Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 15, Issue 5
Suspended Clay Inhibits the Growth of Harmful Algal Bloom-forming Freshwater Cyanobacteria through Physical Interactions
Katherine Tomaska1,2, Guanju Wei1, Sam Nguyen1,2 and Judy Q. Yang1*2Department of Bioproducts and Biosystems Engineering, University of Minnesota, Minneapolis Minnesota, USA
Received: 21-Aug-2023, Manuscript No. JMBT-23-22880; Editor assigned: 24-Aug-2023, Pre QC No. JMBT-23-22880 (PQ); Reviewed: 07-Sep-2023, QC No. JMBT-23-22880; Revised: 14-Sep-2023, Manuscript No. JMBT-23-22880 (R); Published: 21-Sep-2023, DOI: 10.35248/1948-5948.23.15.578
Abstract
Many theories exist to predict the growth of Microcystis, one major type of toxic freshwater cyanobacteria that form harmful algal blooms. However, the impacts of suspended particles, which are ubiquitous in freshwater, on Microcystis growth have not been fully understood. Here, we show that smectite clay can inhibit the growth of Microcystis aeruginosa through physical clay-cell interactions. We grow M. aeruginosa under identical growth conditions in three nutrient solutions: one pure solution, one with synthetic and transparent clay, and another one chemically modified by clay but with clay particles removed. Cells in pure solution and chemically-modified solution grow equally well, while cells in solutions with the physical presence of clay do not grow nor produce pigments. Microscopic imaging of clay-cell interactions suggests that the inhibition of the growth of M. aeruginosa by clay is due to the physical encapsulation of cells in clay. This previous unappreciated mechanism, the inhibition of cell growth due to encapsulation by clay, is likely widely present in natural aquatic environment where suspended clay and microbial cells are common.
Keywords
Harmful algal blooms; Microcystis; Clay; Suspended sediment; Encapsulation; Cell density
Introduction
Harmful Algal Blooms (HABs) in aquatic ecosystems can harm human health through a variety of exposure pathways, including consumption of contaminated water and fish direct contact with water and exposure to aerosolized bacteria [1-4]. Additionally, HABs cause hypoxic conditions and reduce water clarity, which threaten fish and invertebrate habitats [5,6]. Microcystis, a genus of toxin-producing cyanobacteria, forms HABs in freshwater lakes [7]. Microcystis blooms frequently occur in the Great Lakes over the summer, which threaten local fishing economies and have led to short-term “do not drink” advisories for local residents [8,9]. Microcystis blooms-produced toxins have been associated with a variety of negative health effects, including vomiting, throat irritation, gastrointestinal illness, and skin irritation [10]. These negative impacts of Microcystis blooms are becoming increasingly threatening under climate change, as more frequent and/or long-lasting blooms have been recorded in inland lakes due to increasing temperatures [6,7,11,12]. Knowledge about factors that control the growth of Microcystis cells is needed to predict the occurrence and frequency of Microcystis blooms and develop HABs mitigation strategies. There are many theories to predict the growth of Microcystis that take into account factors such as salinity nutrient availability and temperature [13-15]. However, the impacts of suspended particles, which are ubiquitous in nature, on Microcystis growth have not been fully understood. Field investigations show that two rivers with similar nutrient conditions can have dramatically different levels of HABs due to difference in suspended sediment concentrations [16]. For example, the Illinois River which has abundant nutrients and appears muddy had no algal blooms, while its tributary Fox River, which had a similar nutrient level and algal seeding but lacks suspended sediment, had frequent HABs. This observation cannot be explained by current theories which correlate Microcystis growth with temperature, light intensity, and available nutrients [7,9], as well as grazing zooplanktons and turbulence [17,18]. While some papers attribute the impacts of suspended sediment on algal growth to a reduction in light intensity, we anticipate that the impacts of suspended fine sediment, especially clay, on algal growth is not merely through light attenuation, because there are a variety of other potential clay-cell interactions that may impair cell growth [17-20]. First, clay can bond to cell surfaces through electrochemical forces, which cause them to co-aggregate [21]. Secondly, clay has also been used to actively remove harmful algal blooms by binding phosphorus and causing algae cells to aggregate to sediment. Additionally, certain modified clays used in filters have combined the absorptive properties of clay with compounds that limit the photosynthetic activity of cyanobacteria, leading to cell death. Based on these previous studies, we hypothesize that clay can impact the growth of Microcystis cells through electrochemical or physical interactions. Here we investigate the impacts of clay- Microcystis interactions on Microcystis growth. We grew a typical cyanobacterial that causes HABS, Microcystis aeruginosa, in solutions with different concentrations of clay. To eliminate the impacts of clay on light intensity, we used transparent synthetic clay, laponite, which has similar chemical structures as the natural bentonite clay and can be classified as a smectite clay. We grew M. aeruginosa in nutrient solutions with different Concentrations of clay in a controlled light and chemical environment and measured the cell density as a function of time. We further investigated the physical interactions between clay and algal cells by imaging them under a confocal laser scanning microscope. Based on the M. aeruginosa growth curves and the confocal imaging, we show that clay can physically interact with Microcystis cells, prevent their growth, and hinder Microcystis blooms.
