Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 2
An Open-label Clinical Study to Determine the Effect of Enhanced Absorption Formula (MB EnzymePro®) on the Bioavailability of Whey Protein in Healthy Male Subjects
Anupam Trehan, Manoj Kumar Verma, Sameer Maheshwari, Tanyya Khanna, Puja Kumari and Harinder Singh*Received: 18-Feb-2020 Published: 05-Mar-2020, DOI: 10.35248/2157-7110.20.11.820
Abstract
Background: Several reports recommend that the dietary supplementation with Whey Protein (WP) in liquid form has been a popular choice for most of the individuals, especially athletes. Some product labels suggest a high serving size of 50 g for WP. Nevertheless, due to the limited endogenous digestive enzymes output and fast transit time, the average amount digested and absorbed may be approximately 15 g, thereby restricting protein bioavailability. The objective of this study was to find out whether MB EnzymePro®, a unique patent-pending Enhanced Absorption FormulaTM (EAF) of digestive proteases, significantly increases the digestion and absorption of WP, ultimately increasing bioavailability. The study also investigated its usefulness in significantly altering nitrogen (N2) balance and C-Reactive Protein (CRP) levels.
Methods: An open-label, three-period, crossover, an oral bioavailability study was performed in healthy male subjects under fasting conditions. The study was carried out as per the Institutional Ethics Committee (IEC) approved study protocol and was registered with Clinical Trials Registry-India (CTRI). The study involved twenty-four subjects (age 20-36 years, BMI 20.0-24.0 kg/m2). A single dose of 50 g of WP alone (control), with one capsule (treatment A) and two capsules (treatment B) of MB EnzymePro® was administered to each group comprising 8 subjects. Blood samples were collected at specific time points up to 4 h for amino acid profile and CRP analysis. A total of 17 amino acids were quantified. Urine was collected after up to 24 h for total N2 analysis. Pharmacokinetic parameters were calculated using Phoenix® WinNonlin®.
Results: The overall protein bioavailability enhancement was more than 50% for WP with MB EnzymePro®. A significant increase of more than 60% in BCAAs was noticed in the MB EnzymePro® treatment. The N2 balance and CRP levels were significantly better in the treated groups than the control group.
Conclusion: Compared with the control condition, a patent-pending blend of digestive proteases (MB EnzymePro®) increased the digestion and absorption (bioavailability) of WP with improved N2 balance and reduced CRP levels.
Keywords
Whey protein; MB EnzymePro®; Enhanced Absorption FormulaTM (EAF); Bioavailability
Introduction
Currently, most of the athletes and bodybuilders worldwide consume an increased level of protein, especially for the purpose of muscle building and muscle recovery. Among the various proteins, milk proteins have experienced extensive research related to their prospective roles in augmenting adjustments from exercise training [1,2] with the highest score on the Protein Digestibility Corrected Amino Acid Score (PDCAAS) rating system [3]. Milk proteins, casein and whey contain the highest leucine content of all other protein sources at 9.3% and 11%, respectively. They differ in the digestion rate as well as their impact on protein metabolism [4-6]. Whey Protein (WP) is water soluble, mixes easily, and digested at a faster rate than casein [7]. In comparison, casein is water insoluble, gels in the gut and is digested at a slower rate than whey protein [7]. These facts make WP the most ideal and widely preferred form of protein.
WP variants, especially WP isolate and WP concentrate in liquid form, have become a supplement of choice as these supplements are pure and easily available forms of protein. WP supplementation can improve muscle protein synthesis and therefore aid in exercise recovery and improve performance [8]. Various literature reports established that approximately 30 g of WP in liquid form demonstrated a large, but momentary increase in postprandial plasma Amino Acid (AA) levels within a window of approximately 90 minutes and returned to baseline levels within 5 h. The increase in AAs levels augmented protein synthesis and nitrogen (N2) balance but did not impair wholebody protein catabolism [4,9,10]. To have a positive effect, WP should be broken down into smaller peptides within 90 minutes of ingestion; if not, the protein is excreted from the body undigested. The larger peptides of protein mainly consist of more than seven amino acids that may activate an immune response, inducing irritation and even inflammation in the bowel during digestion. Layman has asserted that the consumption of WP above 1.5 g/kg/ day aided in reducing body fat, building lean body mass and supporting nitrogen (N2) balance [11]. Despite a plethora of research studies exhibiting safety, much concern even exists encompassing the clinical implications of ingesting an increased quantity of protein, predominantly on renal-hepatic health. The scientific literature on this subject consistently reports that increased consumption of protein by competitive sportspersons and active individuals presents no indication of hepato-renal damage [12-14]. Antonio and colleagues published a sequence of original studies that prescribed exceedingly high volumes of protein (~3.4-4.4 g/kg/ day) and have constantly reported no harmful effects [15-18]. Moreover, there were no changes in clinical markers of metabolic processes and blood lipids.
