
| Sulfadiazine 2 | 251.1 | 108 | 20 | 95 | 10 | 30 |
| Sulfamerazine 1 | 265.1 | 92.2 | 20 | 75 | 10 | 35 |
| Sulfamerazine 2 | 265.1 | 108 | 20 | 75 | 10 | 35 |
| Sulfamethazine 1 | 279.2 | 92.1 | 20 | 90 | 10 | 35 |
| Sulfamethazine 2 | 279.2 | 108 | 20 | 90 | 10 | 30 |
| Sulfamethoxazole 1 | 254.1 | 156.1 | 20 | 70 | 10 | 20 |
| Sulfamethoxazole 2 | 254.1 | 108 | 20 | 70 | 10 | 30 |
| Sulfapyridine 1 | 250.1 | 156.1 | 20 | 75 | 10 | 25 |
| Sulfapyridine 2 | 250.1 | 108 | 20 | 75 | 10 | 35 |
| Sulfathiazole 1 | 256.1 | 156.1 | 20 | 75 | 10 | 15 |
| Sulfathiazole 2 | 256.1 | 108 | 20 | 75 | 10 | 25 |
| Trimethoprim 1 | 291.2 | 261.1 | 20 | 80 | 10 | 35 |
| Trimethoprim 2 | 291.2 | 230.1 | 20 | 80 | 10 | 35 |
| Sulfachloropyridazine 1 | 285 | 156 | 20 | 66 | 10 | 23 |
| Sulfachloropyridazine 2 | 285 | 92 | 20 | 66 | 10 | 41 |
| Sulfadimethoxin 1 | 311.07 | 156.1 | 20 | 66 | 10 | 29 |
| Sulfadimethoxin 2 | 311.07 | 92 | 20 | 66 | 10 | 49 |
| Sulfadoxin 1 | 311.06 | 156.04 | 20 | 66 | 10 | 25 |
| Sulfadoxin 2 | 311.06 | 92.1 | 20 | 66 | 10 | 45 |
| Sulfisoxazol 1 | 268.1 | 156 | 20 | 56 | 10 | 21 |
| Sulfisoxazol 2 | 268.1 | 113.1 | 20 | 56 | 10 | 23 |
| Sulfamethoxypyridazin 1 | 281.1 | 156 | 20 | 61 | 10 | 25 |
| Sulfamethoxypyridazin 2 | 281.1 | 92.1 | 20 | 61 | 10 | 43 |
| Sulfaquinoxalin 1 | 301.1 | 156 | 20 | 61 | 10 | 25 |
| Sulfaquinoxalin 2 | 301.1 | 92 | 20 | 61 | 10 | 45 |
| Sulfamethizol 1 | 270.9 | 107.9 | 20 | 50 | 10 | 30 |
| Sulfamethizol 2 | 270.9 | 91.8 | 20 | 50 | 10 | 36 |
| Sulfamoxole 1 | 268 | 156 | 20 | 56 | 10 | 24 |
| Sulfamoxole 2 | 268 | 92 | 20 | 56 | 10 | 42 |
| Sulfaguanidine 1 | 215 | 108.1 | 20 | 40 | 10 | 28 |
| Sulfaguanidine 2 | 215 | 156.1 | 20 | 40 | 10 | 25 |
| Sulfamonomethoxine 1 | 281.1 | 155.8 | 20 | 66 | 10 | 25 |
| Sulfamonomethoxine 2 | 281.1 | 107.9 | 20 | 66 | 10 | 25 |
| Chloramphenicol 1 | 321.1 | 152.2 | 200 | -71 | -25 | -12 |
| Chloramphenicol 2 | 321.1 | 121.2 | 200 | -71 | -35 | -12 |
| Chlortetracycline 1 | 478.926 | 444.011 | 50 | 56 | 31 | 16 |
| Chlortetracycline 2 | 478.926 | 154.017 | 50 | 56 | 41 | 10 |
| Doxycycline 1 | 445.1 | 410 | 50 | 71 | 35 | 12 |
| Doxycycline 2 | 445.2 | 154.059 | 50 | 71 | 43 | 10 |
| Oxytetracycline 1 | 461 | 426.2 | 50 | 101 | 27 | 18 |
| Oxytetracycline 2 | 461 | 154 | 50 | 101 | 39 | 10 |
| Tetracycline 1 | 445.1 | 410.2 | 50 | 66 | 27 | 14 |
| Tetracycline 2 | 445.1 | 154 | 50 | 66 | 41 | 12 |
Table 6: Mass values and Selective Reaction Monitoring (SRM). Q1 mass, Precursor ion; Q3 mass, product ions; DP: DeclusteringPotential; CE: Collision Energy; CXP: Collision Exit Potential.
