Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2016) Volume 8, Issue 1
Urinary Thrombomodulin Levels Were Significantly Higher Following Occupational Exposure to Chemicals, In The Presence of Dipstick Protein, But Not in the Presence of Dipstick Blood
Abstract
Currently, there are no biomarkers which can identify patients with an increased risk of developing urothelial cancer as a result of occupational chemical exposure. The aim of this study was to evaluate the relationships between final diagnosis and 22 biomarkers measured in urine, serum and plasma collected from 156 hematuric patients. Fourteen of the 80 patients (17.5%) with urothelial cancer and 13/76 (17.1%) of the controls were deemed to have a history of chemical exposure. We applied Fisher's exact tests to explore associations between chemical exposure and final diagnosis, and tumor stage and grade, where applicable; ANOVA and t-test to compare age across patients with and without chemical exposure; and Zelen’s exact test to evaluate relationships across final diagnosis, chemical exposure and smoking. Following pre-selection of biomarkers using Lasso, we identified differentially expressed biomarkers across patients with and without chemical exposure using Welch’s t-test. Using a one-sided t-test and considering multiple testing using FDR, we observed that TM levels in urine were significantly higher in samples from patients with a history of chemical exposure regardless of their diagnosis as control or urothelial cancer (one-sided t-test, pUC = 0.014 and pCTL = 0.043); in the presence of dipstick protein and when urinary pH levels ≤ 6 (p = 0.003), but not in the presence of dipstick blood (p = 0.115). Urothelial cancer patients with a history of chemical exposure were significantly younger (64.1 years) than those without chemical exposure (70.2 years) (one-sided t-test p-value = 0.012); and their tumors were higher grade (Fisher’s exact test; p = 0.008). There was a strong association between a history of chemical exposure and smoking in urothelial cancer patients (Zelen’s exact test; p = 0.025). Elevated urinary thrombomodulin levels could have the potential to identify chemical exposure in hematuric patients at highrisk of developing urothelial cancer
Keywords: Thrombomodulin; Smoking; Bladder cancer; Urine; Chemical exposure; Occupation
Abbreviations
CE: Chemical Exposure; UC: Urothelial Cancer; NCE: No Chemical Exposure; CTL: Control Trans; TURB: FDR: Urethral Resection of the Bladder False Detection Rate ; CRP: C-reactive Protein; IL-4: Interleukin-4; TM: Thrombomodulin; MCP-1: Monocyte Chemo Attractant Protein 1; MMP9NGAL: Matrix Metalloproteinase Neutrophil Gelatinase Associated Lipocalin Complex; BPE: Benign Prostate Enlargement; MNI: Non- muscle Invasive; MI: Muscle Invasive
Introduction
Currently, there are no biomarkers which can identify patients who have an increased risk of developing UC as a result of occupational exposure to chemicals. Chemical exposure remains a significant risk factor for urothelial cancer (UC) in developed countries despite the laws which limit occupational exposure to harmful chemicals. This is attributable to both the latency periods that often exceed 20 years [1-3], and the significant range of chemical agents associated with increased risk of bladder cancer [3-7]. Recent evidence suggests that metal workers, car mechanics, plumbers [8], those exposed to intermediates in rubber and plastics manufacture [9], and those working in occupations allied to agriculture or medicine and health [10] could be at risk of developing UC. With respect to aromatic amines, risk is highest in those exposed at a younger age, those with over 10 years of exposure [11] and amongst certain categories of painters [12]. Polycyclic aromatic hydrocarbons arising from incomplete combustion of carbon fuels can increase bladder cancer risk, e.g. in those exposed to diesel fumes [13] and amongst asphalt pavers [14]. Dermal absorption and inhalation of oils, fumes and metals may be one reason for the significantly increased risk of bladder cancer reported for employees who have worked for over 20 years on the assembly line in a car factory [15]. Smoking is regarded as the most significant risk factor for UC. Smokers are at least three times more likely to develop UC and their risk increases with increased packyears of cigarette smoking [16,17]. However, the association between smoking and prognosis in patients diagnosed with UC is unclear [18].