Kim and colleagues stated that a 70 g protein promoted a further favorable net balance of protein in comparison to a 40 g protein due to a stronger attenuation of rates of muscle protein catabolism [19]. To reach these high grades of protein intake, one must face upwards to some authentic source of protein that is easily usable and fit/safe for everyday use. WP is the best appropriate option for this class of individuals, particularly athletes who desire to increase their daily protein intake to build lean muscle bulk. Various aspects can limit protein digestion and absorption including limited endogenous enzyme output at this extreme level of intake. WP is a highly purified protein prepared from milk using industrial techniques, such as spraydrying and pressure microfiltration, which may have an undesirable impact on digestive enzymes in vitro due to overprocessing [20,21]. To avert this problem, the use of proteolytic enzymes has been described to increase WP digestibility. These enzymes are also known to increase the extent of hydrolysis (DH), solubility and simultaneous in vitro digestibility [22]. One foremost factor in the digestion of WP is its absorption, which may be reduced by the fast transit time through the gastrointestinal tract (g.i.t.), which is on an average equal to 90 minutes for thick dispersions [23]. The high absorption rate of WP has been reported to be (8 to 10) g/h [24]. Based on these facts, the maximum WP that can be absorbed from a thick liquid is approximately 15 g. The combined effects of overprocessing and the increased intake of WP may contribute to inadequate digestion, leading to several side effects, such as bloating, belching, diarrhea, and stomach heaviness [25,26]. These elements will contribute to the reduction in the recuperative effects of a protein-rich diet, including improved lean muscle mass and increased N2 assimilation, along with constructive cardiovascular effects, such as the decline in levels of C-Reactive Protein (CRP) [27-32]. C-Reactive Protein (CRP) is one of the most determined inflammatory indicators in clinical and epidemiologic studies. CRP, a protein made by the liver, is sent into bloodstream in response to inflammation. Inflammation is body's way of protecting tissues if there is injury. Numerous randomized controlled trials have evaluated the level of circulating CRP in reaction to whey supplementation. Resistance exercise frequently leads to skeletal muscle injury and generates inflammatory markers [33]. Protein cures the soreness by stimulating muscle biosynthesis and decreasing inflammatory markers [34].
The popularity of digestive enzymes in sports nutrition commodities has increased during the past few years with several products now containing a blend of proteases and lipases. Proteases can break down proteins into different peptide configurations and possibly single amino acids. Digestive enzymes might potentially work to encourage optimal digestion by permitting upregulation of a variety of metabolic enzymes that may be essential to allow for effective bodily operation [35]. Further, digestive enzymes have been reported to minimize quality differences between variable protein sources [36]. Individuals aspiring to increase plasma peak amino acid concentrations may get help from protein enriched with digestive enzymes.
Improving the digestion of WP before it reaches the caecum may enhance the absorption rate and the anticipated result of a high protein diet. Therefore, we recommend that adding proteindigesting enzymes would improve the digestion of WP, ultimately enhancing the absorption of WP in vivo, leading to a positive N2 balance and reduced CRP levels.
A branded proteolytic enzymatic composition has been established to be used along with a WP supplement to offer predigestion of the protein and to take full benefit of the availability of the essential amino acids to build up muscle and enhance muscle recovery. Increased digestion will also confirm that smaller, nonimmunogenic peptides will be produced, therefore decreasing the potential for discomfort that is frequently associated with protein intake.
The objective of this study was to establish whether MB EnzymePro®, a unique patent awaiting, enhanced absorption formulaTM (EAFTM) (Indian patent application published 201911004951; PCT application filed PCT/IN2019/050865) [37], when coadministered with WP improves the overall digestion of amino acids and reduces the immunogenic responses associated with WP consumption compared to WP alone. MB EnzymePro® comprises different food-grade enzymes obtained from microbial (fungal, bacillus, etc.) and plant sources. An increase in WP absorption was measured by comparing the levels of Total Serum Amino Acids (TSAA) and Individual Amino Acids (IAAs). Other measurable parameters, such as CRP levels and N2 balance, were also determined and equated to the control group to differentiate the positive therapeutic effects of WP.
Materials
MB EnzymePro® was manufactured by Bright Lifecare Pvt Ltd, Gurugram, India. The WP used in this study was manufactured using spray-dried whey (manufactured by Milk Specialties Global, Minnesota, United States). WP samples were supplied in premeasured individual packets of 50 g each by Bright Lifecare Pvt Ltd, Gurugram, India.
Methods
Experimental design
The study was planned as an open-label, balanced, randomized three-arm, three-period, crossover, an oral bioavailability study. The study was performed in healthy, adult, human male subjects in fasting conditions. As per the study protocol approved by the Independent Ethics Committee (IEC) (GSER/2019/CL/049), twenty-four (24) subjects were enrolled in the study.
All subjects were divided into 3 equal groups comprising 8 subjects each. The 3 groups were assigned to either Treatment-A, Treatment-B or the control group and sequenced (ABC and BCA and CAB) in a random order according to the randomization schedule generated using a “ Software SPSS, Version 25.0”. Treatment A consisted of administering 50 g of WP and 1 capsule of MB EnzymePro®, while treatment B consisted of administering 50 g of WP and 2 capsules of MB EnzymePro®. The control group included subjects that received only 50 g of WP. The difference in the rate of WPC absorption was determined by quantifying the serum levels of postprandial amino acids, and the level of urea in urine samples was used to determine total N2 [38,39]. CRP levels were also measured.
Subjects
An adequate number of volunteers were selected randomly from the volunteer database and underwent a standardized screening procedure. Twenty-four healthy, lean, males aged 20-36 years, Body Mass Index (BMI) ranging from 20-24, participated in the study. None of the subjects were taking any specific protein-rich dietary regime, muscle-toning or bodybuilding procedure during the research study. The clinical study was enrolled in the Clinical Trials Registry-India (CTRI/2019/02/017731). Each subject was pre-informed of the objective, methods, and probable risks associated with the experimental study, and informed consent was signed by each participant.
