Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Image Article - (2021) Volume 12, Issue 3
Study on screening Vietnamese herbs with antiviral activity to create products to support treatment of some diseases caused by RNA-virus
Quang Huan Le1*, The Quan Nguyen1, Hoang Minh Duc1, Thi Tam Quyen Doan1, Thi Huyen Trang Chu2, Thu Thao Dao2 and Thi Thanh Huong Ha32HUS High School of Gifted Students, Vietnam, 18 Hoang Quoc Viet, CauGiay, Hanoi, Vietnam
3Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, Vietnam
Received: 13-Feb-2021 Published: 23-Mar-2021, DOI: 10.35248/2157-7439.21.12.562
Abstract
Using information technology and traditional oriental medicine research, we have screened 5 Vietnamese herbs that contain active ingredients with antiviral effects. Andrographispaniculata, Syzygiumaromaum, Zingiber officinale Rose, Houttuyniacordata, Glycyrrhizauralensis Fisch. The active ingredients in herbs are extracted by ultrasound in a water-ethanol solvent system and made in the form of a nanometer complex, then mixed in specified proportions to form the product. The product was evaluated cytotoxicity by MTT (3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) assay and evaluated the growth inhibition effect of H5N1 virus. Result: The complex of active ingredients in the composition has the nanometer size, the main size is 443 nm, the zeta potential is -11.9 mV. The inhibitory activity of the H5N1 virus was dose-dependent, and a concentration of 3 mg/mL completely inhibited the growth of the H5N1 virus in the erythrocyte agglutination test.
Keywords
Nanotechnology; VIPDERVIR; Herbal; Ultrasound; Virus H5N1
Introduction
Viruses are the cause of many diseases, including cancer. Some difficult and complex syndromes such as Alzheimer's disease, type 1 diabetes and HCC have been linked to viral infections.
An estimated 250,000-500,000 annual deaths are caused by seasonal diseases and major pandemics. The Spanish flu of 1918 caused 20-40 million deaths. Currently, the COVID-19 epidemic in China has spread to many countries around the world. According to information from the Ministry of Health, until September 9th, epidemic COVID-19 worldwide has recorded 27,502,063 cases, 893,130 died in 215 countries and territories; To date, the total number of recovered COVID-19 patients is 19,372,831 and 7,034,907 are on treatment, of which 60,028 are critical [https://ncov.moh.gov.vn/].
The epidemic caused by COVID-19 is still happening in a very complicated way, causing social instability and great economic losses [Yin et al., 2020]. According to researchers around the world, it will take a few months for the vaccine to prevent SARS CoV-2 virus. Although the current technology of making vaccines is very modern, it still has not met the prevention needs.
Vaccine is the most effective preventive measure against diseases caused by viruses, but until now, there are still many diseases caused by viruses but there is still no vaccine, especially for RNAviruses strains with high mutation frequency, such as seasonal influenza virus, respiratory disease virus, SARS virus, SARS CoV- 2 virus. Sometimes a vaccine against a virus (such as respiratory virus, SARS), after being created, tested, and put into use, the virus changes into a new strain so that vaccine does not still works. That means vaccine creation seems to be not proactive, sometimes very passive.
Currently, several antiviral drugs have been introduced. But due to the high risk of mutation resistance, with many drugs being put into use, the effectiveness of treatment has been significantly reduced. The rapid growth of virus strains with recent outbreaks (such as H5N1, H1N1, H7N9) [Cao et al., 2009; World Health Organization, 2014] has caused extremely negative impacts on social life as well as economic development.
Therefore, research and search for new antiviral drugs from highly effective and affordable herbal sources for timely treatment and disease control when there is a lack or no creation of a vaccine for virus prevention [Lim et al., 2018; Choe JY and Kim SK, 2017; Yu et al., 2020] is essential. Timeliness is extremely necessary and urgent. Research on antiviral drugs is focused in the following directions [1]. Preventing virus adhesion to host cells [2] Prevent the replication of viral genetic material in infected host cells [3]. Activate cells of the immune system to increase resistance to the patient [Qamar et al., 2020; Khaerunnisa et al., 2020].