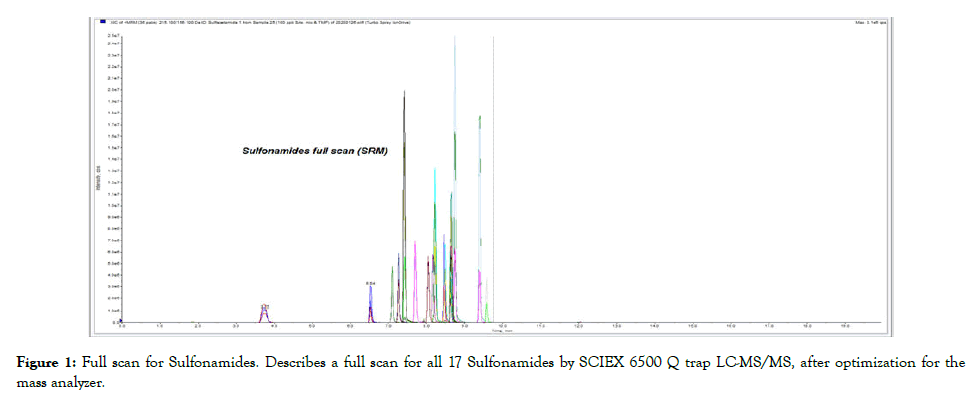
Figure 1: Full scan for Sulfonamides. Describes a full scan for all 17 Sulfonamides by SCIEX 6500 Q trap LC-MS/MS, after optimization for the mass analyzer.
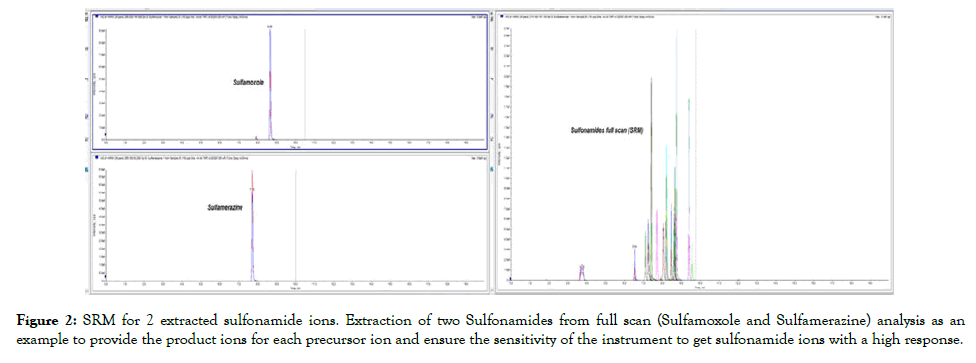
Figure 2: SRM for 2 extracted sulfonamide ions. Extraction of two Sulfonamides from full scan (Sulfamoxole and Sulfamerazine) analysis as an example to provide the product ions for each precursor ion and ensure the sensitivity of the instrument to get sulfonamide ions with a high response.