Materials and Methods
Bacteria growth
We grew Microcystis Aeruginosa (UTEX LB2385) in 100 mL of BG- 11 solution (20 mL cell solution with 80 mL BG-11 solution). The BG-11 solution consists of 16 mL of 50X- concentration BG-11 solution diluted with 784 mL DI water (Milli-Q), as well as 0.4 g of HEPES buffering agent. We placed the flask with the cyanobacteria+nutrient solution on a shaker set at 110 rpm in an incubator set at 24°C. The incubator was set on a 12 hr to 12 hr light: dark cycle at approximately 2000 lumens. One 4100 K Cool white color temperature fluorescent tube light (Philips F20T12) was placed at the top of the incubator. The tube light was covered in a semi-transparent white cloth to dissipate the light, providing a consistent light intensity to the entire shaker surface. During experimentation, the light intensity reaching the flasks ranged from 1800 lumens to 2200 lumens. The pH remained between 6.5 and 8. We kept the M. aeruginosa in the growth phase by renewing its nutrient solution on a monthly basis.
Clay-cyanobacteria experiment set-up
To investigate the impacts of clay on Microcystis growth, we grow M. aeruginosa in BG-11+HEPES solution with varying amounts of laponite RD (BYK USA Inc.), a transparent and synthetic smectite clay, and monitor the growth of the cells over time by counting the cell density using a hemocytometer. First, 10 ml M. aeruginosa solutions, obtained from the same cell culture in the growth phase (the 16th day of growth), were added to flasks with 90 ml three different growth solutions: one is 90 ml BG-11+ HEPES solution without clay, the second is 90 ml BG-11+ HEPES solution with 1% (weight ratio) clay, and the third is BG-11+ HEPES solution modified by 1% clay, as shown in Table 1. The 1% clay solution was made by adding 10 grams of limonite clay to 1000 mL of BG- 11+HEPES solution, mixing the solution and letting it sit for two days, and mixing again and letting it sit for another two days. This procedure allows the chemical composition between clay and the solution to reach equilibrium. The solution modified by 1% clay was made by first making the 1% clay solution and then removing the clay particles through centrifugation. Specifically, the 1% clay solution made from the procedure described above was shaken and transferred into many 50 mL centrifuge tubes and centrifuged at 3000 rpm for 30 minutes. After the clay particles settled into the bottom of the centrifuge tubes, the upper solution was then transferred to a culture flask and referred to as a clay modified solution. Visual examination of the clay modified solution using a microscope shows that there were no clay particles in the solution. Three replicates were made for each growth solution (Table 1). All solutions were autoclaved before usage, and the solution with clay were exposed to UV light for 30 minutes for additional sterilization. To further eliminate the impacts of light intensity due to the transparent clay on bacterial growth, we placed a flask with clay and 100 ml BG-11 solution but lacking cells on top of the flask with cells growing in the BG-11 solution. For the flasks with cells growing in BG-11 solution with 1% clay and in the solutions modified by clay, we placed flasks with pure BG-11 solution and lacking cells on top. The stacking of two flasks (Figure 1), eliminates the impacts of light attenuation due to clay on cell growth, because if light attenuation exists for the transparent clay, then the cells in pure BG-11 solution with clay solution on top will also receive less light. Similar to the initial preparation of the bacteria Solution, the experimental flasks were placed in an incubator at 24°C, shaken at a rate of 110. Rpm and exposed to approximately 2000 lumens of light in a 12:12 light: dark cycle (Table 1 and Figure 1).

