Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 11, Issue 3
Tissue Biomineralization as a Mechanism Leading to Cancer Development
Maciej Pawlikowski*Received: 24-Mar-2020 Published: 20-Apr-2020, DOI: 10.35248/2157-2518.20.11.347
Abstract
This article summarizes the results of many years of studies on biomineralization in chosen cancers. They were conducted on cancers of skin, lungs, mouth, kidney, prostate, thyroid, connective tissue and others, using modern methods of mineralogy and histology.
Obtained results indicate that two types of biomineralization are present in cancer areas: hidden and visible biomineralization. Hidden biomineralization does not manifest as grains or mineral crystals in the tissue. It is present in the form of elevated levels of elements and compounds in the body fluids as well as elements incorporated into the atomic structures of the tissue.
Visible biomineralization is the next stage. It builds up as a result of continued development of hidden biomineralization, leading to the formation of mineral grains, crystals, etc.
Tissue biomineralization with various substances, including so called carcinogens, can lead to DNA modification in the section responsible for cell multiplication. That in turn can cause a DNA defect, accelerating the cell proliferation rate. The result is uncontrolled cell multiplication, causing development of cancerous tissue in the organ.
Depending on which cells are mutated, which DNA section gets defected, and what substance (carcinogen) is built into the DNA during cell proliferation, a wide spectrum of cancers can develop even within one organ. Described phenomenon results in huge variety of types of cancer.
Keywords
Biomineralization; Energy spectrum; Carcinoma basocellulare solidum; Cell proliferation
Introduction
Tissue biomineralization with various substances, including so called carcinogens, can lead to DNA modification in the section responsible for cell multiplication. That in turn can cause a DNA defect, accelerating the cell proliferation rate. The result is uncontrolled cell multiplication, causing development of cancerous tissue in the organ [1-5].
Depending on which cells are mutated, which DNA section gets defected, and what substance (carcinogen) is built into the DNA during cell proliferation, a wide spectrum of cancers can develop even within one organ. Described phenomenon results in huge variety of types of cancer [6-8].
Materials and Methods
The results presented below have been selected from 30 years of studies on cancer biomineralization. Biomineralogical research was preceded by histological diagnosis. The biomineralogical studies were conducted using appropriate mineralogical research techniques allowing us to determine chemical composition and – where possible – phase composition (presence of mineral substances) of cancerous tissue as shown in Figure 1 and 2 [9-11].
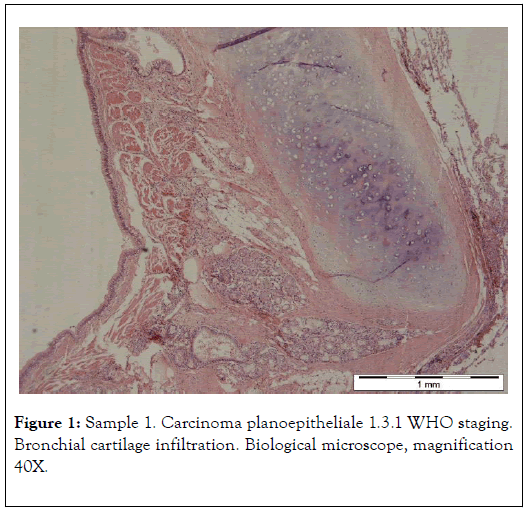
Figure 1. Sample 1. Carcinoma planoepitheliale 1.3.1 WHO staging. Bronchial cartilage infiltration. Biological microscope, magnification 40X.
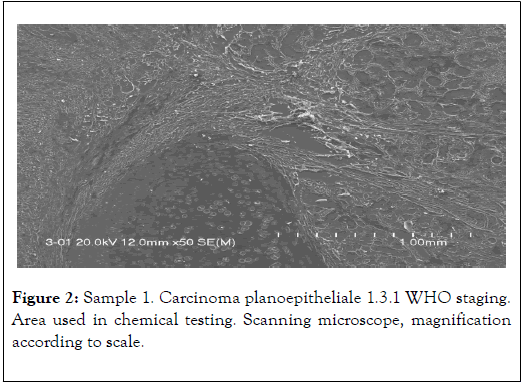
Figure 2. Sample 1. Carcinoma planoepitheliale 1.3.1 WHO staging. Area used in chemical testing. Scanning microscope, magnification according to scale.
Mainly electron (scanning) microscopy was used for that purpose, allowing us to obtain photomicrographs of cancer tissues and conduct chemical analyses on them in 2-3 μm2 area (EDS method). Jeol 560 and FEI QUANTA 200 FEG microscopes were used. Both quality analyses (energy spectra) and quantity analyses with appropriate standards were conducted. Materials used for studies were tissues removed during surgeries explained by Table 1 and Figure 3 [11-13].
