Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2021) Volume 12, Issue 1
A Case of HIV and Clinical Confirmed Covid-19
Cansu Özgen, Ülkü Arslan, Simay Karaduman and Hülya Sungurtekin*Received: 30-Dec-2020 Published: 21-Jan-2021, DOI: 10.36648/2155-9597.21.12.385
Abstract
Understanding the clinical conditions and outcomes of Covid-19 infected patients with immunodeficiency like HIV will be an information for improving management and treatment modalities. It was reported a patient of HIV plus clinical confirmed Covid-19 in this presentation.
Keywords
Covid-19; Coronavirus infection; SARS-CoV-2
Introduction
Covid-19, a novel coronavirus infection that causes severe acute respiratory syndrome (SARS-CoV-2), is a global health crisis. In the disease caused by SARS-CoV-2, the virus disrupts the host immune system by avoiding the immune response. Cellular immune response is an adaptive immune mechanism. Cellular immunity may be seen inside infected cells mediated by T-lymphocytes. Clearing and killing viral infected cells made by cytotoxic T cells. The overall adaptive immune response is directed by helper T cells. CD4 T cells provide B cell help for antibody production and organize the response of other immune cells. CD8 T cells kill infected cells to decrease the viral load. It was shown that the occurrence of lymphopenia with reduced numbers of both CD4 and CD8 T cells in Covid-19 cases [1,2].
SARS-CoV-2 immunopathology studies are still being studied worldwide. Residual immune dysregulation phenomenon is seen in HIV patients. HIV is harmful for the immune system [3]. Decreased CD4/CD8 ratio is related with higher morbidity and mortality and is an indicator for antiretroviral therapy for HIV patients. Here we report a case of SARS-CoV-2 infected HIV patients in our clinic.
Case Study
A 37 years-old male referred from another hospital with complaints of high temperature, dry cough, fatigue, perspiration for about 5 days at 10th April 2020. He engaged in tourism business for about three years. He was diagnosed for HIV on 23th March 2020. He was heterosexual. His antiviral therapy starts on 31th March 2020 with Genvoya® (Elvitegravir 150 mg+ Cobicistat 150 mg+ Emtricitabine 200 mg+ Tenofovir 10 mg) once a day with ceftriaxone 1 gr once a day and tetracycline 100 mg once a day. Anti-HIV titer before admission was 778 and HIV-RNA level was 165960 copies/mL.
The patient was admitted to the intensive care unit with respiratory failure. At admission, the patient’s respirator rate was 25 per minute, pulse 116 per minute, temperature 37.2°C, and peripheral oxygen saturation was 77% while taken oxygen 6 liters per minute via face mask. Therefore, he was intubated and started mechanical ventilation. The patient’s clinical characteristics, blood gas, and laboratory parameters were given (Table 1). The patient presented with leukopenia, lymphopenia, and neutrophilia and increased NLR levels. Procalcitonin, C-reactive protein levels were also elevated.
| Properties | 1st day | 3rd day | 7th day | 10th day | 25th day |
|---|---|---|---|---|---|
| Heart rate | 62 | 56 | 67 | 77 | 98 |
| Temperature (°C) | 37.2 | 38.5 | 36.3 | 37.1 | 37.1 |
| Mean arterial pressure (mm Hg) | 89 | 102 | 98 | 101 | 90 |
| Mechanical ventilation mod | SMIV/PCV | SMIV/PCV | SMIV/PCV | SMIV/PCV | SIMV/PCV |
| FiO2 | 60 | 60 | 50 | 50 | 50 |
| PEEP | 8 | 12 | 8 | 8 | 10 |
| TV | 450 | 460 | 450 | 430 | 450 |
| pH | 7.29 | 7.44 | 7.42 | 7.48 | 7.49 |
| PaCO2 (mm Hg) | 56.4 | 50.03 | 44.8 | 57.4 | 48 |
| PaO2 (mm Hg) | 99.6 | 77.8 | 83 | 91.5 | 78.1 |
| HCO3 (mmol/L) | 24.3 | 31.6 | 27.7 | 39 | 35.4 |
| Beb (mMol/L) | -0.2 | 7.8 | 3.6 | 15.4 | 11.7 |
| Lactate | 0.98 | ||||
| PaO2/FiO2 | 166 | 155.6 | 166 | 183 | 156.2 |
| White blood cell count (K/uL) | 15.28 | 12.62 | 13.27 | 12.66 | 12.29 |
| Lymphocyte (K/uL) | 2.9 | 1.66 | 1.88 | 2.85 | 2.65 |
| Neutrophil (K/uL) | 13.62 | 10.45 | 10.7 | 9.17 | 8.51 |
| Neutrophil to Lymphocyte ratio (NLR) | 4.7 | 6.3 | 5.69 | 3.22 | 3.21 |
| Hemoglobin (gr/dL) | 10.6 | 11.4 | 9.9 | 10.5 | 9.5 |
| Platelets (K/uL) | 400 | 325 | 368 | 387 | 430 |
| D-dimer (ng/mL) | 936 | 2023 | 2768 | 1875 | 1044 |
| Fibrinogen (mg/dL) | 784 | 906 | 350 | 433 | 693 |
| International normalized ratio (INR) | 1.27 | 1.32 | 1.19 | 1.23 | 1.2 |
| Ferritin (ug/L) | 355.4 | 570.4 | 381.8 | 485 | 1596 |
| Procalcitonin (ng/mL) | 3.08 | 0.86 | 0.1 | 0.1 | 0.5 |
| C-reactive protein (mg/L) | 261.6 | 318 | 11.3 | 14.2 | 121.1 |
Table 1: Clinical and laboratory data of the patient.