Figure 1: Experimental set-up. M. aeruginosa cells were grown in three growth solutions’ described in Table 1 in the lower flasks. Flasks filled with solutions with and without clay were placed on top of the lower flasks to create identical light conditions for all cases. The flasks were placed on a shaker set to 110 rpm within an incubator with white fluorescent light illuminating from the top of the flasks at 12 h:12h light/dark cycle.
| Nutrient solution +1% clay | Nutrient solution | Nutrient solution | |
|---|---|---|---|
| Upper flask | Modified clay nutrient solutiona, seeded with M. | ||
| Nutrient solution+1% clay, seeded with | aeruginosa | ||
| Lower flask | Nutrient solution seeded with M. aeruginosa | M. aeruginosa | |
| Quantity | 3 | 3 | 3 |
Note: A modified clay nutrient solution was made by removing clay particles from the 1% clay solution through centrifuge and filtration.
Table 1: Setup of clay-cell culture experiments
Density measurements and confocal microscopic imaging
We measured cell density three times a week. An absorbance versus wavelength graph was created from samples in each flask on the spectrophotometer. We performed a cell count under a Nikon Eclipse E400 microscope with a hem cytometer at 40X magnification. To compensate for the reduction in solution volume due to measurements and the evaporation of the bacterial solution, we added additional BG-11+HEPES solution every 4 weeks to keep the volume of solution in each flask at 100 mL. On weeks where the cyanobacteria solution was diluted with BG- 11+HEPES solution, we took the cell density measurements before dilution occurred. We calculated the dilution factor for each flask by dividing the volume of the solution by 100 mL. In subsequent weeks, this factor was multiplied by the cell density to compensate for the dilution effect due to the added solution. In addition to cell density measurements, the physical. Interactions between cyanobacteria and clay particles were visualized using a confocal laser scanning microscope (Nikon C2 plus). Each image is around 2048 by 2048 pixels at a resolution of 0.08 um/pixel. We used a 20X objective magnification. A sequence of images was taken at 10-second intervals for 5 minutes. The laser used for excitation has a wavelength of 488 nm (FITC) and the emission wavelength is 525 nm.
Results
During the Microcystis clay culture experiments, we observed significant growth of M. aeruginosa in both the pure BG-11+HEPES solution (Figure 2a), and the modified clay solution (Flasks S and MS in Figures 2b and 2c). In contrast, no significant growth was observed in the 1% clay solution (Flask C in Figures 2b and 2c). The measured M. aeruginosa cell density in the three different solutions over 42 days is shown in Figure 2a. The initial cell density on Day 2 is between 300 cells/mm3 and 700 cells/mm3 for all three solutions. M. aeruginosa in the pure solution and modified clay solution grow to tens of thousands of cells/mm3. Specifically, the cell density of M. aeruginosa in the pure solution peaked on Day 35 with a density of 18,856 cells/mm3. The cell density within the modified clay solution also peaked on Day 35 with a density of 22,484 cells/mm3. In contrast, on Day 35, the cell density in 1% clay solution was only 90.2 cells/mm3. The peak density measurement for the 1% clay solution occurred on Day 21, when the measured cell density was 1,980 cells/mm3, one order of magnitude smaller than in pure solution and in clay-modified solution. The suppression of M. aeruginosa growth in the solution with clay, compared with growth in solutions without the physical presence of clay (pure solution and clay modified solution), suggests that clay slows down bacterial growth through physical interactions rather than light attenuation and chemical interactions. First, the fast growth of cells in the pure solution with a flask containing 1% clay solution on top, Figure 2a, suggests that the suppression of cell growth is not due to light attenuation because if the transparent laponite clay attenuates light, the light in the pure solution would also be attenuated by the solution with clay on top. Second, the fast growth of cells in the clay modified solution suggests that the suppression of cell growth is not due to the chemical interactions, or the sorption of nutrients to clay, because if the sorption of nutrients by clay was what slows down cell growth, then the cell growth in the clay modified solution would also be slow. Based on these two reasoning’s, we hypothesize that the impacts of clay on the growth of M. aeruginosa is likely through physical interactions, i.e., forming clay- cell aggregates which have often been observed in both the field and experiments (Figure 2).