Dietary protocol
All the participants followed a specified, well-adjusted diet of 2200 Kcal/day during the stay at the clinic facility. An overnight fast of at least 12 h was mandatory before every treatment. All participants were advised to resume the diet after the last blood sample. The nutrient composition comprised 40% carbohydrate, 25% protein, and 35% fat. The precise weight and constitution were established for everyone by a registered dietician at the Auriga Research Private Limited, New Delhi, India. Participants were instructed to strictly follow the prescribed diet and dietary restrictions as per the approved study protocol.
Study protocol
Subjects received treatment in a crossover design under fasting conditions as per the randomization schedule. All subjects received a 50 g of WP with 360 mL of drinking water either alone (control group) or with treatment A (one capsule of MB EnzymePro®) or treatment B (two capsules of MB EnzymePro®) at room temperature in sitting position and under the supervision of qualified study personnel after an overnight fast of at least 12 h in each period. The time of dosing was recorded. The dosing was performed under the supervision of the investigator, authorized study personnel and Quality Assurance (QA) personnel. Subjects were not allowed to drink water for 1 h before dosing until 2 h postdose. Food was prohibited until 4 h after dosing. Subjects remained in the seated or semi-inclined position until 2 h after dosing. The two treatment days were separated by a washout period of 5 days. The administration of the investigated product was performed as per the randomization schedule generated using “ Software SPSS, Version 25.0” and as authorized by a Biostatistician.
A predose blood sample of 6 mL plus 9 postdose blood samples of 6 mL each were collected using a Serum Separator Tube (SST) vacutainer from each subject in each period for analysis of amino acids (AAs). A predose blood sample of 4 mL plus 01 postdose blood samples of 4 mL each at 4 h was also collected using a SST vacutainer from each subject in each period to analyze the serum inflammatory marker C-Reactive Protein (CRP). Predose blood samples were withdrawn at 30 ± 10 minutes prior to dosing in each period. Whereas, the postdose blood samples were taken at 00.25, 00.50, 00.75, 01.00, 01.25, 01.50, 02.00, 03.00 and 04.00 h postdose in each period within a time frame of ± 02 minutes from its scheduled time. Blood samples were analyzed to measure TSAA, IAA and CRP levels. Urine samples were collected over a period of 24 h postdose for each subject in each period to evaluate the amount of nitrogen excreted.
Sample collection and preparation
Blood samples were collected in pre-labeled SST vacutainers and gently manually inverted 5 times to mix the tube clot activator. Then, the blood was allowed to clot by leaving it in an upright position and undisturbed at room temperature for at a minimum of 30 minutes until centrifugation. The blood samples were centrifuged at a temperature of 2 to 1°C at 4000 ± 100 rpm for 10 minutes to separate the serum and remove the clot. After centrifugation, the separated serum was transferred to suitably pre-labeled polypropylene tubes in duplicate, placed in a wet ice bath and stored upright in a deep freezer at -20 ± 5°C for interim storage. Finally, the samples were transferred to the bioanalytical laboratory and stored in a deep freezer at -70°C ± 15°C until the completion of the analysis. Blood samples collected in 4 mL SST vacutainers were transferred to the clinical laboratory for analysis of serum inflammatory marker CReactive Protein (CRP) in each period.
Analytical method
To remove any analytical bias, blinded samples of urine and serum were submitted to the laboratory. Amino Acid (AA) analysis consisted of the quantification of seventeen individual serum AAs for each subject at each time point. The analysis was performed on a Waters Xevo TQS Micro LC/MS/MS coupled with a Waters UPLC system. Quantification was performed by comparing with reference standard and control mixtures of known quantities of all 17 AAs (Waters AccQ-Tag Ultra Amino Acid Standard Kit). AA levels are reported in nmol/mL and as the percent increase in AUC. TSAA levels were reported as the sum total of all seventeen AAs. The AOAC Kjeldahl digestion flask method was used to determine urinary nitrogen [40]. CRP was measured by a highly sensitive protein precipitation extraction and chromatographic separation technique (Waters, India) by derivatizing each amino acid with a specific compound. For all samples, within-run variations in control values of greater than ± 20% required reanalysis.
Statistical analysis
All significance and power testing on the results were performed at a level of alpha=0.05. The within-group analysis was performed between the control and treatment groups. The mean area under the concentration-time curve (AUC) and other pharmacokinetic parameters were calculated using Phoenix® WinNonlin®. Two-way Analysis Of Variance (ANOVA-2) was performed on TSAA levels between control and treatment groups. ANOVA-1 was performed on TSAA levels between 0 h and each time point within the control and treatment groups and on the percent increase in AUC of each IAAs level at 0 h and 4 h. ANOVA-2 was used on the urine N2 and CRP levels between control and treatment groups. The results are expressed as means ± SEM. Statistics were performed using a commercially available software program (SPSS Statistics, version 25.0).
Results
TSAA levels
Analysis of the TSAA kinetic profiles of the treatment groups showed a progressive, time-dependent increase for approximately 1.5 h and then a constant decrease until 4 h. Statistical comparison of means between the treatment groups and the control group showed significant differences among the groups at all time points (p=0.05). Statistical power of 1.0 was reached between all comparisons.