Vietnam has a diverse source of medicinal herbs, many herbs containing antiviral active ingredients that have been used in traditional medicine such as a remedy for dengue fever, a remedy for colds, and a remedy for inflammation. Therefore, we do research to screen and select Vietnamese herbs that contain active ingredients that have anti-adhesion effects, inhibiting the multiplication of viruses, especially viruses of RNA nature [4] to create highly effective treatment products for a number of diseases caused by these viruses, especially those that cause respiratory diseases such as influenza virus, SARS CoV-2 virus.
Materials and Methods
Andrographispaniculata, Syzygiumaromaum, Zingiberofficinale Rose, Houttuyniacordata, Glycyrrhizauralensis Fisch were obtained from Vietnam Pharmaceutical Company; Cell line HCT116 (Colon cancer cell line), AGS (gastrointestinal carcinoma cell lines); HCl, NP40, isopropanol; cell culture medium DMEM, Fetal bovine serum (FBS), Phosphate buffered saline, Ethanol, dH2O, and 05 vials containing 5 ml MTT (3-[4,5-dimethylthiazol-2-yl]- 2,5-diphenyltetrazolium bromide) (1x), at 5 mg/ml in phosphate buffered saline; NaCl, Na2HPO4, KH2PO4, Destroza, NaCitrat ,Axit Citric, NaCl, dH2O.
Virus H5N1 NIBRG-14, working seed 200407; Clean Egyptian eggs have embryos from the Thuy Phuong chicken breeding center;
Detection particle size and ζ-potential of product
The measurements of the average particle size and ζ-potential of the aqueous suspensions of product were carried out on a Malvern Zetasizer Nano-ZS dynamic light scattering (DLS) analyzer (Malvern Instruments Ltd., Malvern, UK)
Survey on the effect of product on the life of some cancer cell lines
Introduction Measurement of cell viability and proliferation forms the basis for numerous in vitro assays of a cell population’s response to external factors. The reduction of tetrazolium salts is now widely accepted as a reliable way to examine cell proliferation. The yellow tetrazolium MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) is reduced by metabolically active cells, in part by the action of dehydrogenase enzymes, to generate reducing equivalents such as NADH and NADPH. The resulting intracellular purple formazan can be solubilized and quantified by spectrophotometric means. The MTT Cell Proliferation Assay measures the cell proliferation rate and conversely, when metabolic events lead to apoptosis or necrosis, the cell viability decreases. The MTT Reagent yields low background absorbance values in the absence of cells. For each cell type the linear relationship between cell number and signal produced is established, thus allowing an accurate quantification of changes in the rate of cell proliferation.
Prepare cells
Colon cancer cell line HCT116 and gastrointestinal carcinoma AGS cell lines were transplanted into 96-well plates with a density of 104 cells/well. HCT116 was cultured in DMEM, 10% FBS, 1% PS, and AGS cells were cultured in RPMI medium, 10% FBS, 1% PS. Maintain plates in incubators of 5% CO2, 37oC for 24 hours.
Prepare the test product
• Solution for test-product is prepared with the ratio of H2O:DMSO is 30:1 (v/v).
• 500 mg of test-product diluted in 10 mL above solution.
• Centrifuge to absorb floating fluid, and Supernatant ultraviolet light for 2 hours.
• The test-product is diluted in culture medium corresponding to each cell line with concentration ranges of 10, 30, 50, 100, 200, 500, 800, 1000, 1200, 1500 (μg/mL).
• After 24 hours of incubation in plate 96, the cells were treated with the above dilution range. Each concentration was repeated 3 times on 3 wells.
• Maintain the plate in the culture cabinet for 48 hours.
MTT assay
• After 48 incubation, the cell wells were added with 10 μL of MTT (5 mg/mL).
• Incubate for 3 hours in the incubator. Absorb fluid in each well.
• Add 100 μL DMSO. Gently shake to create a homogeneous solution in each well.
• Measure the OD at a wavelength of 570 nm with the ELISA disc reader.
Determination of viral growth inhibitory activity
To evaluate the virus-killing ability of the produced product, we have evaluated the antiviral effect of the product made according to formula 1 and formula 2 on H5N1 virus. This is the RNA virus and also the virus that caused the H5N1 influenza epidemic in poultry and in humans.
Transplant influenza virus into chicken eggs
• The influenza virus is diluted in sterile 0.01M PBS solution to a concentration of about 100 - 1000 of EID50. Disinfect the injection site with iodine alcohol, then inject 0.2 mL into the urinary cavity of incubation chickens for 10-11 days.