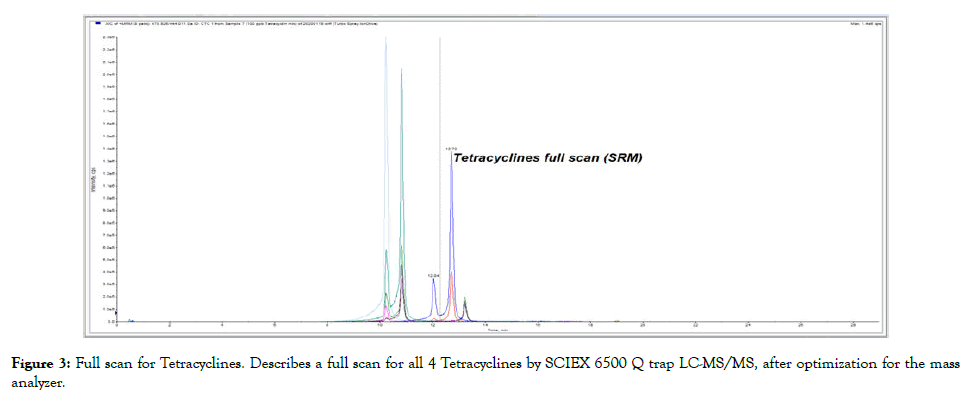
Figure 3: Full scan for Tetracyclines. Describes a full scan for all 4 Tetracyclines by SCIEX 6500 Q trap LC-MS/MS, after optimization for the mass analyzer.
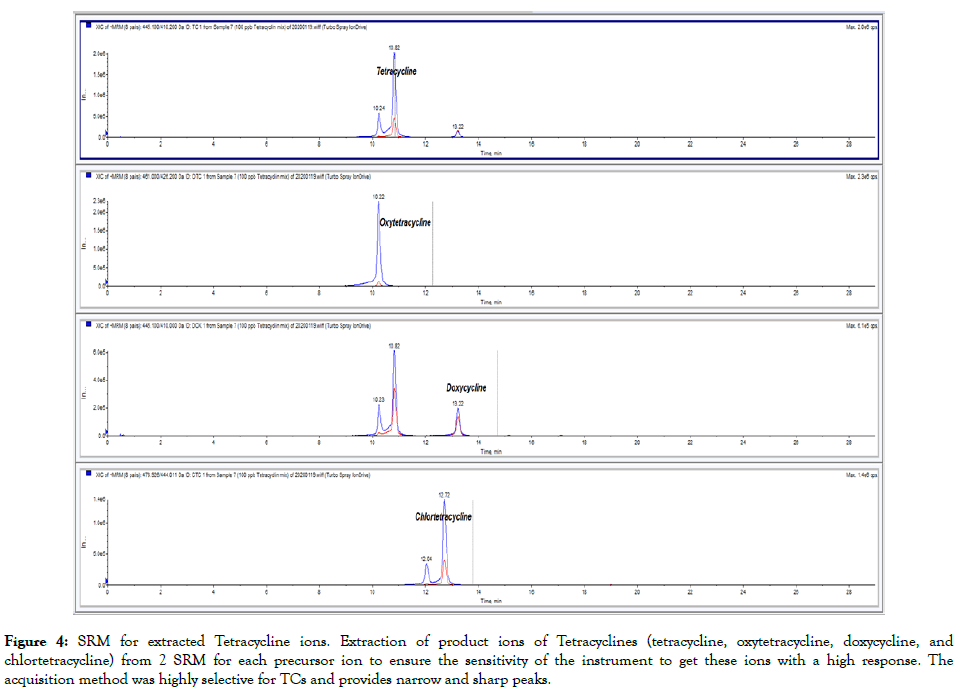
Figure 4: SRM for extracted Tetracycline ions. Extraction of product ions of Tetracyclines (tetracycline, oxytetracycline, doxycycline, and chlortetracycline) from 2 SRM for each precursor ion to ensure the sensitivity of the instrument to get these ions with a high response. The acquisition method was highly selective for TCs and provides narrow and sharp peaks.