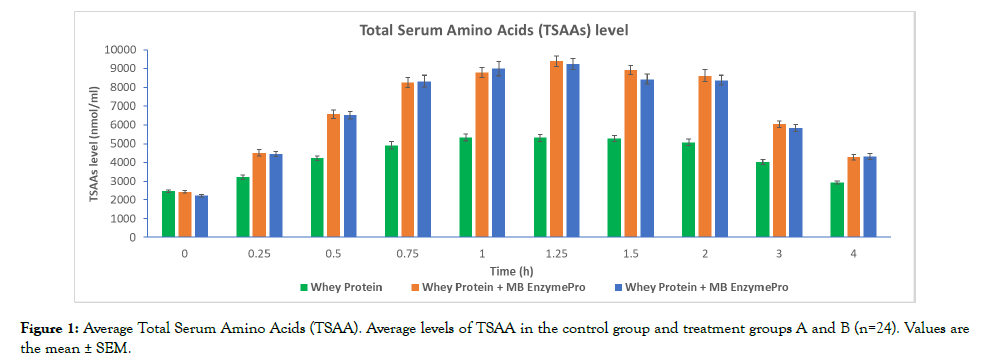
Comparison of average TSAA levels between 0 h and each time point revealed significant (p=0.05) increases in all treatment groups. In the control group, the mean TSAA level increased significantly at each time point and reached a maximum level at 1.25 h (2.39 times than the initial level at 0 h). In treatment group A, the mean TSAA level increased significantly at each time point and reached a maximum level at 1.25 h (3.81 times than the initial level at 0 h) (Figure 1). In treatment group B, the mean TSAA level increased significantly at each time point, and reach a maximum level at 1.25 h (3.80 times that of the initial level at 0 h) (Figure 1).
Figure 1: Average Total Serum Amino Acids (TSAA). Average levels of TSAA in the control group and treatment groups A and B (n=24). Values are the mean ± SEM.
Individual amino acid levels
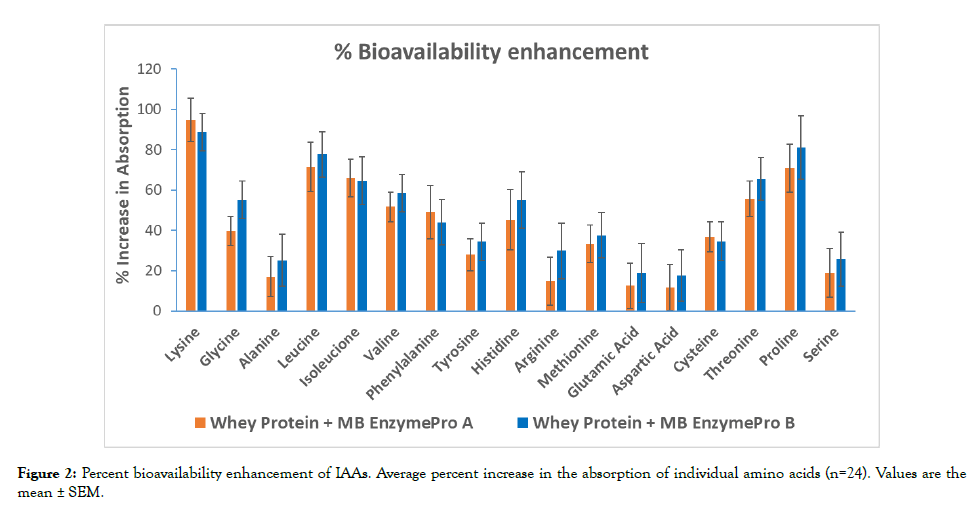
After standard subtraction, the relative percent increase in the AUC of each of the seventeen AAs analyzed in the treatment groups and the control group is shown in Figure 2. There was an overall increase in the Individual Amino Acid (IAA) level. There were statistically significant differences between the control and treatment groups for cysteine, isoleucine, leucine, lysine, phenylalanine, proline, threonine, tyrosine, methionine and valine (10 of 17). The amino acids alanine, glycine, histidine, arginine aspartic acid, glutamic acid, and serine were not significantly different between the treatment and control groups.
Figure 2: Percent bioavailability enhancement of IAAs. Average percent increase in the absorption of individual amino acids (n=24). Values are the mean ± SEM.
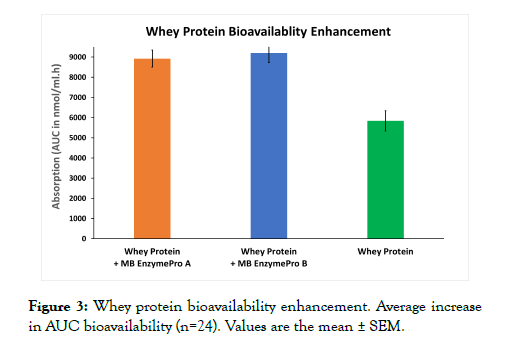
The sum of the AUC of all seventeen amino acids was considered the whey protein bioavailability of treatment A and treatment B, which was 1.53 and 1.57 times greater than that of the control group (Figure 3). The sum of the AUC was significantly greater in each treatment group than in the control group (p=0.05). The significant difference between the two treatments was not significant.
Figure 3: Whey protein bioavailability enhancement. Average increase in AUC bioavailability (n=24). Values are the mean ± SEM.