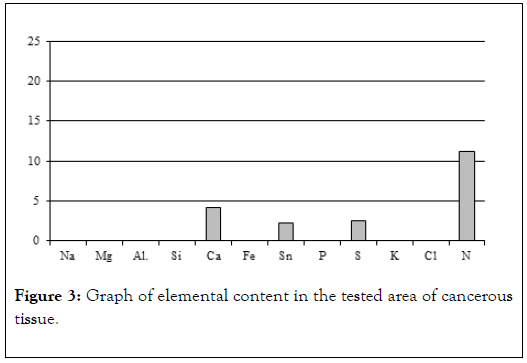
Figure 3. Graph of elemental content in the tested area of cancerous tissue.
| Element | Content (weight %) |
|---|---|
| Na | 0 |
| Mg | 0 |
| Al | 0 |
| Si | 0 |
| Ca | 4.17 |
| Fe | 0 |
| Sn | 2.25 |
| P | 0 |
| S | 2.56 |
| K | 0 |
| Cl | 0 |
| N | 11,2 |
Table 1: Results of chemical analysis of sample 1.
Examples Of Cancer Biomineralization
Lung cancers
Prostate cancers
EDS energy spectrum of the prostate area affected by morphological changes. Visible energy peaks of Mg, Ca, Na and a weak peak of P indicate incorporation of those elements into the biological structures of prostate tissues, i.e. hidden biomineralization of the prostate as shown in Figures 4-6 and Table 2 [12].
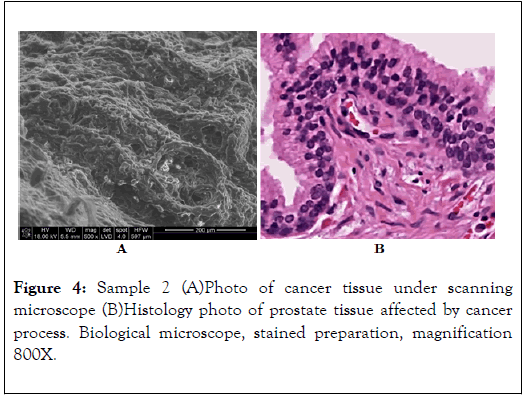
Figure 4. Sample 2 (A)Photo of cancer tissue under scanning microscope (B)Histology photo of prostate tissue affected by cancer process. Biological microscope, stained preparation, magnification 800X.
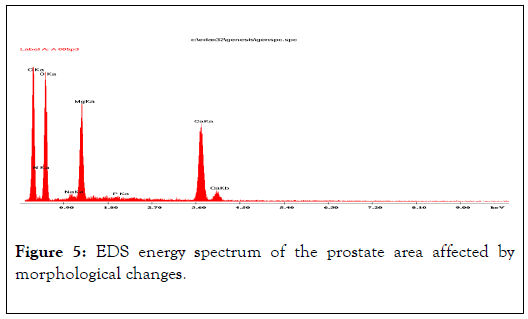
Figure 5. EDS energy spectrum of the prostate area affected by morphological changes.
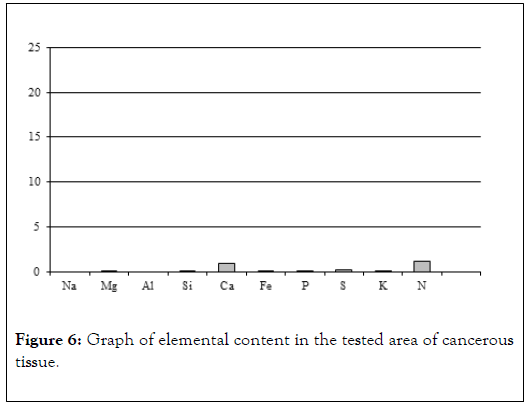
Figure 6. Graph of elemental content in the tested area of cancerous tissue.
| Element | Content (weight%) |
|---|---|
| Na | 0 |
| Mg | 0.13 |
| Al | 0 |
| Si | 0.1 |
| Ca | 0.87 |
| Fe | 0.15 |
| P | 0.1 |
| S | 0.19 |
| K | 0.08 |
| N | 1.13 |
Table 2: Results of chemical analysis of sample 2.
Skin cancers
Visible are well delimited cancer cell nests with a palisaded perimeter. Preparation stained with hematoxylin and eosin. Polarizing microscope 1 N, magnification 60x. Selected area is shown in Figure 7 [13].