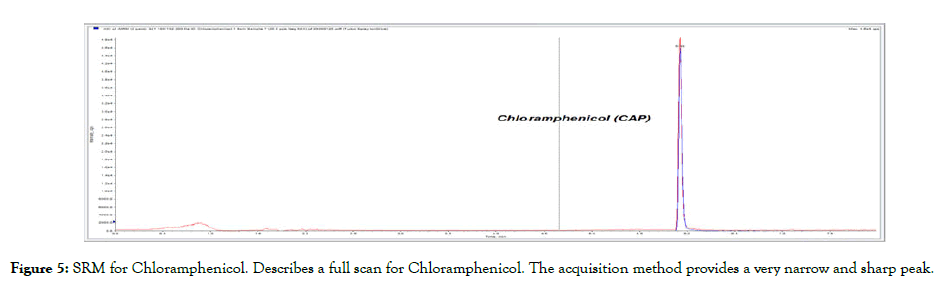
Figure 5: SRM for Chloramphenicol. Describes a full scan for Chloramphenicol. The acquisition method provides a very narrow and sharp peak.
Method optimization and accuracy
Validation protocol was performed for analysis of veterinary drugs in the chicken sample using specific guidelines for the evaluation of method performance [21,22]. Some parameters were evaluated to ensure the method accuracy such as linearity, specificity, recovery, and Limit of Quantitation (LOQs).
Linearity: Instrument linearity (linear range): Instrument linearity was studied to determine the Lowest Calibration Level (LCL) and highest calibration level which can be determined by the instrument. The quantitation is based on the multilevel calibration curve that established to include LOQ, MRL and working range for studied drugs in chicken samples.
Method linearity: Method linearity was tested by performing recovery tests at four different concentration levels on the chicken sample. LOQ, MRL and working range for studied drugs in chicken samples have been included in that selection.
Recovery method: The estimation of recovery for all studied drugs was tested by performing 10 replicates of spike chicken samples at the expected level. The selection has based on LOQ, MRL and working range for studied drugs in chicken samples.
Limit of Quantitation (LOQ): The limit of quantitation is the minimum concentration of the analyte in the test sample that can be determined with acceptable precision and recovery under the stated conditions of the test. The LOQ value was estimated by using 10 repeated spiked samples at the expected lowest quantitation level on chicken samples. European Union stated that CAP has been prohibited in the food of animal origin due to health concern; a legislative Minimum Required Performance Level (MRPL) of 0.30 μg/kg has been issued for CAP which means all methods used in the analysis of this compound should be able to, at least, achieve this level [23].
Results and Discussion
Method evaluation
Linearity: Instrument linearity (linear range): The evaluation of the linearity was achieved using different concentrations to select the Lowest Calibration Level (LCL) and Highest Calibration Level (HCL) that can be used for quantitation. Results showed that the accurate lowest calibration level was 1 μg/L for SAs and TCs and 0.1 μg/L for CAP. The linearity range was from (1 to 100) μg/kg for SAs and TCs compounds. On the other hand, CAP linearity was from (0.1 to 20) μg/kg to cover the lowest concentration (LOQ); Maximum Residue Limits (MRL) and working range for target analytes. The correlation coefficients (R2) were higher than 0.995 for all the compounds.
Method linearity: Method linearity was tested at 4 different concentration levels including LOQ and MRL as well as the more frequency appearance concentration levels in routine samples. For SAs and TCs, 10 replicates for these concentrations (10, 50, 100 and 200) μg/kg were spiked in chicken samples, but for CAP 10 replicates for these concentrations (0.2, 5.0, 10 and 20) μg/kg. The method showed to be linear for all SAs and TCs from the LOQ 10 μg/kg up to 200 μg/kg. Also, was linear in case of CAP from LOQ 0.2 μg/kg up to 20 μg/kg The Recovery results for the different three levels are shown in Table 7.