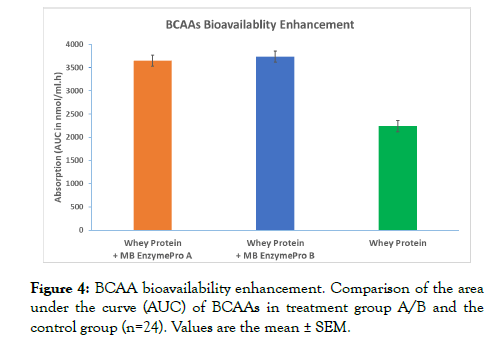
There was a significant increase of 163% and 167% in the AUC of BCAAs (sum of the AUC of leucine, isoleucine, and valine) in treatment groups A and B, respectively, compared to the control group (Figure 4). The difference between the two treatments was not significant.
Figure 4: BCAA bioavailability enhancement. Comparison of the area under the curve (AUC) of BCAAs in treatment group A/B and the control group (n=24). Values are the mean ± SEM.
Nitrogen excretion
The average quantity of N2 excreted in 24 h was determined as urea in both treatment groups and the control group.
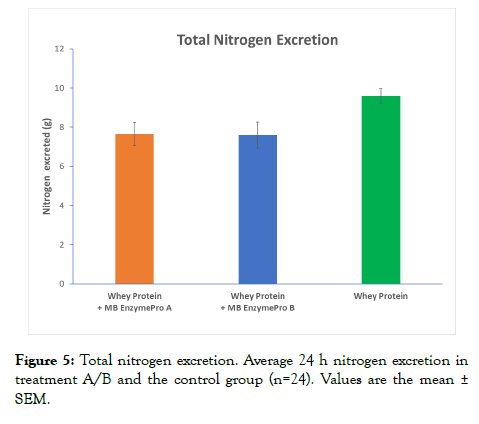
ANOVA-2 showed that the average N2 excretion declined significantly (p<0.05) in each treatment group contrasted to the control group. The mean difference between treatment groups and the control group, as well as the interaction between both treatment groups, was not significant (Figure 5). In treatment groups A and B, the average amount of N2 excreted over 24 h was 7.65 g and 7.60 g, respectively. In the control group, the average amount of N2 excreted over 24 h was 9.59 g. In summary, total nitrogen excretion decreased significantly with both treatments A and B, which included MB EnzymePro®.
Figure 5: Total nitrogen excretion. Average 24 h nitrogen excretion in treatment A/B and the control group (n=24). Values are the mean ± SEM.
C-reactive protein
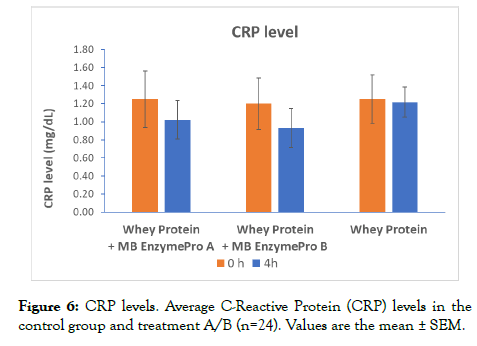
CRP was measured at 0 h and 4 h in each treatment group and in the control group (Figure 6). ANOVA-2 comparison of the means between the control group and treatment groups showed significant differences (p=0.05). No significant differences were found when evaluating the means of both treatment groups. The interaction between time points was not significant. No significant changes were found between 0 h and 4 h in either treatment group. Significant (p=0.003) reductions were found between 0 h and 4 h in each treatment group compared to the control group. Compared to the control group, treatment group A showed a reduction of 16.15%, and treatment group B showed a reduction of 23.61% at 4 h.
Figure 6: CRP levels. Average C-Reactive Protein (CRP) levels in the control group and treatment A/B (n=24). Values are the mean ± SEM.
Discussion
The objective of this clinical study was to establish whether MB EnzymePro® (EAFTM), a patent-pending blend of digestive proteases, could significantly affect the quantity of WP metabolized in vivo and if any effect could significantly and adequately alter N2 balance and CRP levels from the control group levels. Comparing the levels of TSAA after consumption with and without MB EnzymePro® would be an indicator of the efficacy of the EAFTM to increase the amount of WP digested and absorbed. Along with other benefits, WP is stated to have positive effects on cardiovascular disease risk factors as described by several scientific studies [41,42].
The results demonstrated that postprandial TSAA levels were significantly improved by treatment with a 50 g dose of WP with MB EnzymePro® compared with WP alone (Figure 1). TSAA levels in each treatment group appeared to be prolonged much longer than in earlier findings using WP isolates from milk [4,10]. Supplementation with MB EnzymePro® increased the digestion of WP, leading to an increase in its digestion and absorption rate. This finding was additionally supported by significant (1.53 and 1.57 times) increase in the sum of the AUC of IAAs (Figure 3) in the treatment groups contrasted to the control group. After baseline subtraction, no significant differences were found between the AUC of treatment A and B, indicating that the WP absorption rate may be close to maximum in treatment A. Additional studies may validate this finding by increasing the number of treatment groups consuming different amounts of MB EnzymePro®.
MB EnzymePro® profoundly increased the level of amino acids, especially BCAAs. BCAAs are of special significance for bodybuilders and athletes since these amino acids are used by the body to build up the protein for muscle synthesis, muscle repair, etc. [43]. BCAAs are essential amino acids that are not manufactured by the body; these amino acids must be obtained from the diet or from nutritional supplements. BCAAs are metabolized in the muscle instead of the liver; consequently, the effect of these BCAAs is much quicker and more efficient than that of any other amino acid. If the diet is balanced, BCAAs will be utilized for protein synthesis, which is critical for endurance athletes and strenuous exercises. BCAAs are used to lessen fatigue in both anaerobic and endurance sports [44-46].