Material and Methods
Patients
One hundred and fifty-six patients with haematuria were recruited from Belfast City and Craigavon Area Hospitals to a case-control study, conducted according to Standards for the Reporting of Diagnostic accuracy studies guidelines, between November 2006 and October 2008. The study was approved by the Office for Research Ethics Committees Northern Ireland (ORECNI 80/04), reviewed by hospital review boards and registered (ISRCTN registry – 30128). The inclusion criteria were 1) patients with haematuria who have undergone cystoscopy; and 2) patients must be able to understand the study procedures and willing to give informed consent. The exclusion criteria were 1) patients who have not had a flexible cystoscopy; 2) patients who did not present with haematuria; 3) patients with UTI destined to undergo TURB; and 4) patients currently suffering from clinically evident alcoholism and or drug dependency. After written informed consent, a clean catch/midstream specimen of urine voided into a sterile collecting container (~50 ml), serum (2 ml) and plasma (2 ml) samples were collected from each patient and stored at -80°C until analysis [19]. Patients (98%) were recruited during normal working hours.
Chemical exposure
Occupations that were classed as high risk included painters [12], dye workers [20], foundry workers [7], hair dressers [21], lorry drivers [22], miners, metal workers, concierges and janitors [23]. Patients with no occupational risk and no known history of exposure to chemicals were assigned as “no chemical exposure” (NCE).
Biomarker analysis
Biomarker analyses (n = 22) in urine, serum and plasma were carried out as described previously. Briefly, scientists blinded to patient data, carried out the analysis of biomarkers at Randox Laboratories Ltd, County Antrim, Northern Ireland, using biochip array technology (BAT) (Randox Evidence © and Randox Investigator©) and ELISA, as previously reported. All analysis was carried out in triplicate (mean ± SD) [19].
Pathology diagnostic review
Following approval from the Northern Ireland Biobank (NIB13- 0065), DOR (Consultant Pathologist), undertook a diagnostic review of 20 patients with UC; 12 who were assigned as CE and eight who were assigned as NCE.
Statistical analyses
We undertook the following analyses: Fisher's exact tests to explore the associations between CE, diagnosis and tumor stage and grade; ANOVA, prior to t-test, to investigate age within UC and control (CTL) subpopulations across CE and NCE; and Zelen’s exact test [24] using the NSM3 R package [25] to explore the three way association across final diagnosis (UC vs CTL), chemical exposure (CE vs NCE) and smoking (non-smoker vs smoker).
Associations between biomarker levels and CE
We pre-selected biomarkers with non-zero coefficients using Lasso in conjunction with the glmnet function in the R package [26,27], repeating each bootstrap analysis 100 times. The minimal λ (0.03636) was chosen by a 10 fold cross-validation procedure. Levels of the pre-selected biomarkers were log10 transformed prior to analyses for differential levels across the CE and NCE subpopulations and across dipstick urine analyses categories using Welch’s t-test and applying a false detection rate (FDR) method.
Results
Chemical risk across UC and control patients
Twenty-seven of the 156 patients (17%) were assigned as CE. Fourteen of these patients had UC (52%) with the following stages: pTa UC (n = 5), pT1 UC (n = 6), pT3a (n = 1), pT3b (n = 1) and pT4a (n = 1). Thirteen of these patients (48%) were CTLs. The occupational histories for the patients assigned as CE are detailed in Table 1.