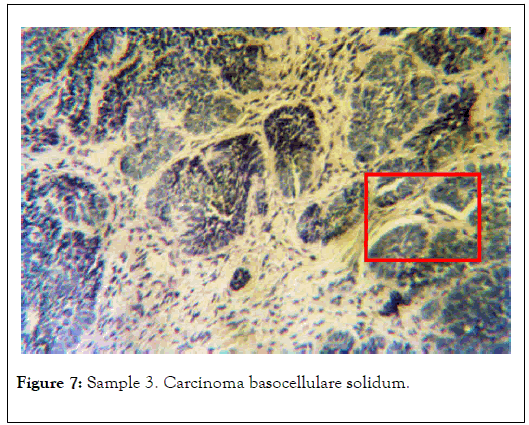
Figure 7. Sample 3. Carcinoma basocellulare solidum.
In 1, analysis of the tissue affected by mineralization was conducted. In 2, comparative analysis of non-mineralized tissue, where no elevated levels of elements were found, was conducted and shown in Figure 8 and Table 3.
| Element | Content (weight%) |
|---|---|
| Na | 0.73 |
| Mg | 0.49 |
| Al. | 0.12 |
| Si | 2.17 |
| Ca | 1.61 |
| Fe | 0 |
| Sn | 0 |
| P | 0 |
| S | 1.23 |
| K | 0.5 |
| Cl | 1.45 |
| Na | 0 |
Table 3: Results of chemical analysis of sample in 1 of Figure 8.
| Element | Content (weight%) |
|---|---|
| Na | 0 |
| Mg | 0 |
| Al | 0 |
| Si | 0.21 |
| Ca | 6.93 |
| Fe | 0.12 |
| P | 3.92 |
| S | 0 |
| K | 0.1 |
| N | 0 |
Table 4: Results of chemical analysis of sample S1-1
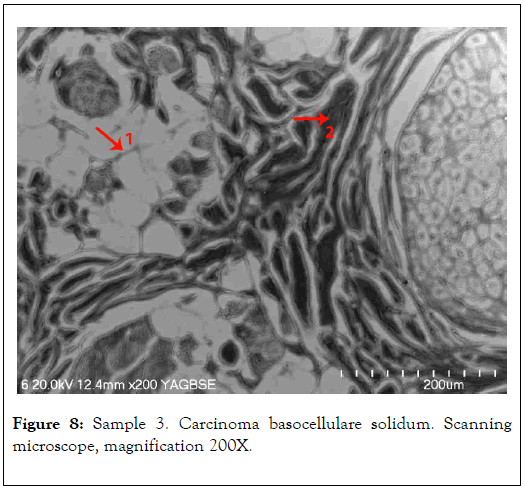
Figure 8. Sample 3. Carcinoma basocellulare solidum. Scanning microscope, magnification 200X.
Oral cancers
Biological microscope, preparation stained H-E, magnification 420X as shown in Figures 9 and 10. Analyzed spot marked with an arrow SEM as shown in Figure 11 [8,9].
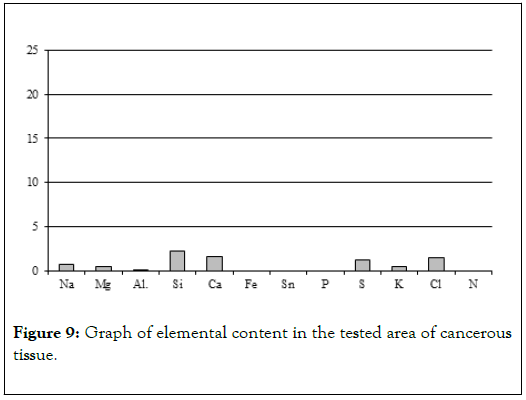
Figure 9. Graph of elemental content in the tested area of cancerous tissue.
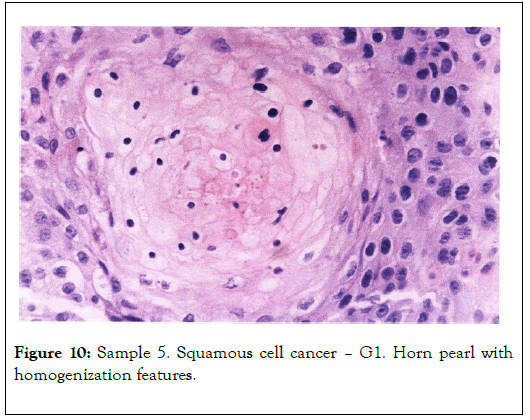
Figure 10. Sample 5. Squamous cell cancer – G1. Horn pearl with homogenization features.