The strongest BCAA is leucine, which is accountable for regulating the levels of blood sugar and the growth and repair of tissues such as skin, bones and skeletal muscles. Leucine facilitates healing wounds, regulating energy and averting the breakdown of muscle tissue. Due to its anti-catabolic properties and its vital role in protein synthesis, leucine is one of the extremely critical BCAAs [47]. Isoleucine is another BCAA that promotes muscle recovery following physical exercise. Individually, isoleucine is desirable for the creation of hemoglobin and supports the regulation of blood sugar and energy levels. Valine contributes to the repair and growth of muscle tissue, as commonly attributed to BCAAs. Valine is not metabolized by the liver; rather, it is actively absorbed by the muscles. Valine maintains N2 balance and preserves the use of glucose [47].
Arginine has marked nitrogen retention ability. The key element in muscle protein synthesis is nitrogen. Arginine is also a precursor of very vital molecules like creatine and Gamma- Aminobutyric Acid (GABA, a neurotransmitter in the brain). Creatine supports muscle growth and improves high-intensity exercise performance. Lysine is an essential amino acid that is required for proper growth. Lysine is essential to produce carnitine, a nutrient that converts fatty acids into energy and helps to reduce cholesterol. Threonine is an essential amino acid which is not synthesized within the body. The two most crucial binding substances, collagen, and elastin are formed by threonine. Arguably its most valuable property, threonine improves the absorption of several nutrients, making protein sources containing threonine more bioavailable as compared to others. Like threonine, proline is also required to produce collagen and cartilage. Proline retains muscles and joint flexibility and helps to reduce sagging and wrinkling, which accompanies UV exposure and leads to the normal aging of the skin.
The significantly elevated levels of AAs in the treatment groups may be linked to a significant rise in N2 retention and a reduction in CRP levels. The TSAA levels remained higher in each treatment group as compared to the control group throughout the 4 h period in this study, as described in previous studies employing a 30 g dose of WP isolate from milk [4,9,10]. Peak postprandial plasma AA levels in these studies were described at approximately 1.5 h, and the levels returned to baseline values in approximately 5 h. In the present study, postprandial TSAA levels peaked at approximately 1.25 h and returned to baseline at approximately 4 h. Numerous factors may account for these changes, involving the amount ingested and over-processing. The amount of WP ingested may have saturated endogenous proteolytic enzymes, which is the ratelimiting step in the digestion process in vivo. These factors inhibit the absorption rate, which may restrict the amount of protein digested and absorbed before reaching the caecum. The addition of MB EnzymePro® provided additional proteolytic enzymes, thus probably increasing the digestion and ultimately absorption rate through the same time period. Whether the addition of MB EnzymePro® has an impact on one or more of these factors in this study cannot be determined at this point; nevertheless, the general effect appears to be a substantial increase in WP digestion along with the significant increase in absorption rate and amount.
The IAA results indicate that MB EnzymePro® supplementation may contribute to reduced whole-body protein metabolism as reported by West et al. [8]. The results show substantially less fluctuation in these crucial AAs between 0 h to 4 h in each treatment group than in the control group, indicating decreased whole-body protein metabolism. These results, together with the significant rise in TSAAs, indicate that MB EnzymePro® supplementation may create optimal requirements for protein synthesis and growth. This finding is in accordance with a considerable decrease in the average N2 excretion in the treatment groups compared to the control group. This array of evidence offers additional support that whole-body protein metabolism was reduced in each treatment group. In a nutshell, whey protein supplementation enhances whole-body anabolism and may improve acute recovery of exercise performance after a strenuous bout of resistance exercise [8]. Additional studies can evaluate this hypothesis by subtracting baseline urinary N2 levels and determining N2 excretion each h during the entire period of the study and post-study for several hours. These findings also suggest increased protein utilization.
CRP levels decreased substantially between 0 h and 4 h in the treatment groups compared to the control group. This result may be related to the differences in the amount of WP digested and absorbed between the control group and treatment groups. Earlier studies have shown that peptides produced from the in vitro enzymatic hydrolysis of WP are bioactive and reduce CRP [31,32]. Supplementing WP with MB EnzymePro® may contribute to the significant decrease in CRP levels bys producing bioactive peptides in vivo. Kerasioti et al. [48] studied the anti-inflammatory effect of whey protein fortified carbohydrate cake intake before 2 h intense exercise and reported a 46% reduction in CRP level. Another study reported a significant reduction in the serum CRP level after dietary supplementation of whey protein. The mechanism was unraveled to be due to the generation of peptide metabolites from the ingested protein [49-52].
Conclusion
The International Society of Sports Nutrition recommended that most exercising individuals should take a minimum of approximately 1.4 to 2.0 g of protein/kg of body weight per day to augment exercise training-induced adaptations. Importantly, this endorsement also falls within the Institute of Medicine’s Acceptable Macronutrient Distribution Range (AMDR) of 10-35% protein. Such high protein intake, limited endogenous enzyme output, and different processing methods may contribute to the decreased digestion and absorption rate of WP and lower than anticipated blood levels of individual amino acids along with digestive side effects as mentioned earlier in the introduction. The results of this clinical study demonstrated that compared with the control condition, supplementing 50 g of WP with MB EnzymePro®, a unique patent-pending, EAFTM for usage as a digestive aid, significantly enhanced the digestion and absorption rate of WP. This effect was reflected by statistically significant (p=0.05) rise in postprandial AUC, TSAA levels, ISAA levels, and N2 balance. A significant decrease in CRP levels is also reported which may be due to the production of bioactive peptide production in vivo. The level of amino acids found in the blood with WP and the increase observed with MB EnzymePro® is consistent with the study and benefits of whey hydrolysate. Among those amino acids are BCAAs, which have been shown to play a vital role in muscle synthesis and recovery. Furthermore, the key findings of this clinical study indicated the immense need for additional research in the field of enzyme supplementation for more comprehensive digestion of protein and possibly other protein-rich foods.