| Diagnosis | Present Occupation | Past occupation(s) |
|---|---|---|
| UC | Timber yard – painter /sprayer | Supervisor - Hardware Store |
| UC | Retired | Car painter |
| UC | Retired | Marine engineer |
| UC | Retired | Landscape contractor |
| UC | Retired | Lorry driver/agricultural sprayer |
| UC | Engineering-mechanical | Engineer |
| UC | Retired | Process operator - car manufacture |
| UC | Retired | Fireman |
| UC | Production worker | |
| UC | Retired | Lorry driver |
| UC | Retired | Service engineer |
| UC | Retired | Mechanical engineer |
| UC | Unemployed | Cable joiner |
| UC | Retired | School caretaker/ tyre making |
| control | Retired | Road service maintenance |
| control | Retired | Building - skilled labourer |
| control | Retired | Foreman – works ministry |
| control | Window blind fitter | Fabric factory – mixed dye |
| control | Retired | Newspaper - production photographer |
| control | Recreation park - attendant | Coal man |
| control | Taxi driver | Moulding factory |
| control | Retired | Carpet cleaner |
| control | Clergyman | TV technician/ RAF radio inspector |
| control | Joiner | |
| control | Warehouse | Plastics manufacturer |
| control | Lorry driver | Car mechanic/car valeting |
| control | Retired | Labourer |
Each patient’s occupational history was reviewed by three authors (MR, CR and KW) and assigned low risk (score 1), moderate risk (score 2), or high risk (score 3). Scores were averaged. Patients deemed to have an occupational history associated with a high risk of bladder cancer; or patients reporting that they had been exposed to chemicals, passive smoking, weed-killers, dyes, paints, leather, metal, rubber, vehicle oils/fumes or other described carcinogens were assigned as CE.
Table 1: Occupational histories for CE patients.
Higher tumor grade in patients with a history of chemical exposure
Grade 1 (n = 4) and grade 2 tumors (n = 39) were combined (n = 43) for comparison to grade 3 tumors (n = 35). There was a significant association between grade and CE (Fisher’s one sided exact test p = 0.008). The proportion of grade 1 and 2 tumors combined for NCE UC patients was 40/64 (63% (CI 51% to 74%)) in comparison to 3/14 (21% (CI 0% to 42%)) for CE UC patients; none of the UC patients with a history of chemical exposure had Grade 1 tumors (Table 2).
| CTL | UC | Total (% cohort) | |||
|---|---|---|---|---|---|
| NCE | CE | NCE | CE | ||
| 63 | 13 | 66 | 14 | ||
| Age | |||||
| Age (mean (SD)) | 54.3 (19.1) | 54.1 (16.7) | 70.2 (8.1)** | 64.1 (8.4)* | |
| Final diagnosis | (% column) | (% column) | (% column) | (% column) | |
| No diagnosis | 27 (42.9) | 9 (69.2) | 0 | 0 | 36 (37.5) |
| Benign pathologies | 5 ( 7.9) | 1 (7.7) | 0 | 0 | 6 ( 3.8) |
| Stones and inflammation | 15 (23.8) | 1 (7.7) | 0 | 0 | 16 (10.3) |
| BPE | 10 (15.9) | 2 (15.4) | 0 | 0 | 12 ( 7.7) |
| Other cancers | 6 ( 9.5) | 0 | 0 | 0 | 6 ( 4.2) |
| NMI UC | 0 | 0 | 51 (77.3) | 11 (78.6) | 62 (39.7) |
| MI UC | 0 | 0 | 15 (22.7) | 3 (21.4) | 18 (11.5) |
| Grade 1/2 UC | 40 (62.5) | 3 (21.4) | 43 (27.5) | ||
| Grade 3 UC | 24 (37.5) | 11 (78.5)** | 35 (22.4 )** | ||
| Dipstick blood | |||||
| -ve | 23 (36.5) | 5 (38.5) | 12 (18.2) | 3 (21.4) | 43 (25.6) |
| + | 13 (20.6) | 5 (38.5) | 24 (36.4) | 2 (14.3) | 44 (28.2) |
| ++ | 9 (14.3) | 0 | 4 (6.0) | 1 ( 7.1) | 14 ( 9.0) |
| +++ | 17 (27.0) | 3 (23.0) | 22 (33.3) | 5 (35.7) | 47 (30.1) |
| ++++ | 1 ( 1.6) | 0 | 4 (6.0) | 3 (21.4) | 8 ( 5.0) |
| Smoking | |||||
| Smokers | 37 (58.7) | 4 (30.8) | 47 (71.2) | 13 (92.9) | 101 (64.7) |
| Smoking years (mean (SD)) | 26.0 (16.2) | 21.3 (8.5) | 35.4 (14.5) | 37.4 (9.6) | N/A |
| TM (ng/ml) (mean) | 4.5 | 6.2*** | 4.5 | 7.1*** | |
*UC patients with CE were significantly younger than UC patients with NCE.**UC patients with CE had higher grade tumors than UC patients with NCE. ***patients with CE had significantly higher levels of urinary TM than NCE patients.