| Compound | Spiking Level (µg/kg) | Number of replicates (n) | Mean Recoveries (%) | SD | RSD (%) |
|---|---|---|---|---|---|
| Sulfacetamide | 10 | 10 | 82.27 | 6 | 7 |
| 50 | 10 | 103.45 | 9 | 9 | |
| 200 | 10 | 101.55 | 9 | 9 | |
| Sulfadiazine | 10 | 10 | 85.93 | 7 | 9 |
| 50 | 10 | 98.61 | 9 | 9 | |
| 200 | 10 | 99.53 | 9 | 9 | |
| Sulfamerazine | 10 | 10 | 82.97 | 6 | 8 |
| 50 | 10 | 95.52 | 7 | 8 | |
| 200 | 10 | 104.33 | 9 | 8 | |
| Sulfamethazine | 10 | 10 | 82.35 | 7 | 8 |
| 50 | 10 | 98.57 | 8 | 8 | |
| 200 | 10 | 103.09 | 9 | 8 | |
| Sulfamethoxazole | 10 | 10 | 79.9 | 5 | 7 |
| 50 | 10 | 98.17 | 8 | 8 | |
| 200 | 10 | 104.65 | 9 | 9 | |
| Sulfapyridine | 10 | 10 | 83.43 | 6 | 8 |
| 50 | 10 | 96.32 | 6 | 6 | |
| 200 | 10 | 102.45 | 9 | 9 | |
| Sulfathiazole | 10 | 10 | 87.52 | 7 | 8 |
| 50 | 10 | 96.43 | 9 | 9 | |
| 200 | 10 | 101.27 | 10 | 10 | |
| Sulfachloropyridazine | 10 | 10 | 80.46 | 5 | 6 |
| 50 | 10 | 96.22 | 9 | 9 | |
| 200 | 10 | 102.25 | 9 | 9 | |
| Sulfadimethoxine | 10 | 10 | 91.7 | 9 | 10 |
| 50 | 10 | 100.5 | 10 | 10 | |
| 200 | 10 | 102.26 | 10 | 9 | |
| Sulfadoxine | 10 | 10 | 85.6 | 12 | 14 |
| 50 | 10 | 93.5 | 13 | 14 | |
| 200 | 10 | 91.05 | 8 | 9 | |
| Sulfisoxazole | 10 | 10 | 109.21 | 4 | 4 |
| 50 | 10 | 84.01 | 13 | 15 | |
| 200 | 10 | 91.05 | 8 | 9 | |
| Sulfaquinoxaline | 10 | 10 | 109.21 | 4 | 4 |
| 50 | 10 | 84.01 | 13 | 15 | |
| 200 | 10 | 91.05 | 8 | 9 | |
| Sulfamethizole | 10 | 10 | 109.21 | 4 | 4 |
| 50 | 10 | 84.01 | 13 | 15 | |
| 200 | 10 | 91.05 | 8 | 9 | |
| Sulfamoxole | 10 | 10 | 109.21 | 4 | 4 |
| 50 | 10 | 84.01 | 13 | 15 | |
| 200 | 10 | 91.05 | 8 | 9 | |
| Sulfaguanidine | 10 | 10 | 109.21 | 4 | 4 |
| 50 | 10 | 84.01 | 13 | 15 | |
| 200 | 10 | 91.05 | 8 | 9 | |
| Sulfamonomethoxine | 10 | 10 | 109.21 | 4 | 4 |
| 50 | 10 | 84.01 | 13 | 15 | |
| 200 | 10 | 91.05 | 8 | 9 | |
| Sulfamethoxypyridazine | 10 | 10 | 109.21 | 4 | 4 |
| 50 | 10 | 84.01 | 13 | 15 | |
| 200 | 10 | 91.05 | 8 | 9 | |
| CAP | 0.2 | 6 | 111.11 | 0.01 | 8.77 |
| 5 | 6 | 105.46 | 0.19 | 7.18 | |
| 10 | 6 | 96.02 | 2.04 | 2.12 | |
| Chlortetracycline | 10 | 10 | 89.16 | 5.47 | 6.14 |
| 60 | 10 | 77.7 | 5.32 | 6.48 | |
| 200 | 10 | 99.24 | 5.87 | 5.91 | |
| Oxytetracycline | 10 | 10 | 105.21 | 4.03 | 3.83 |
| 60 | 10 | 79.3 | 2.53 | 3.19 | |
| 200 | 10 | 97.89 | 2.74 | 2.8 | |
| Doxycycline | 10 | 10 | 86.94 | 2.36 | 2.71 |
| 60 | 10 | 75.46 | 4.53 | 6.01 | |
| 200 | 10 | 106.9 | 11.42 | 10.68 | |
| Tetracycline | 10 | 10 | 90.29 | 2.61 | 2.89 |
| 60 | 10 | 77.37 | 2.59 | 3.34 | |
| 200 | 10 | 97.68 | 1.92 | 1.96 |
Table 7: Recovery tests.