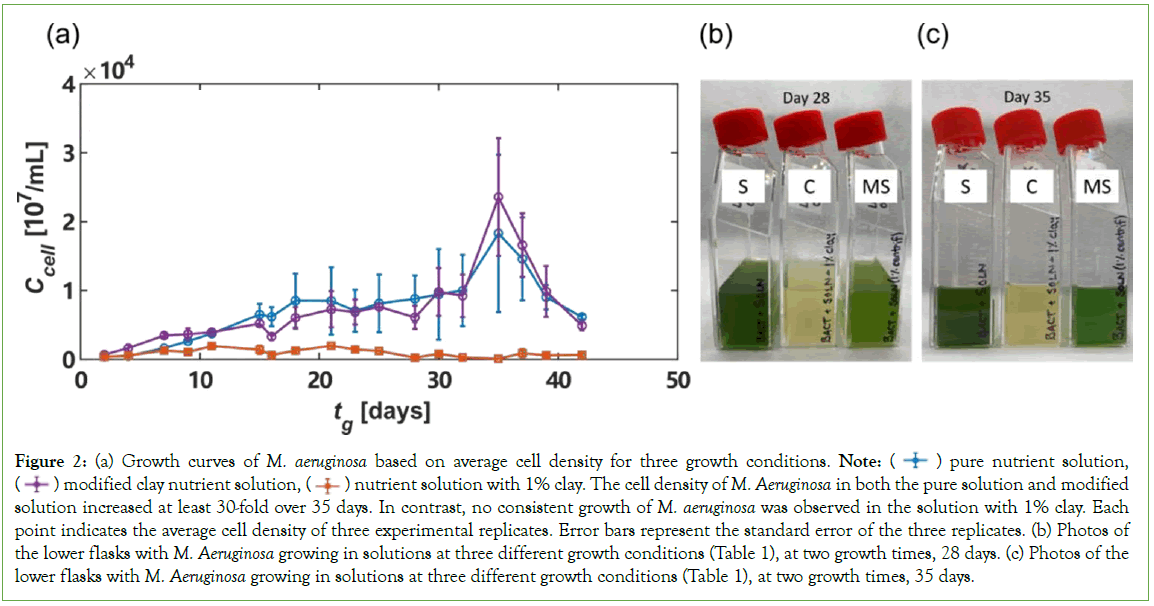
Figure 2: (a) Growth curves of M. aeruginosa based on average cell density for three growth conditions. Note:  pure nutrient solution,
pure nutrient solution,  nutrient solution with 1% clay. The cell density of M. Aeruginosa in both the pure solution and modified solution increased at least 30-fold over 35 days. In contrast, no consistent growth of M. aeruginosa was observed in the solution with 1% clay. Each point indicates the average cell density of three experimental replicates. Error bars represent the standard error of the three replicates. (b) Photos of the lower flasks with M. Aeruginosa growing in solutions at three different growth conditions (Table 1), at two growth times, 28 days. (c) Photos of the lower flasks with M. Aeruginosa growing in solutions at three different growth conditions (Table 1), at two growth times, 35 days.
nutrient solution with 1% clay. The cell density of M. Aeruginosa in both the pure solution and modified solution increased at least 30-fold over 35 days. In contrast, no consistent growth of M. aeruginosa was observed in the solution with 1% clay. Each point indicates the average cell density of three experimental replicates. Error bars represent the standard error of the three replicates. (b) Photos of the lower flasks with M. Aeruginosa growing in solutions at three different growth conditions (Table 1), at two growth times, 28 days. (c) Photos of the lower flasks with M. Aeruginosa growing in solutions at three different growth conditions (Table 1), at two growth times, 35 days.