• Urinary fluid was collected from non-dead eggs after incubation at 48 hours at 35°C for influenza A. The collected urine fluid was determined by the erythrocyte agglutination (HA) titre.
Determination of erythrocyte agglutination titres (Hem Aglutination, HA)
Prepare a 0.5% chicken erythrocyte suspension
• Use a sterile syringe with 10 mL capacity to pre-suck 3 mL of alsever anticoagulant into the pump, then use a syringe to suck 3 mL of venous blood from the chicken, pump into a test tube that has 5ml alsever solution available.
• Red blood cells were washed 3 times in 0.01M PBS by centrifuging 1500 rpm for 5 minutes. For the last time, centrifuge 10 minutes. Remove the supernatant water, dissolve the red blood cell residue into a 0.5% suspension in PBS.
Titration of the HA antigen
• Red cell agglutination (HA) antigen is titrated in 96-well microplastics plates with a U-bottom, using an 8-channel or 12-channel pipette for dilution:
• Add to all wells, each well 50 μl PBS 0.01M.
• Secondary dilution of the antigen begins with a 1/10 dilute, the final dilution depends on the estimated titer of the antigen used in the reaction (1/10 - 1/1280). Row 12 as a control for small red blood cells using 50 μl PBS.
• Add 50 μL of 0.5% red blood cell suspension in PBS into all wells.
• Shake well, leave the plastic sheet at laboratory temperature for about 30 minutes until the red blood cells (in row 12) settle well, read the results.
• The highest dilution of the antigen that still causes erythrocyte agglutination per unit agglutination. The opposite value of dilution is the antigen's precipitation titre (in units of agglutination). For example, at 1/1280 dilution the erythrocytes are still agglutinating, the antigen titre is 1280 units of HA.
• Seed storage: Choose the urinary fluid with the highest and most beautiful brand name (not cloudy, the clear fluid is light yellow color), divide into 2 mL Cryo Tubes and store in -70-80oC.
Evaluate the effectiveness of virus elimination
Experiment 1
Dissolve 0.5g of VIPDERVIR in 10 mL PBS1x, vontex, and centrifuge to collect supernatant (Solution 1). Proceed to test VIPDERVIR at different concentrations:
• 1.5 mg: 30 μL of solution 1+ 170 μL PBS 1x + 100 μL virus, mix well
• 3 mg: 60 μL of solution 1+ 140 μL PBS 1x + 100 μL virus, mix well
• 5 mg: 100 μL solution 1+ 100 μL PBS 1x + 100 μL virus, mix well
Experiment 2
Suck 1ml Solution 1 + 9 mL PBS1x and mix well (Solution 2)
• 0.15 mg: 30 μL of solution 2+ 170 μL PBS 1x + 100 μL virus, mix well
• 0.3 mg: 60 μL of solution 2+ 140 μL PBS 1x + 100μL virus, mix well
• 0.5 mg: 100 μL solution 2+ 100 μL PBS 1x + 100μL virus, mix well
Experiment 3
Suck 1mL Solution 2 + 4 mL PBS1x, mix well (Solution 3).
• 0.03 mg: 30 μL solution 3+ 170 μL PBS 1x + 100μL virus, mix well
• 0.06 mg: 60 μL solution 3+ 140 μL PBS 1x + 100μL virus, mix well
• Inject each concentration of medication into 6 eggs
Control
• Viral fluid + PBS
• After injection, eggs were incubated at 33.5oC for 3 days
• Carry out oocyte collection to check the agglutination of erythrocytes
• Proceed to collect egg solution: 10 l egg suspension + 90 μL PBS, mix well and then dilute according to step 2. Add 50 μL of 0.5% chicken erythrocytes to each well.
• After 1 hour of reading the results of the HA reaction
Results and Discussions
Make nanoparticle for the active ingredients
The active ingredients are extracted and nanosized by ultrasonic technology when combined with the surfactants: Polyvinylpyrrolidone (PVP), Tween-80, Sodium dodecyl sulfate (SDS) and lactose with ratio 1:1:1:0.2. The result, the product (named VIPDERVIR) haved average particle size is 8299 nm. In that, peak 1: size 443.7 nm with account 100%; PI dispersion value = 0.243; Zeta potential: -11.9 mV (Figure 1).