Recovery study: Data in Table 7 shows the mean recovery of spiked chicken samples at a concentration level from (10 to 200) μg/L for SAs and TCs and from (0.2 to 10) μg/L for CAP. Method validation and quality control procedures for veterinary drug residues analysis in the food of animal origin stipulated the acceptable range of recovery between 70% and 120% [22] and the other regulatory agencies considered these values. The mean recoveries of veterinary drugs studied in chicken were ranged between (70%-120%) Accordingly, none of these compounds was out of the acceptable range of recovery percentage.
Limit of Quantitation (LOQ): Results showed that the lowest practical limit of quantitation for SAs and TCs compounds was 10 μg/kg and was 0.20 μg/kg for CAP with accepted recoveries and precision as shown in Table 6.
Method accuracy: The trueness of methods has been studied by analysis of many proficiency test samples (PT) as shown in Table 8.
| Compounds | PT Assigned value (µg/kg) | Accepted range (µg/kg) | PT Results (µg/kg) |
|---|---|---|---|
| CAP | 1.01 | 0.567-1.460 | 0.71 |
| Sulfadiazine | 49 | 27.40-70.60 | 32 |
| Sulfamethazine | 55.7 | 31.20-80.30 | 37.44 |
| Sulfaquinoxaline | 62.6 | 35.10-90.20 | 87.35 |
| Total Sulfonamides | 203 | 121.0-286.0 | 217.36 |
| Doxycycline | 148 | 85.0-211.0 | 117.84 |
| Oxytetracycline | 143 | 81.8-205.0 | 159.63 |
Table 8: Results of the Performance Test (PT).
Monitoring of veterinary drugs in chicken samples: The present study was conducted in continuation with the previous studies to evaluate the current residue level of VD residues in chicken samples collected during one successive year. Sixty samples were randomly collected from several chicken farms from January 2018 to December 2018. These samples were analyzed using the accredited methods of analysis as previously described [20].
Table 9 showed the minimum and maximum of monitoring amounts of VD residues in chicken samples in addition to the frequency and violation of contaminated samples.
| VD Compounds | Monitoring amounts of VD (µg/kg) | Frequency | Violation | ||
|---|---|---|---|---|---|
| Minimum | Maximum | Mean | |||
| DOX | 57 | 76 | 66.5 | 2 | 0 |
| CTC | --- | --- | --- | --- | --- |
| OXT | --- | --- | --- | --- | --- |
| TC | --- | --- | --- | --- | --- |
| SAs | --- | --- | --- | --- | --- |
| CAP | --- | --- | --- | --- | --- |
Table 9: Monitoring amounts of VD residues in chicken samples during 2018.
The present study indicated that the collected chicken samples were free from anyss VD residues except two out of sixty chicken samples, one from Kotor and the other from Kafr-Elsheikh were contaminated by doxycycline but lower than the MRL (100 ppb).
In the same way in Europe, the proportion of non-compliant results for antibiotic residues in food was only 0.27% (out of 750 000 analyzed samples) in the 27 countries members of the European Union [24].
In Kuwait, the result of a recent study of antibiotic residues in animal products showed that 5% of chicken samples were noncompliant [25].
In Hanoi, a study of 3 Tetracyclines residue (tetracycline, oxytetracycline, and chlortetracycline) in pork sold in the markets showed that only 5.5% of the samples (16 of 290 samples analyzed) were contaminated with Tetracyclines including 2 samples containing tetracycline at a concentration higher than MRLs [26] which is in agreement with our results. On the other hand, the previously published researches on VD residues in broiler chicken samples and other food of animal origin indicated that the presence of VD residues is quite common especially tetracycline compounds. Many studies have been carried out in different countries on broiler chicken samples. In Vietnam, a study conducted to monitor VD residues in pork and chicken meat, showed that twenty-six different antibiotics were used in pig and chicken production mostly contaminated with Chloramphenicol and tetracycline [27].