The green color is representative of the amount of green pigment produced by the cells for each of the three M. aeruginosa growth conditions: pure nutrient solution (S), nutrient solution with 1% clay (C), and Modified clay nutrient Solution (MS) In addition to measuring cell density, we also imaged the flasks containing the three solutions with growing cells during the experiments. Figures 2b and 2c, shows representative images of the three bacterial solutions at 28 days and 35 days after the experiments started. On both days, the flasks with pure nutrient solution (labeled as “S”) and modified clay solution (labeled as “MS”) appear green, indicating that green pigment has been produced due to M. aeruginosa growth. In contrast, the color of the bacterial solution with 1% clay (labeled as “C”), appears yellow instead of green, further suggesting that the physical presence of clay significantly reduced the growth of M. aeruginosa cells and their production of pigments and also likely toxins. To test our hypothesis that the physical interactions between clay and the cells slow down the growth of M. aeruginosa, we imaged the M. aeruginosa cells sampled from each growth solution using a confocal laser scanning microscope. Figure 3, shows time-lapse photos of the M. aeruginosa taken on Day 49. Within the clay growth solution, two M. aeruginosa cells are visible, encapsulated in a clay particle. The cells show minimal movement at each of the three- time intervals: 0 seconds, 155 seconds, and 310 seconds. In contrast, cell movement is observed in the time lapse images taken of M. aeruginosa growing in both pure nutrient solution and modified clay solution. The cell densities are also notably higher in the two solutions lacking the physical presence of clay than that in the solution with clay. This observation suggests that clay and the cyanobacterial cells indeed physically interact with each other and such interactions reduce the movement and possibly replication capability of the cells, leading to the suppression of cell growth (Figure 3).
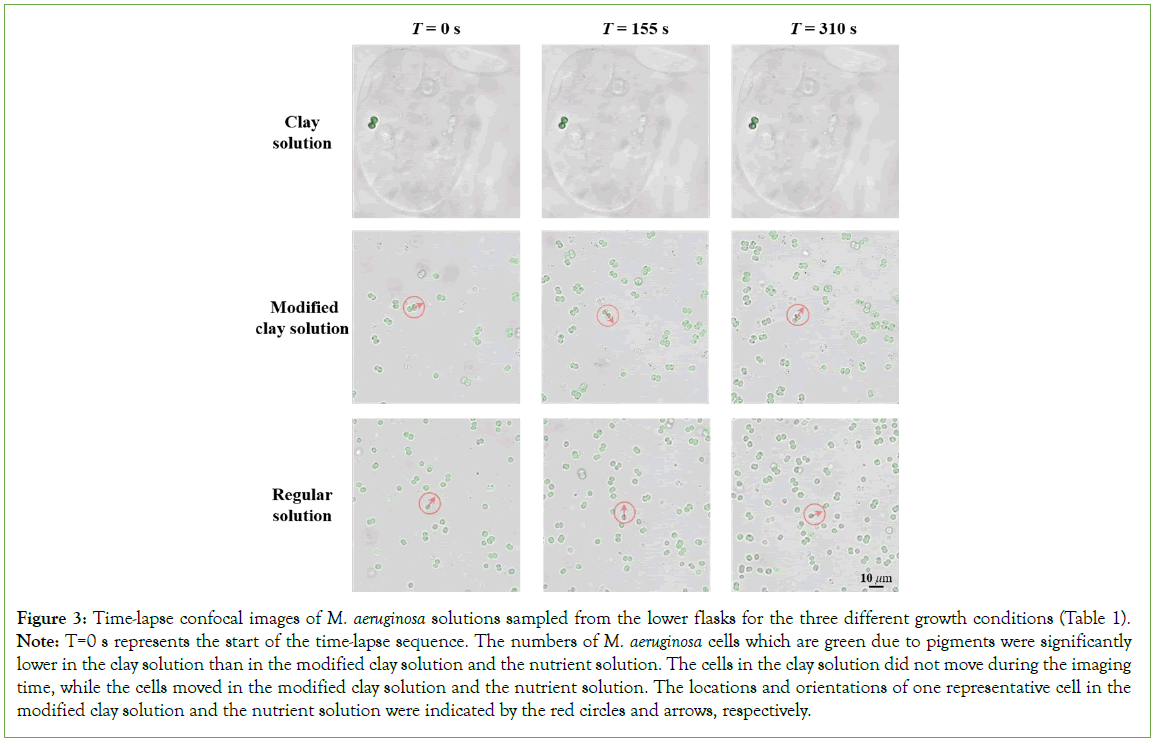
Figure 3: Time-lapse confocal images of M. aeruginosa solutions sampled from the lower flasks for the three different growth conditions (Table 1).