Table 2: Patient characteristics (n = 156).
Younger age of UC patients with a history of chemical exposure
UC patients with a history of CE were significantly younger (median = 64 years (IQR 59 to 71)) than NCE UC patients (median = 71 years (IQR 65 to 76)) (ANOVA; p < 0.001) (t-test; p = 0.012). Further, there was no statistically significant difference in age when CE CTL and NCE CTL patients were compared; both groups had an average age of 54 years (Table 2).
The relationship between CE and smoking across controls and UC patients
The proportion of patients with CE who also smoked (13/14 (93%) was significantly higher in the UC subpopulation (hypergeometric p-value (greater) p2 = 0.080; odds ratio = 5.18) in comparison to the CTL subpopulation (4/13 (31%)) (hypergeometric p-value (less) p1 = 0.062; odds ratio = 0.32) (p = 0.025 (Zelen’s test)). It is noteworthy that the odds ratios were not significant when the joint effect of smoking and CE was assessed independently for CTL and UC using a Fisher’s exact test.
Biomarkers with differential expression across CE vs NCE patients
Four urinary biomarkers, i.e. C-reactive protein (CRP), interleukin- 4 (IL-4), Thrombomodulin (TM), and IL-2 together with plasma biomarkers, monocyte chemo attractant protein 1 (MCP-1) and matrix metalloproteinase neutrophil gelatinase associated lipocalin complex (MMP9NGAL) were pre-selected using Lasso. Urinary IL-2, and the two plasma biomarkers, MCP-1 and MMP9NGAL, were less stable (bootstrap ≤ 0.11) than the three urinary biomarkers TM, CRP and IL-4 (bootstrap ≥ 0.34). Urinary TM was the only biomarker that was differentially expressed across the NCE and CE subpopulations (mean NCE = 4.5 ng/ml; mean CE = 6.8 ng/ml) (Welch’s t-test; p = 0.002). Further, urinary TM was significantly higher in CE patients across both subpopulations, i.e. UC (mean NCE = 4.5 ng/ml; mean CE = 7.1 ng/ml) (one-sided t-test; p = 0.014) versus CTL (mean NCE = 4.5 ng/ml; mean CE = 6.2 ng/ml) (one-sided t-test; p = 0.043) groups demonstrating that the differential expression was independent of UC.
Thrombomodulin levels were higher in patients with a history of chemical exposure
Using a one-sided t-test and considering multiple testing using FDR, we observed that TM levels in urine were significantly higher in CE patients, in the presence of dipstick protein and when urinary pH levels ≤ 6 (p = 0.003), but not in the presence of dipstick blood (p = 0.115). TM levels corrected using creatinine (p = 0.029) were significantly higher in patients with a history of chemical exposure. TM and creatinine levels measured in urine were significantly correlated (Pearson correlation; r = 0.72, p < 0.001). For the remaining biomarkers r = < 0.5 (Pearson correlation).