Acknowledgements
The authors are thankful to the Research and Development scientists and other employees of Bright Lifecare Pvt Ltd (Healthkart), Gurugram, India who have supported in the successful completion of this project.
Authors Contributions
AT, MKV and SM were involved with project conception, planning, interpretation and giving final approval of the manuscript. TK, PK, and HS were involved in formulation development, drafting, and revision of the manuscript.
Ethics Approval and Consent to Participate
The study design and its procedures were approved by the Independent Ethics Committee (Good Society Ethical Research, New Delhi India). All participants completed an informed consent form prior to their participation in this study.
Consent for Publication
Not Applicable
Availability of Data and Materials
All data pertaining to the conclusions of the study are found in the article. Please contact the author for any data related query.
Competing Interests
The authors declare no competing interests.
Funding
Not applicable
REFERENCES
- Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107:987-992.
- Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373-381.
- Norton L, Wilson GJ. Optimal protein intake to maximize muscle protein synthesis. Agro Food Ind Hi-Tech. 2009;20:54-57.
- Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci. 1997;94:14930-14935.
- Dangin M, Boirie Y, Guillet C, Beaufrère B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr. 2002;132:3228S-3233S.
- Dangin M, Guillet C, Rodenas CG, Gachon P, Demange CB, Magnani KR, et al. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol. 2003;549:635-644.
- Wilson J, Wilson GJ. Contemporary issues in protein requirements and consumption for resistance trained athletes. J Int Soc Sports Nutr. 2006;3:7.
- West DW, Sawan SA, Mazzulla M, Williamson E, Moore DR. Whey protein supplementation enhances whole body protein metabolism and performance recovery after resistance exercise: A double-blind crossover study. Nutrients. 2017;9:735.
- Bendtsen LQ, Thorning TK, Reitelseder S, Ritz C, Hansen ET, van Hall G, et al. Human muscle protein synthesis rates after intake of hydrolyzed porcine-derived and cows’ milk whey proteins-a randomized controlled trial. Nutrients. 2019;11:989.
- Dangin M, Boirie Y, Rodenas CG, Gachon P, Fauquant J, Callier P, et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340-348.
- Layman DK. Protein quantity and quality at levels above the RDA improves adult weight loss. J Am Coll Nutr. 2004;23:631S-636S.
- Martin WF, Armstrong LE, Rodriguez NR. Dietary protein intake and renal function. Nutr Metab. 2005;2:25.
- Poortmans JR, Dellalieux O. Do regular high protein diets have potential health risks on kidney function in athletes? Int J Sport Nutr Exerc Metab. 2000;10:28-38.
- Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373-385.
- Antonio J, Ellerbroek A, Silver T, Orris S, Scheiner M, Gonzalez A, et al. A high protein diet (3.4 g/kg/d) combined with a heavy resistance training program improves body composition in healthy trained men and women-a follow-up investigation. J Int Soc Sports Nutr. 2015;12:39
- Antonio J, Ellerbroek A, Silver T, Vargas L, Peacock C. The effects of a high protein diet on indices of health and body composition-a crossover trial in resistance-trained men. J Int Soc Sports Nutr. 2016;13:3.
- Antonio J, Ellerbroek A, Silver T, Vargas L, Tamayo A, Buehn R, et al. A high protein diet has no harmful effects: a one-year crossover study in resistance-trained males. J Nutr Metab. 2016;2016:9104792.
- Antonio J, Peacock CA, Ellerbroek A, Fromhoff B, Silver T. The effects of consuming a high protein diet (4.4 g/kg/d) on body composition in resistance-trained individuals. J Int Soc Sports Nutr. 2014;11:19.
- Kim IY, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab. 2016;310:E73-E80.
- Henares JAR, Andrade CD, Pérez SJ, Morales FJ. Assessing nutritional quality of milk-based sport supplements as determined by furosine. Food Chem. 2007;101:573-578.
- Almeida CC, Monteiro ML, Lima BRC, Alvares TS, Junior CAC. In vitro digestibility of commercial whey protein supplements. LWT-Food Sci Technol. 2015;61:7-11.
- Sindayikengera S, Xia WS. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex. J Zhejiang Univ Sci B. 2006;7:90-98.
- KIM SK. Small intestine transit time in the normal small bowel study. Am J Roentgenol. 1968;104:522-524.
- Bilsborough S, Mann N. A review of issues of dietary protein intake in humans. Int J Sport Nutr Exerc Metab. 2006;16:129-152.
- Giezenaar C, Marsh NDL, Hutchison AT, Lange K, Hausken T, Jones KL, et al. Effect of gender on the acute effects of whey protein ingestion on energy intake, appetite, gastric emptying and gut hormone responses in healthy young adults. Nutr Diabetes. 2018;8:1-2.