Note: T=0 s represents the start of the time-lapse sequence. The numbers of M. aeruginosa cells which are green due to pigments were significantly lower in the clay solution than in the modified clay solution and the nutrient solution. The cells in the clay solution did not move during the imaging time, while the cells moved in the modified clay solution and the nutrient solution. The locations and orientations of one representative cell in the modified clay solution and the nutrient solution were indicated by the red circles and arrows, respectively.
Discussion
Our results show that the growth of M. aeruginosa was significantly reduced, by an order of magnitude, due to the physical presence of transparent synthetic spectate clay (laponite). First, our bacterial- growth experiments show that M. aeruginosa growth is limited when grown in a nutrient solution containing 1% clay. In contrast, the cells grew fast in a flask with pure nutrient solution, which was placed under another flask of abiotic solution containing 1% clay. Therefore, the decrease in cell growth is not caused by the light attenuation by clay because the pure solution also has clay on top of it. Second, the cells grew well in the clay modified solution, which was mixed with 1% clay for 4 days but with clay filtered out afterwards. Such growth suggests that clay did not prevent cyanobacterial growth by absorbing key nutrients. Only when the M. aeruginosa was grown in physical contact with clay was static growth recorded. Therefore, the physical interactions between the laponite clay particle and the M. aeruginosa are what limit cell growth. The relationship between turbidity or clay concentration and algal bloom concentration may not be explained by nutrient absorption, or a difference in light intensities, but rather by the physical encapsulation of the algal cells by clay. Our result explains previous field observations that the Illinois River, which has abundant nutrients and appears muddy, had no algal blooms, while its tributary Fox River, which had a similar nutrient level and algal seeding but lacks suspended sediment, had frequent HABs [16]. The exchange of air and carbon dioxide (CO2) between the water and the atmosphere is another important factor that can affect algal growth. Algae, including M. aeruginosa, require CO2 for photosynthesis, and the availability of CO2 in the water is dependent on the rate of gas exchange with the atmosphere. However, it is worth noting that in the case of the experiment described in this study, the impact of air/CO2 exchange may be negligible. Due to the fact that the experimental conditions were identical for all treatments, any differences in algal growth likely resulted from other variables, such as the physical interactions between M. aeruginosa and laponite clay particles.
Conclusion
To fully understand the role of air/CO2 exchange in algal bloom formation, future research should investigate how clay particles influence these mechanisms in natural systems. The mechanisms of cell-clay interactions revealed in this study will improve future predictions of the occurrence of HABs in aquatic environments with varying turbidity and development of strategies to prevent HABs. Specifically, our results demonstrate that spectate clay can slow down the growth of Microcystis cells through physical encapsulation, suggesting that clay can potentially be used to reduce the growth of harmful algal cells and the development of HABs in aquatic environment.
Declaration of Competing Interest
The Authors declare no competing financial interest.
References
- Zamyadi A, Dorner S, Sauvé S, Ellis D, Bolduc A, Bastien C, et al. Species-dependence of cyanobacteria removal efficiency by different drinking water treatment processes. Water Res. 2013;47(8):2689-700.
[Crossref] [Google Scholar] [PubMed]
- Hardy FJ, Johnson A, Hamel K, Preece E. Cyanotoxin bioaccumulation in freshwater fish, Washington State, USA. Environ Monit Assess. 2015;1(87):1-5.
[Crossref] [Google Scholar] [PubMed]
- Watson SB, Miller C, Arhonditsis G, Boyer GL, Carmichael W, Charlton MN, et al. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae. 2016.44-66.
[Crossref] [Google Scholar] [PubMed]
- Chapra SC, Boehlert B, Fant C, Bierman Jr VJ, Henderson J, Mills D, et al. Climate change impacts on harmful algal blooms in US freshwaters: A screening-level assessment. Environ Sci Technol. 2017;51(16):8933-8943.
[Crossref] [Google Scholar] [PubMed]
- Mancuso JL, Weinke AD, Stone IP, Hamsher SE, Woller-Skar MM, Snyder EB, et al. Bloom and bust: Historical trends of harmful algal blooms in Muskegon Lake, Michigan, a Great Lakes estuary. Freshw Sci. 2021;40(3):463-477.