It was revealed that oxytetracycline was the most predominant antibiotic detected among the four studied antibiotics and followed by Sulfadiazine [10].
While in Egypt many studies have been conducted to monitor VD residues in chicken. A study had results opposed to our results showed that the proportion of non-compliant samples for the presence of Tetracyclines residues in chicken meat was more than 7% and a total of 12 (8%), 13 (7.33%) and 20 (13.33%) samples of breast, thigh, and liver, respectively, had TC residues above the MRL [28]. Higher results were obtained, 14 (56%), 11 (44%) and 14 (56%) which contained antibiotic residues in breast muscles, thigh muscles, and liver, respectively [10]. While another study recorded 13 (39.4%) in liver samples and 7 (20.4%) in muscle samples were positive for the presence of residues [29]. Other studies revealed a detectable level of oxytetracycline residues which confirm widespread misuse of antibiotic especially oxytetracycline in farms and lack application of recommended withdrawal times [30]. A study reported that the residues in all muscle and liver samples were higher than the maximum residue limits of both FAO and FDA reports for DOC [31].
Conclusion and Future Perspectives
The objective of this study was to develop and optimize a highly sensitive analytical method for various groups of veterinary drugs in chicken samples to be used for monitoring chicken samples from different poultry farms in Egypt and to ensure the safety of chicken for humans. By evaluating the data from this study the results showed that methods were fit for purpose, able to get valid results within an acceptable range for studied veterinary drugs in chicken samples from the Egyptian market. Subsequently, the study results showed that no veterinary drugs were higher than the Maximum Residue Limits (MRLs) determined by European regulation. Accordingly, no potential risk was related to the studied veterinary drugs however more studies can be conducted in this field to cover new generations of veterinary drugs.
REFERENCES
- El Nagar A, Ibrahim A. Case study of the Egyptian poultry sector. InProceedings of the International Poultry Conference. 2007:31.
- Maged O, Hamdey E. The analysis of livestock industry frame in Egypt: proposal in the light of bird flu crisis. IDSC: Ministerial Cabinet Information and Designing Making Supporting Center: report. 2006;29:2006.
- Donoghue DJ. Antibiotic residues in poultry tissues and eggs: human health concerns? Poult Sci. 2003;82:618-621.
- Crawford LM. The impact of residues on animal food products and human health. Rev Sci Tech. 1985;4:669-723.
- American Veterinary Medicine Association (AVMA). Antimicrobial use and antimicrobial resistance. 2015 https://www.avma.org/resources-tools/one-health/antimicrobial-use-and-antimicrobial-resistance.
- Health Canada. List of Maximum Residue Limits (MRLs) for veterinary drugs in foods. 2013.
- World Health Organization. Antimicrobial resistance: global report on surveillance. World Health Organization, 2014.
- Beyene T, Kemal A, Jibat T, Tadese F, Ayana D, Feyisa A. Assessment on chemicals and drugs residue in dairy and poultry products in Bishoftu and Modjo, central Ethiopia. J Nutr Food Sci. 2015:1.
- Macarov CA, Tong L, Martínez MH, Hermo MP, Chirila E, Wang YX, et al. Multi residue determination of the penicillins regulated by the European Union, in bovine, porcine and chicken muscle, by LC-MS/MS. Food Chem. 2012;135:2612-2621.
- Shareef AM, Jamel ZT, Yonis KM. Detection of antibiotic residues in stored poultry products. Iraqi J Vet Sci. 2009;23:45-48.
- Dey BP, Thaker NH, Bright SA, Thaler AM. Fast antimicrobial screen test (FAST): improved screen test for detecting antimicrobial residues in meat tissue. J AOAC Int. 2005;88:447-454.
- Kozarova I, Janosova J, Mate D, Tkacikova S. Evaluation of three different microbial inhibition tests for the detection of sulphamethazine residues in the edible tissues of rabbit. Addit Contam Part A Chem Anal Control Expo Risk Assess. 2009;26:978-987.