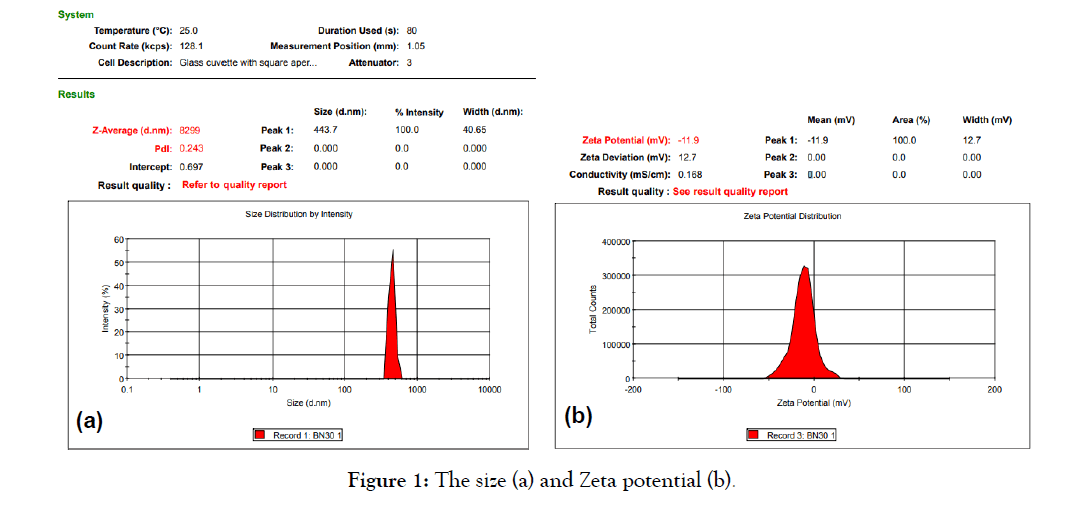
Figure 1: The size (a) and Zeta potential (b).
Investigate the effects of the VIPDERVIR on cell lines
From a concentration of 200 μg/mL, test-product started to inhibit proliferation and cell death compared to the control. With the HCT 116 cell line, the percentage of living cells was about 17% lower than that of the control. For the AGS strain, this figure was 4% compared to the control.
For HCT strain 1116, at concentrations less than 200 μg/mL, the percentage of viable cells was higher than the control at 10 μg/mL and not significantly different from the control at all concentrations. 30-100 μg/mL (Figure 2).
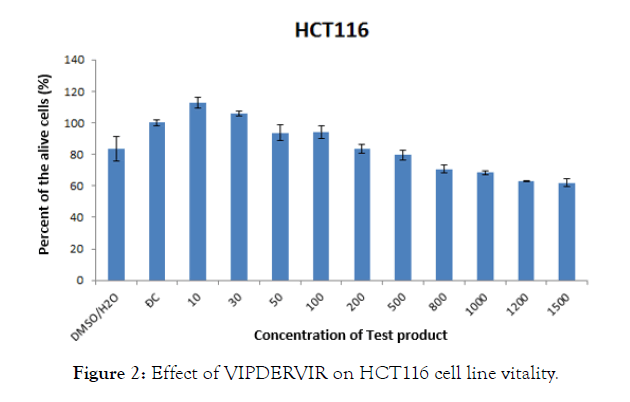
Figure 2: Effect of VIPDERVIR on HCT116 cell line vitality.
For AGS cell lines, a higher percentage of living cells compared to the control was noted at concentrations between 10-100 μg/ mL. From a concentration of 200 μg/mL, the percentage of viable cells decreased compared with the control (Figure 3). The level of inhibition increases with increasing concentration.
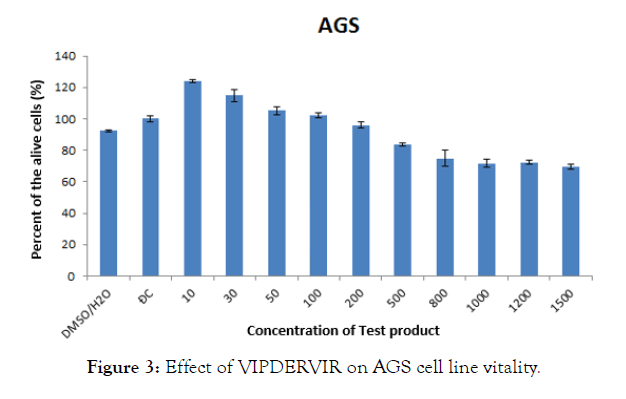
Figure 3: Effect of VIPDERVIR on AGS cell line vitality.