- Gill D, Rowe M, Joshi SJ. Fishing in greener waters: Understanding the impact of harmful algal blooms on lake erie anglers and the potential for adoption of a forecast model. J Environ Manage. 2018;227:248-255.
[Crossref] [Google Scholar] [PubMed]
- Levy S. Microcystis rising: Why phosphorus reduction isn’t enough to stop cyanoHABs. 2017;A34-A39.
- Mchau GJ, Makule E, Machunda R, Gong YY, Kimanya M. Harmful algal bloom and associated health risks among users of Lake Victoria freshwater: Ukerewe Island, Tanzania. J Water Health. 2019 Oct 1;17(5):826-36.
[Crossref] [Google Scholar] [PubMed]
- Deng J, Qin B, Paerl HW, Zhang Y, Ma J, Chen Y. Earlier and warmer springs increase cyanobacterial (Microcystis spp.) blooms in subtropical Lake Taihu, China. Freshw Biol. 2014;59(5):1076-1085.
- Osburn FS, Wagner ND, Taylor RB, Chambliss CK, Brooks BW, Scott JT. The effects of salinity and N: P on N‐rich toxins by both an N‐fixing and non‐N‐fixing cyanobacteria. Limnol Oceanogr Lett. 2023;8(1):162-172.
[Crossref] [Google Scholar] [PubMed]
- You J, Mallery K, Hong J, Hondzo M. Temperature effects on growth and buoyancy of Microcystis aeruginosa. J Plankton Res. 2018;40(1):16-28.
- Amano Y, Machida M, Tatsumoto H, George D, Berk S, Taki K. Prediction of Microcystis blooms based on TN: TP ratio and lake origin. Sci World J. 2008;8:558-572.
[Crossref] [Google Scholar] [PubMed]
- Wang W. Effect of turbidity on algal growth (Circular no. 121).1974.
- Dzialowski AR, Smith VH, Wang SH, Martin MC, Jr FD. Effects of non-algal turbidity on cyanobacterial biomass in seven turbid Kansas reservoirs. Lake Reserv Manag. 2011;27(1):6-14.
- Chan F, Pace ML, Howarth RW, Marino RM. Bloom formation in heterocystic nitrogen‐fixing cyanobacteria: The dependence on colony size and zooplankton grazing. Limnol Oceanogr. 2004;49(6):2171-2178.
- Smith VH. Effects of nutrients and non-algal turbidity on blue-green algal biomass in four North Carolina reservoirs. Lake Reserv Manag. 1990;6(2):125-131.
- Knowlton MF, Jones JR. Non-algal seston, light, nutrients and chlorophyll in Missouri reservoirs. Lake Reserv Manag. 2000;16(4):322-332.
- Liu H, Yuan P, Liu D, Zhang W, Tian Q, Bu H, et al. Insight into cyanobacterial preservation in shallow marine environments from experimental simulation of cyanobacteria-clay co-aggregation. Chem Geol. 2021;55(77):120-285.
- Lürling M, Faassen EJ. Controlling toxic cyanobacteria: effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins. Water Res. 2012;46(5):1447-1459.
[Crossref] [Google Scholar] [PubMed]
- de Magalhães L, Noyma NP, Furtado LL, Drummond E, Leite VB, Mucci M,et al. Managing eutrophication in a tropical brackish water lagoon: testing lanthanum-modified clay and coagulant for internal load reduction and cyanobacteria bloom removal. Estuaries Coast. 2019;4(2):390-402.
- Sukenik A, Viner-Mozzini Y, Tavassi M, Nir S. Removal of cyanobacteria and cyanotoxins from lake water by composites of bentonite with micelles of the cation Octadecyltrimethyl Ammonium (ODTMA). Water Res. 2017;120:165-173.
[Crossref] [Google Scholar] [PubMed]
Citation: Tomaska K, Wei G, Nguyen S, Yang JQ (2023) Suspended Clay Inhibits the Growth of Harmful Algal Bloom-forming Freshwater Cyanobacteria through Physical Interactions. J Microb Biochem Technol. 15:578.
Copyright: © 2023 Yang JQ, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