- Giezenaar C, Trahair LG, Luscombe-Marsh ND, Hausken T, Standfield S, Jones KL, et al. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women. Am J Clin Nutr. 2017;106:865-877.
- Johnston CS, Tjonn SL, Swan PD. High-protein, low-fat diets are effective for weight loss and favorably alter biomarkers in healthy adults. J Nutr. 2004;134:586-591.
- Devries MC, Sithamparapillai A, Brimble KS, Banfield L, Morton RW, Phillips SM. Changes in kidney function do not differ between healthy adults consuming higher-compared with lower-or normal-protein diets: a systematic review and meta-analysis. J Nutr. 2018;148:1760-1775.
- Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Speizer FE, et al. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr. 1999;70:221-227.
- Giezenaar C, Van der Burgh Y, Lange K, Hatzinikolas S, Hausken T, Jones KL, et al. Effects of substitution, and adding of carbohydrate and fat to whey-protein on energy intake, appetite, gastric emptying, glucose, insulin, ghrelin, cck and glp-1 in healthy older men-A randomized controlled trial. Nutrients. 2018;10:113.
- Fekete AA, Givens DI, Lovegrove JA. The impact of milk proteins and peptides on blood pressure and vascular function: a review of evidence from human intervention studies. Nutr Res Rev. 2013;26:177-190.
- Ehlers PI, Kivimäki AS, Turpeinen AM, Korpela R, Vapaatalo H. High blood pressure-lowering and vasoprotective effects of milk products in experimental hypertension. Br J Nutr. 2011;106:135313-135363.
- Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J Int Soc Sports Nutr. 2012;9:20.
- Volek JS, Volk BM, Gómez AL, Kunces LJ, Kupchak BR, Freidenreich DJ, et al. Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr. 2013;32:122-135.
- Jäger R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, et al. International society of sports nutrition position stand: protein and exercise. J Int Soc Sports Nutr. 2017;14:1-25.
- Minevich J, Olson MA, Mannion JP, Boublik JH, McPherson JO, Lowery RP, et al. Digestive enzymes reduce quality differences between plant and animal proteins: a double-blind crossover study. J Int Soc Sports Nutr. 2015;12:P26.
- Trehan A, Verma MK, Singh H, Khanna T, Maheshwari S. Proteolytic enzyme composition. Indian patent application number 201911004951.
- Bos C, Metges CC, Gaudichon C, Petzke KJ, Pueyo ME, Morens C, et al. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J Nutr. 2003;133:1308-1315.
- van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. J Nutr. 2015;145:1981-1991.
- Devani MB, Shishoo CJ, Shah SA, Suhagia BN. Spectrophotometric method for microdetermination of nitrogen in kjeldahl digest. J Assoc Off Anal Chem. 1989;72:953-956.
- Fekete ÁA, Giromini C, Chatzidiakou Y, Givens DI, Lovegrove JA. Whey protein lowers systolic blood pressure and Ca-caseinate reduces serum TAG after a high-fat meal in mildly hypertensive adults. Sci Rep. 2018;8:1-9.
- Tahavorgar A, Vafa M, Shidfar F, Gohari M, Heydari I. Beneficial effects of whey protein preloads on some cardiovascular diseases risk factors of overweight and obese men are stronger than soy protein preloads-A randomized clinical trial. J Nutr Intermed Metab. 2015;2:69-75.
- Blomstrand E, Eliasson J, Karlsson HK, Köhnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136:269S-273S.
- Newsholme EA, Blomstrand E. Branched-chain amino acids and central fatigue. J Nutr. 2006;136:274S-276S.
- Tsuda Y, Yamaguchi M, Noma T, Okaya E, Itoh H. Combined effect of arginine, valine, and serine on exercise-induced fatigue in healthy volunteers: A randomized, double-blinded, placebo-controlled crossover study. Nutrients. 2019;11:862.
- Hsueh CF, Wu HJ, Tsai TS, Wu CL, Chang CK. The effect of branched-chain amino acids, citrulline, and arginine on high-intensity interval performance in young swimmers. Nutrients. 2018;10:1979.
- Gorissen SH, Phillips SM. Branched-chain amino acids (Leucine, Isoleucine, and Valine) and skeletal muscle. Nutr Skeletal Muscle. Academic Press. 2019:283-298.
- Kerasioti E, Stagos D, Jamurtas A, Kiskini A, Koutedakis Y, Goutzourelas N, et al. Anti-inflammatory effects of a special carbohydrate-whey protein cake after exhaustive cycling in humans. Food Chem Toxicol. 2013;61:42-46.
- Lands LC, Iskandar M, Beaudoin N, Meehan B, Dauletbaev N, Berthiuame Y. Dietary supplementation with pressurized whey in patients with cystic fibrosis. J Med Food. 2010;13:77-82.
- Wolfe RR, Cifelli AM, Kostas G, Kim IY. Optimizing protein intake in adults: interpretation and application of the recommended dietary allowance compared with the acceptable macronutrient distribution range. Adv Nutr. 2017;8:266-275.
- Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90:106-115.
- Børsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648-E657.
Citation: Trehan A, Verma MK, Maheshwari S, Khanna T, Kumari P, Singh H (2020) An Open-label Clinical Study to Determine the Effect of Enhanced Absorption Formula (MB EnzymePro®) on the Bioavailability of Whey Protein in Healthy Male Subjects. J Food Process Technol 11:820. doi: 10.35248/2157-7110.20.11.820
Copyright: ©2020 Trehan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.