Micropapillary variant of UC in patients with a history of chemical exposure
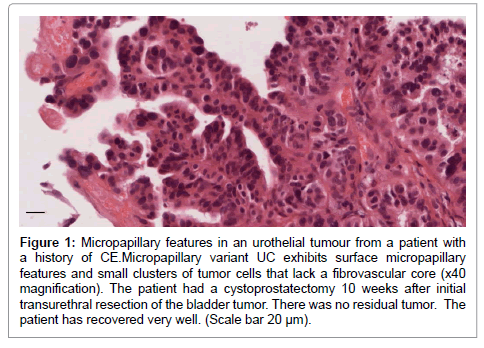
Interestingly, there were features of the micropapillary variant of UC [28] in the four biopsies available for review from 3 patients with a history of CE. The first biopsy from one of these patients was a pT2G1 tumor which displayed micropapillary features. Further, micropapillary and nested patterns were observed in the recurrence from the same patient which was reviewed as a pT2G3. Carcinoma in situ (CIS) and lymphovascular invasion were observed in 3/4 (75%) biopsies [29] with micropapillary features (Figure 1). Notably these three patients had smoked for more than 10 years. Eight of the 12 CE patients (66%) had recurrences, four of which were higher grade/stage disease (Table 3).
Figure 1: Micropapillary features in an urothelial tumour from a patient with a history of CE.Micropapillary variant UC exhibits surface micropapillary features and small clusters of tumor cells that lack a fibrovascular core (x40 magnification). The patient had a cystoprostatectomy 10 weeks after initial transurethral resection of the bladder tumor. There was no residual tumor. The patient has recovered very well. (Scale bar 20 μm).
| Patient | Age | Smoking years | Grade | Stage | Inflammation | Pattern | CIS | LVI |
|---|---|---|---|---|---|---|---|---|
| 1 | >60* | <10 | 2 # | TA | 3 | sessile | absent | no |
| 2 | TA | 0 | papillary | absent | no | |||
| 3 | TA | 0 | papillary | present | no | |||
| 2 | >60* | >10 | 2 | TA | 0 | papillary | absent | no |
| 2 | TA | 2 | papillary | absent | no | |||
| 3 | >60* | >10 | 1 # | TA | 0 | papillary | absent | no |
| 2 | TA | 0 | papillary | absent | no | |||
| 4 | >60 | >10 | 2 # | TA | 0 | papillary | absent | no |
| 3 | T1 | 2 | papillary | present | yes | |||
| 5 | >60 | >10 | 3 | T1 | 1 | micro-papillary | present | yes |
| 6 | <60 | >10 | 3 | T4A | 1 | nested | absent | yes |
| 7 | >60 | >10 | 3 | T2 | 1 | micro-papillary | present | yes |
| 3 | T2 | 3 | micro-papillary and nested | present | yes | |||
| 8 | <60 | >10 | 0 | 0 | papillary | absent | no | |
| 3 | TA | 0 | papillary | absent | no | |||
| 9 | <60 | >10 | 0 # | TA | 0 | papillary | absent | no |
| 1 | TA | 1 | papillary | absent | no | |||
| 3 | TA | 3 | papillary | absent | no | |||
| 10 | >60 | 0 | 3 | T2 | 1 | nested | absent | no |
| 3 | T2 | 1 | squamous diff | absent | no | |||
| 11 | <60 | >10 | 3 | TA | 1 | micro-papillary | absent | no |
| 12 | <60 | >10 | 2 | TA | 0 | papillary | absent | no |
Table 3: Pathological review of 22 biopsies from 12 UC patients with CE. Following approval from the Northern Ireland Biobank (NIB13-0065), DOR (Consultant Pathologist), undertook a diagnostic review of all tumor biopsies that were available within the Belfast City Hospital archive for each of the 12 patients with UC who were assigned as CE. Eight of the 12 (66%) patients with a history of CE had recurrences, four of which were higher grade/stage disease #. INF inflammation: 0 absent, 1 = +, 2 = ++, 3 = +++; LVI lymphovascular invasion*patients with incomplete biomarker data who were not included in the biomarker analyses
There was no evidence of micropapillary features in the biopsies from the eight patients with NCE and no recurrences were recorded for these patients. However, 2/8 (25%) of these patients had tumors which displayed a nested pattern (Table 4).