At admission, nasopharyngeal swap sample was used for reversetranscriptase– polymerase-chain-reaction (RT-PCR) assay for Covid-19. At admission, in chest tomography CT scan, the patient had patchy shadowing, ground glass opacities, and local consolidation distributed peripheral zones that compatible with SARS-CoV-2 (Figure 1). Chest radiography of the patient at admission and at 10th day were given (Figures 2 and 3).
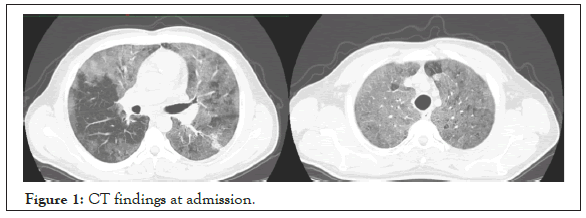
Figure 1: CT findings at admission.
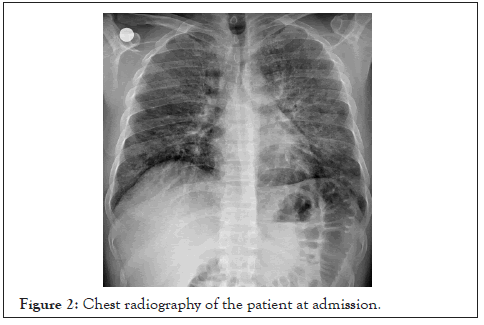
Figure 2: Chest radiography of the patient at admission.
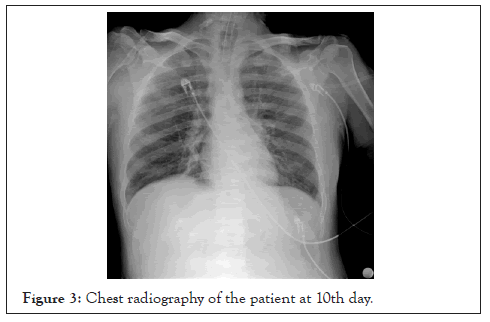
Figure 3: Chest radiography of the patient at 10th day.
It was started SARS-CoV-2 treatment regimen. Hydroxychloroquine was given 400 mg twice a day with a loading dose on the first day and then 200 mg twice a day, Favipiravir 600 mg twice a day after loading dose of 1600 mg twice a day and ceftriaxone 1 gr iv twice daily for one week.
He also received his routine antiviral regimen Genvoya® (Elvitegravir 150 mg+Cobicistat 150 mg+Emtricitabine 200 mg+Tenofovir 10 mg) for three days. On the third day of admission, his body temperature was progressively increasing Table 1. Prevention of Pneumocystis pneumonia (PCP), prophylaxis dose of trimethoprim/sulfamethoxazole (15 mg/kg for trimethoprim component) started three times a day.
Enoxaparin 0.6 unite once a day, pantoprazole 40 mg once a day, vitamin C 1.5 gr/day, and acetylsalicylic acid 100 mg per day also were given. Tocilizumab therapy was given at the 3rd day of ICU with a dose of 400 mg/day IV infusion then a dose of 240 mg/day for 4th day.
Microbial cultures from tracheal aspirate, blood, and urine were taken at admission and in need of clinical situation. On the 15th day of admission, Stenotrophomonas Maltophilia was identified in the tracheal aspirate cultures. Micafungin 100 mg/day and Teicoplanin loading dose of 400 mg twice a day for 2 days and a maintenance dose of 400 mg/day were received.
On The 7th day, patients’ vital signs were stable, GCS was E4M6VE and PaO2/FiO2 was 166, then the patient extubated. High flow oxygen therapy was received. The patient was intubated again at the third hour of extubation because of the signs of respiratory failure.