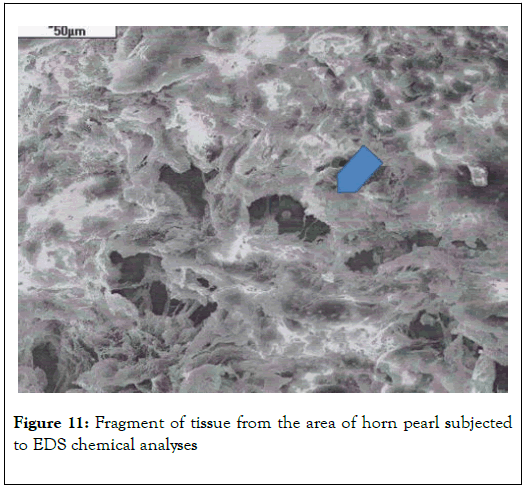
Figure 11. Fragment of tissue from the area of horn pearl subjected to EDS chemical analyses
Chemical analyses conducted in several spots of the tissue showed (Table 4) elevated levels of not just calcium and phosphorus, but also iron, potassium and silica (Figure 9). That indicates that main elements mineralizing tissue in the area of horn pears are calcium phosphates. The other elements probably do not form separate mineral phases but are present in the biological structures incorporated into atomic structures of biological components illustrated by Figure 12 [11-13].
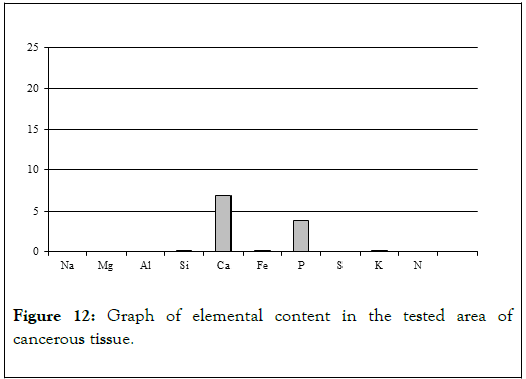
Figure 12. Graph of elemental content in the tested area of cancerous tissue.
Discussion
Studies of cancer tissue mineralization conducted over the last 30 years are basis for an attempt to explain the role of mineralization in cancer development. The diagram of this phenomenon is presented in Figure 13 [12,13].
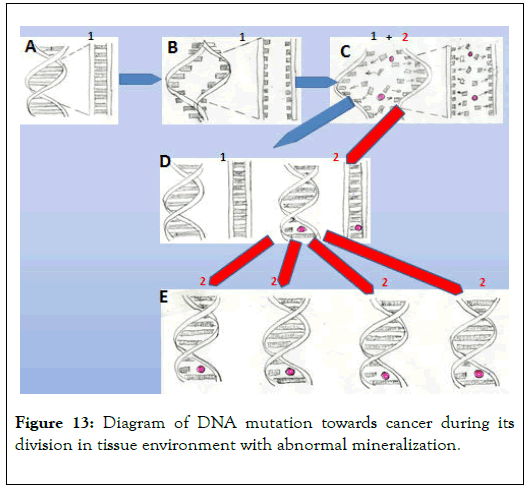
Figure 13. Diagram of DNA mutation towards cancer during its division in tissue environment with abnormal mineralization.
A – Diagram of DNA section responsible for proliferation of a cell
B – Initial division stage of the DNA section
C – Main division stage of the DNA section in an environment with abnormal mineralization. Red dot – carcinogen (element or compound). It can be incorporated into different places in the described DNA section. Variety of cells in tissues, variety of carcinogens, and the possibility of them being incorporated into different parts of the DNA section are all reasons for the variety of types of cancer. It is also possible for more than one carcinogen to be incorporated into newly formed DNA at the moment of division.
D – Stage where two cells are created. One of them (1) contains normal DNA, while in the other (2) the DNA fragment responsible for cell proliferation is mutated.
E – Further proliferation of cancer cells and development of cancer tissue.
Conclusion
All of the studied preparations contained tissues with various stages of cancer process and were affected by weaker or stronger mineralization, or otherwise represented comparative material – healthy tissues. Tissues representing the earliest stage of the cancer process were not studied because cancer changes are often elusive at that point.
Obtained results suggest that biomineralization of the tissue environment, including body fluids, as well as substitution of elements in biological structures, participates in creation of DNA defects, leading to formation of modified cancer cells.
It was observed that in cancer tissues, in comparison with healthy tissue, uneven distribution and elevated content of certain elements appear. Concentrations of mineral micrograins are present. They are variably mineralized with different elements, especially calcium, but to a smallest degree also phosphorus, sulfur and others.