| Patient | Age | Smoking years | Grade | Stage | Inflammation | Pattern | CIS | LVI |
|---|---|---|---|---|---|---|---|---|
| 1 | <60 | 0 | 3 | T1 | 1 | nested | present | no |
| 2 | >60 | >10 | CIS | TIS | 1 | CIS | present | no |
| 3 | >60 | 0 | 3 | T2 | 1 | papillary | absent | no |
| 4 | >60 | 0 | 3 | T1 | 1 | papillary | present | no |
| 5 | >60 | >10 | 3 | TA | 2 | papillary | absent | no |
| 6 | >60 | >10 | 2 | TA | 0 | papillary | absent | no |
| 7 | >60 | >10 | 3 | T1 | 2 | papillary | present | no |
| 8 | >60 | >10 | 3 | T3A | 1 | nested | present | yes |
Table 4: Pathological review of 8 tumors from 8 UC patients with NCE. Following approval from the Northern Ireland Biobank (NIB13-0065), DOR (Consultant Pathologist), undertook a diagnostic review of all tumor biopsies that were available within the Belfast City Hospital archive for each of 8 patients with UC who were assigned as NCE.No recurrences were recorded for the eight NCE patients.INF inflammation: 0 absent, 1 = +, 2 = ++, 3 = +++; LVI lymphovascular invasion
Discussion
TM is a glycoprotein expressed on the surface of endothelial cells, on the membranes of the transitional epithelium and in the cytoplasmic region of umbrella cells [30]. Positive TM immunoreactivity has been shown to have an inverse correlation with cancer progression and metastasis [31,32]. Previously, we reported that the immunodetection of cytoplasmic membrane-bound TM in UC, squamous cell carcinoma and adenocarcinoma formalin fixed paraffin embedded tissue microarrays was independent of grade and stage. Furthermore, TM immunostaining was significantly stronger in the UC tissue sections with respect to both adenocarcinoma and SCC sections which stained weakly [33]. TM expression has also been shown to be increased in the transitional epithelium in patients with cystitis [32].
Although increased levels of plasma TM are considered to reflect endothelial damage [34], the physiological significance of soluble TM in UC is unknown [35]. TM is shed into the circulation following its proteolytic cleavage from endothelial surfaces and increased TM levels have been associated with endothelial dysfunction [36]. Endothelial shedding is common in patients with hypertension, hypercholesterolemia, diabetes, atherosclerotic disease, and in chronic smokers [35,37]. Further, TM has anti-inflammatory properties and is known to increase during inflammatory conditions [38]. It is therefore conceivable that by-products from CE could potentially induce endothelial shedding or irritation within the bladder which could result in an increased level of urinary TM.
While health and safety regulations may limit occupational exposure to harmful chemicals, related cases of bladder cancer remain high as the latency period for cancer development after exposure may be as long as 20 years [20]. Similarly to our findings of aggressive UC tumors in those exposed of chemicals, Noon et al. [20] reported that patients exposed to crack detection dye penetrants normally present with highgrade tumors with associated panurothelial carcinogenesis, in contrast to most UC which are unifocal and low grade.
In our study, urinary TM was the only biomarker that was differentially expressed across the NCE and the CE subpopulations. Urinary TM levels were significantly higher in CE patients, in the presence of dipstick protein, but not in the presence of dipstick blood. Furthermore, UC patients with a history of both smoking and CE tended to have more aggressive tumours, and at a significantly younger age, in comparison to UC patients with no history of CE.
There is a need for new approaches to identify patients who are at risk of serious disease. Urinary TM may be a novel biomarker that could be used to screen patients in potentially ‘at risk’ occupations. Where levels of urinary TM levels are found to be elevated, further investigations are warranted. It should be noted that our study is limited by the small numbers of patients in each of the groups and our conclusions should be viewed in this context. As such, a larger study is required to validate our findings.