- Niewold TA. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult Sci. 2007;86:605-609.
- Huyghebaert G, Ducatelle R, Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 2011;187:182-188.
- IARC. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-present, 1990.
- Joint FA, WHO Expert Committee on Food Additives. Specifications for identity and purity and toxicological evaluation of some antimicrobials and antioxidants. Food and Agriculture Organization of the United Nations, 1965.
- Gentili A, Perret D, Marchese S. Liquid chromatography-tandem mass spectrometry for performing confirmatory analysis of veterinary drugs in animal-food products. Trends Analyt Chem. 2005;24:704-733.
- Samsonova JV, Cannavan A, Elliott CT. A critical review of screening methods for the detection of Chloramphenicol, thiamphenicol, and florfenicol residues in food stuffs.
Crit Rev Anal Chem. 2012;42:50-78. - Hanekamp JC, Bast A. Antibiotics exposure and health risks: Chloramphenicol. Environ Toxicol Pharmacol. 2015;39:213-220.
- Khabeer MA, Gouda GAR, Ryad L, Alabdulmalik N, Souaya ER. Method optimization and performance characteristics for the determination of twelve veterinary drugs in food of animal origin using modified Quechers and LC-MS/MS QTRAP Detection. Int J Chromatogr Sep Tech. 2018.
- CRL. Guidelines for the Validation of Screening Methods for Residues of Veterinary Medicines (Initial Validation and Transfer). Community Reference Laboratories Residues (CRLs). 2010:1-8.
- Magnusson B. The fitness for purpose of analytical methods: a laboratory guide to method validation and related topics (2014). Eurachem. 2014:57.
- European Community. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Official J Eur Union. 2003;268:29-43.
- European Food Safety Authority (EFSA). EFSA in focus-Alimentation. European Union. Ed. 7 2010.
- Al-Mazeedi HM, Abbas AB, Alomirah HF, Al-Jouhar WY, Al-Mufty SA, Ezzelregal MM, et al. Screening for tetracycline residues in food products of animal origin in the State of Kuwait using Charm II radio-immunoassay and LC/MS/MS methods. Food Addit Contam. 2010;27:291-301.
- Van Nhiem D, Paulsen P, Suriyasathaporn W, Smulders FJ, Kyule MN, Baumann MP, et al. Preliminary analysis of tetracycline residues in marketed pork in Hanoi, Vietnam. Ann N Y Acad Sci . 2006;1081:534-542.
- Thuat DT, Tuan NN, An VTT, Hien LT, Lam VB, Ninh KT. Study on antibiotic use and antibiotic residues in pork and broiler meat of Binh Duong province. J Vet Sci Technol. 2002:50-57.
- Salama NA, Abou-Raya SH, Shalaby AR, Emam WH, Mehaya FM. Incidence of tetracycline residues in chicken meat and liver retailed to consumers. Food Addit Contam Part B. 2011;4:88-93.
- Shahid MA, Siddique M, Rehman US, Hameed S, Hussain A. Evaluation of a microbiological growth inhibition assay as a screening test for the presence of antibiotic residues in poultry meat. Am J Food Technol. 2007;2:457-461.
- Hussein MA, Khalil S. Screening of some antibiotics and anabolic steroids residues in broiler fillet marketed in El-Sharkia governorate. Life Sci J. 2013;10:2111-2118.
- Abdel-Mohsein HS, Mahmoud MA, Ibrahim A. Tetracycline residues in intensive broiler farms in Upper Egypt: Hazards and Risks. J Worlds Poult Res. 2015;5:48-58.
Citation: Elkhabeer MA, Gouda GAR, Ryad L, Souaya ER (2020) Analytical Method Optimization and Determination of Sulfonamides, Chloramphenicol and Tetracyclines Drug Residues in Chicken Meat across Egypt. J Food Process Technol 11:825. doi: 10.35248/2157-7110.20.11.825
Copyright: © 2020 Elkhabeer MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