Obtained results suggest that the presence of relatively rich mineralization of cancer areas can also be at least partially connected with functioning of developed cancer phenomena. Such environment additionally favors further cellular modifications and development of other types of cancer in a place already affected by cancer.
Studies show that recognized level of cancer tissue mineralization is more strongly connected with the stage of the cancer process than with the patient’s age.
In the first stage, mineralization of the cancer-affected area is hidden and does not manifest as grains, but is only visible in chemical analyses as elevated levels of elements in tissue. That means that elements such as calcium, phosphorus, sulfur and others get incorporated into biological structures of the tissue.
Hidden mineralization can (but does not have to) further develop to transform into visible mineralization, e.g. concentrations of mineral micrograins. Such grains, up to 10.0 μm in size, are usually located near the cells. Observations of interdependence of calcium and phosphorus show that such grains are built either of calcium phosphates or calcium carbonates.
The studies were conducted in a very specific and unconventional way, using electron beam. Analyses were performed on dewaxed histological cuttings, approximately 20-25 μm thick. Obtained results prove that research technique is fully useful to study the level of mineralization in cancer tissue.
It was determined that structures of cancer tissue are less resistant to testing in a vacuum (scanning), which means that they are structurally weaker.
Summarizing the studies, a hypothesis can be formed that both hidden tissue mineralization and visible mineralization, including so called calcifications, may favor structural DNA defects developing during the chromosome division in the process of cell proliferation. Cell division environment with abnormal content of elements (both too low and too high) and presence of carcinogens (elements and compounds) may favor modifications in the DNA section responsible for the rate of mitotic divisions. The exact spot of DNA mutation is probably incidental. It might, however, be genetically conditioned.
Studies indicate that along with the development of cancer process, mineralization of the tissue can, but does not have to increase. Due to intense metabolism of the mutated cells, there may be excess of e.g. carbon dioxide in the tissues that the circulatory system may struggle to transport. It leads to local acidification of cancer tissue.
Modification of the tissue environment to lightly acidic favors development of angiogenesis, i.e. proliferation of blood vessels within cancer areas.
Similarly, angiogenesis develops in the areas that are hypoxic with elevated lever of CO2 near arterial blockage (heart attacks – collateral circulation), or in the areas of bone breaks where mechanical destruction of blood vessels often occurs. Presented results and discussion are the foundation for research on cancer prevention, especially methods of eliminating cells with mutated DNA, i.e. methods of cancer treatment.
REFERENCES
- Boyle P, Doré JF, Autier P, Ringborg U. Cancer of the skin: a forgotten problem in Europe. Annals of Oncology. 2005;16(5):5-6.
- Chichel A, Skowronek J. Contemporary skin cancer treatment - dermatology, surgery or radiation therapy? Contemporary Oncology. 2005;9(10):429-435.
- Daniel L, Leśniewski Kmak K. Treatment of long-term basal cell carcinoma of the skin that destroys half of the face: Case report. Contemporary Oncology. 2005;(9)10:440-442.
- Kokot F. Water-electrolyte and acid-base economy in physiology and pathology. Warszawa. 1993;2(6):150-170.
- Miśkowiak B. Basics of histology and histological technique. Poznań University Publishing House, Warszawa, Poland. 1996. pp:25-37.
- Pawlikowski M. Crystals in the human body. Krakow Publishing and Printing House, Krakow, Poland. 1993. pp:46-89.
- Pawlikowski M. Mineralization of the living human body (Human Mineralogy). Centrum Publishing House, Krakow, Poland. 1987. pp: 103-125.
- Pawlikowski M. Sekrety mineralizacji tkanek. (Secrets of human body mineralization) Centrum Publishing House, Krakow, Poland.1995. pp:96-120.
- Pawlikowski M. Biomineralogical Studies of Oral Squamous Cell Carcinoma. Arch Community Fam Med. 2019;2(1):18-32.
- Pawlikowski M, Miler M. Biomineralogy of Selected Skin Cancers. SM Dermatology Journal. 2017;3(3):1017.
- Pawlikowsk M. Biomineralogy of Cancer. Lupus Open Access. 2018;3(1):1-2.
- Pawlikowski M. Biomineralogical Studies of Oral Squamous Cell Carcinoma. Arch Community Fam Med. 2019;2(1):19-33.
- Szymański A. Biomineralizacja i biomateriały. Poznań University Publishing House, Warszawa, Poland. 1991.pp:120-160.
Citation: Pawlikowski M (2020) Tissue Biomineralization as a Mechanism Leading to Cancer Development. J Carcinog Mutagen. 11:347. DOI: 10.35248/ 2157-2518.20.11.347.
Copyright: © 2020 Pawlikowski M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