Acknowledgements
MWR and CNR are employees of Randox Laboratories Ltd who undertook the biomarker analyses using multianalyte biochip array technology. RdMS, FES, BD, MWR, CNR and KEW have been or will be named on patents: 1. Methods for the detection of, or risk of, bladder cancer (JWJ01571US January 2013 and JWJ01571GB August 2009). 2. A method of defining the likelihood of a subject having bladder cancer JWJ01839GB October 2012. 3. A method for detecting exposure to a hazardous chemical JWJ01877GB (on going). Randox Laboratories Ltd paid the salary of Mr Funso Abogunrin who recruited the patients over two years. Invest Northern Ireland RD0412515 funded RdMS and GC.
References
- Dietrich H, Dietrich B (2001) Ludwig Rehn (1849-1930)--pioneering findings on the aetiology of bladder tumours. World J Urol 19: 151-153.
- Matsumoto K, Irie A, Satoh T, Kuruma H, Arakawa T, et al. (2005) Occupational bladder cancer: from cohort study to biologic molecular marker. Med SciMonit 11: RA311-315.
- Jacobs BL, Lee CT, Montie JE (2010) Bladder cancer in 2010: how far have we come? CA Cancer J Clin 60: 244-272.
- Richardson K, Band PR, Astrakianakis G, Le ND (2007) Male bladder cancer risk and occupational exposure according to a job-exposure matrix-a case-control study in British Columbia, Canada. Scand J Work Environ Health 33: 454-464.
- Bi W, Hayes RB, Feng P, Qi Y, You X, et al. (1992) Mortality and incidence of bladder cancer in benzidine-exposed workers in China. Am J Ind Med 21: 481-489.
- Sorahan T, Hamilton L, Jackson J (2000) A further cohort study of workers employed at a factory manufacturing chemicals for the rubber industry, with special reference to the chemicals 2-mercaptobenzothiazole (MBT), aniline, phenyl-beta-naphthylamine and o-toluidine. Occup Environ Med 57: 106-115.
- Gaertner RR, Thériault GP (2002) Risk of bladder cancer in foundry workers: a meta-analysis. Occup Environ Med 59: 655-663.
- Colt JS, Karagas MR, Schwenn M, Baris D, Johnson A, et al. (2011) Occupation and bladder cancer in a population-based case-control study in Northern New England. Occup Environ Med 68: 239-249.
- Zahm SH, Hartge P, Hoover R (1987) The National Bladder Cancer Study: employment in the chemical industry.J Natl Cancer Inst 79: 217-222.
- Cassidy A, Wang W, Wu X, Lin J (2009) Risk of urinary bladder cancer: a case-control analysis of industry and occupation. BMC Cancer 9: 443.
- Pira E, Piolatto G, Negri E, Romano C, Boffetta P, et al. (2010) Bladder cancer mortality of workers exposed to aromatic amines: a 58-year follow-up. J Natl Cancer Inst 102: 1096-1099.
- Guha N, Steenland NK, Merletti F, Altieri A, Cogliano V, et al. (2010) Bladder cancer risk in painters: a meta-analysis. Occup Environ Med 67: 568-573.
- Silverman DT, Hoover RN, Mason TJ, Swanson GM (1986) Motor exhaust-related occupations and bladder cancer.Cancer Res 46: 2113-2116.
- Burstyn I, Kromhout H, Johansen C, Langard S, Kauppinen T, et al. (2007) Bladder cancer incidence and exposure to polycyclic aromatic hydrocarbons among asphalt pavers. Occup Environ Med 64: 520-526.
- Kobrosly RW, Meliker JR, Nriagu JO (2009) Automobile industry occupations and bladder cancer: a population-based case-control study in southeastern Michigan, USA. Occup Environ Med 66: 650-656.
- Baris D, Karagas MR, Verrill C, Johnson A, Andrew AS, et al. (2009) A case-control study of smoking and bladder cancer risk: emergent patterns over time. J Natl Cancer Inst 101: 1553-1561.
- Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC (2011) Association between smoking and risk of bladder cancer among men and women. JAMA 306: 737-745.
- Crivelli JJ, Xylinas E, Kluth LA, Rieken M, Rink M, et al. (2014) Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. EurUrol 65: 742-754.
- Abogunrin F, O'Kane HF, Ruddock MW, Stevenson M, Reid CN, et al. (2012) The impact of biomarkers in multivariate algorithms for bladder cancer diagnosis in patients with hematuria. Cancer 118: 2641-2650.
- Noon AP, Pickvance SM, Catto JW (2012) Occupational exposure to crack detection dye penetrants and the potential for bladder cancer Occup Environ Med 69: 300-301.
- Harling M, Schablon A, Schedlbauer G, Dulon M, Nienhaus A (2010) Bladder cancer among hairdressers: a meta-analysis. Occup Environ Med 67: 351-358.
- Smith EM, Miller ER, Woolson RF, Brown CK (1985) Bladder cancer risk among auto and truck mechanics and chemically related occupations. Am J Public Health 75: 881-883.
- Kogevinas M, 't Mannetje A, Cordier S, Ranft U, González CA, et al. (2003) Occupation and bladder cancer among men in Western Europe. Cancer Causes Control 14: 907-914.
- Zelen M (1970) The Analysis of Several 2 x 2 Contingency Tables. Biometrika 58: 129-137
- Hollander M, Wolfe D (1999) Nonparametric statistical methods, Solutions Manual. (2ndedn), John Wiley & Sons, NY.
- Tibshirani R (1996) Regression shrinkage and selection via the lasso. J Royal Stat Soc 58: 267-288.
- Friedman J, Hastie T, Tibshirani R (2010) Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw 33: 1-22.
- Kwon GY, Ro JY (2011) Micropapillary variant of urothelial carcinoma. AdvUrol 2011: 217153.
- Lopez-Beltran A, Montironi R, Blanca A, Cheng L (2010) Invasive micropapillaryurothelial carcinoma of the bladder. Hum Pathol 41: 1159-1164.
- Obama H, Obama K, Takemoto M, Soejima Y, Shirahama T, et al. (1999) Expression of Thrombomodulin in the epithelium of the urinary bladder: a possible source of urinary thrombomodulin. Anticancer Res 19: 1143-1147.
- Manning T, Smoller BR, Horn TD, El Darouti M, Marzouk S, et al. (2004) Evaluation of anti-thrombomodulin antibody as a tumor marker for vascular neoplasms. J CutanPathol 31: 652-656.
- Wu CT, Chang YH, Lin P, Chen WC, Chen MF1 (2014) Thrombomodulin expression regulates tumorigenesis in bladder cancer. BMC Cancer 14: 375.
- Ruddock M, Reid C, McReynolds D, Lamont J, Fitzgerald S et al (2014) Immunodetection of cytoplasmatic membrane-bound thrombomodulin in formalin-fixed paraffin-embedded human tissue microarrays. J MolGenet 8: 1-4.
- Seigneur M, Dufourcq P, Conri C, Constans J, Mercie P, et al. (1993) Plasma thrombomodulin: new approach of endothelium damage. IntAngiol 12: 355-359.
- Suzuki K, Kusumoto H, Deyashiki Y, Nishioka J, Maruyama I, et al. (1987) Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J 6: 1891-1897.
- Münzel T, Sinning C, Post F, Warnholtz A, Schulz E (2008) Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med 40: 180-196.
- Jansson JH, Boman K, Brännström M, Nilsson TK (1996) Increased levels of plasma thrombomodulin are associated with vascular and all-cause mortality in patients on long-term anticoagulant treatment. Eur Heart J 17: 1503-1505.
- Arazi H, Giorgi M, Miriuka S, Caroli C, Carnevalini M et al (2011) Soluble Thrombomodulin Levels are Related to Inflammation after Coronary Bypass Surgery. J Clinic Experiment Cardiol 2: 1-3.
Copyright: © 2015 Ruddock MW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.