RT-PCR assay for detection of coronavirus RNA was repeated the patient’s endotracheal swabs on 3rd-5th-8th day of ICU. There was not a positive result. In the serological tests, there is not an acute onset infectious disease (Table 2).
| Test | Result |
|---|---|
| TPHA | Negative |
| VDRL-RPR | Negative |
| Anti-HIV | Positive |
| HBsAg | Negative |
| Anti HCV | Negative |
| Cmv IgG | Reactive |
| Cmv IgM | Nonreactive |
| Anti Hbc IgG | Negative |
| Anti HBs | Negative |
| Anti-Toxoplasma IgG | Reactive |
| Anti-Toxoplasma IgM | Nonreactive |
| EbcVca IGG | Reactive |
| EBV VCA IgM | Nonreactive |
| EBV EBnA IgG | Reactive |
| Anti-Rubella IgG | Reactive |
| Anti-Rubella IgM | Nonreactive |
Table 2: Serology results of the patient.
The patient’s CD4 T cell count and CD8 T cell count were 10% and 79%, respectively; and CD4/CD8 was 0,126 at 30th March 2020. The patients’ CD4 T cell count and CD8 T cell count were 7% and 80%, respectively; and CD4/CD8 ratio was 0, 08 on 24th April 2020.
The patient were given antiviral, antimicrobial, and antifungal therapies. Patient was tracheotomysed on May 11, 2020. It was started spontaneous breathing trials on May 16. Tracheostomy was closed on May 20th. Patient was discharged to infection ward on May 24, discharged to home June 05 with home physical therapy recommendations. The patient continues to treat Dolutegravir (Tivicay) and 200 mg Emtricitabine/245 mg Tenofovir (Hivent).
Discussion
Covid-19 is a novel infectious disease caused by beta-coronavirus (SARS, COV-2) and related with respiratory symptoms, fever, fatigue, cough, myalgia, arthralgia and weakness. Older age, high SOFA scores, elevated d dimer levels are related with mortality [4]. Our patient had similar symptoms and he was HIV positive, so this was a risk factor for him.
Previous studies suggest that coinfection with HIV in a Covid-19 patients causes a delayed antibody response against SARS-Cov-2 [1]. In addition, the patient’s antiretroviral therapy against HIV might decrease replication of the virus [5,6]. We are in the same opinion with literature because after initial and maintenance therapy the patient’s PCR analyses were persistently negative for Covid-19 and antibody rapid test was also negative for this patient. The normal CD4/CD8 ratio is approximately 1.5 to 2.5 and related with age, gender, and genetics. A low or decreased ratio in HIV infected patients is related with immune dysfunction and also in morbidity and mortality [7]. The decrease in CD4/CD8 rates in the following periods of the treatment also supports our hypothesis.
Zhang et al. declare that IL6 plays a dominant role in the cytokine storm in ARDS and so in Covid-19 infected patients, and blocking IL6R is an effective treatment method [8]. Patients treated by tocilizumab showed significantly improved clinical changes after therapy. In our patient IL-6 levels increased before tocilizumab. After tocilizumab therapy, there was not any significant decrease ın IL-6 levels, neither any symptoms like fever or decrease in FiO2. Despite the second dose of tocilizumab, his clinical status did not change, and the patient’s respiratory tract culture resulted with Stenotrophomonas Maltophilia. Tocilizumab therapy in the early days of the disease might be responsible for this clinical situation [9].
Conclusion
Patients with HIV are more susceptible to respiratory infections while HIV is not well treated. At the same time, immune deficiency status in HIV patients may lead to less detectability of SARS-CoV-2 virus and worse clinical condition.
Competing Interest
The authors declare that they have no competing interests.
Funding
The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in the writing of the manuscript.
Acknowledgements
None.
REFERENCES
- Zhao J, Liao X, Wang H, Wei L, Xing M, Liu L, et al. Early virus clearance and delayed antibody response in a case of COVID-19 with a history of co-infection with HIV-1 and HCV. Clin Infect Dis. 2020;1(1):ciaa408.
- Zhu F, Cao Y, Xu S, Zhou M. Reply to Comments on Co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92(9):1417-1418.
- Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119(1):51-83.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
- Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64(5):e00399-e00420.
- National Health Library and Knowledge Service (HSE).Clinical evidence for the use of antivirals in the treatment of COVID-19. Health Service Executive. 2020.
- McBride JA, Striker R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLOS Pathog. 2017;13(11):e1006624.
- Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954.
- Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for covid-19-induced cytokine release syndrome: A cautionary case report. Chest. 2020;158(1):e15-e19.
Citation: Özgen C, Arslan Ü, Karaduman S, Sungurtekin H (2021) A Case of HIV and Clinical Confirmed Covid-19. J Bacteriol Parasitol. 12: 385.
Copyright: © 2021 Özgen C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