The HA assessment
Experimental results on the ability to inhibit the development of H5N1 virus of the preparation are presented in Table 1, Figures 4 & 5.
| VIPDERVIR concentration (mg) | 5 | 3 | 1,5 | 0,5 | 0.3 | 0,15 | 0.06 | 0,03 | ĐC1 |
| HAU (average) | 0 | 0 | 13 | 17 | 20 | 20 | 26 | 33 | 320 |
Table 1: Results of the HA assessment.
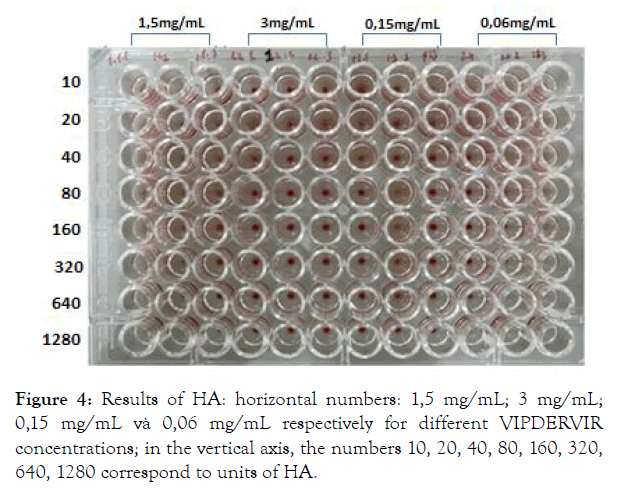
Figure 4: Results of HA: horizontal numbers: 1,5 mg/mL; 3 mg/mL; 0,15 mg/mL và 0,06 mg/mL respectively for different VIPDERVIR concentrations; in the vertical axis, the numbers 10, 20, 40, 80, 160, 320, 640, 1280 correspond to units of HA.
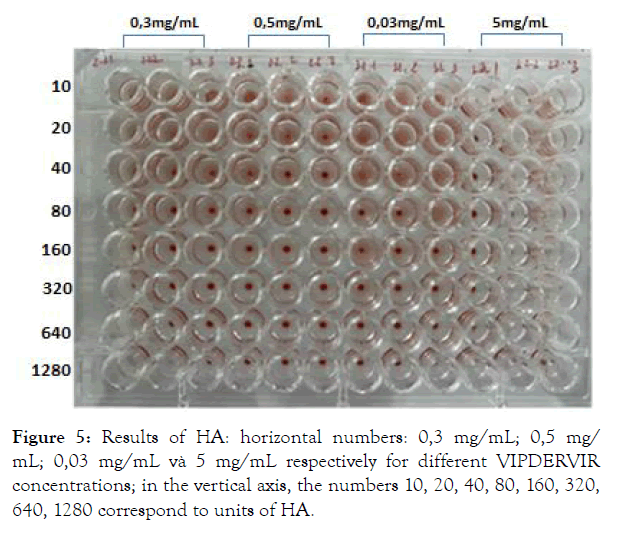
Figure 5: Results of HA: horizontal numbers: 0,3 mg/mL; 0,5 mg/ mL; 0,03 mg/mL và 5 mg/mL respectively for different VIPDERVIR concentrations; in the vertical axis, the numbers 10, 20, 40, 80, 160, 320, 640, 1280 correspond to units of HA.
Discussion
Currently, many studies are searching and filtering active ingredients from herbs that inhibit the virus. In particular, quercetin active ingredient is evaluated to be effective for COVID-19 [5] prevention and treatment. The trial is based on the assumption that quercetin, a potent bactericidal and anti-inflammatory agent, may be effective in the case of COVID-19. Quercetin dosage is planned to be 500– 1000 mg respectively for prevention and treatment, Onal H & Semerci SY [6] Studies on the effects of tannins [7] and extracts from Caesalpiniaspinosa (Molina) Kuntze Manrrique M & Garcia A, [8] have also shown positive results. Scientists' interest in the flavonoid's antiviral ability against human coronavirus infection could benefit from the enormous resources that governments, health agencies and private companies is pouring into this field [9] looking for a cure against SARS-CoV-2 [10]. While waiting for a valid vaccine against COVID-19, the pharmacological approach is preferred and flavonoids can play an important role in inhibiting viral growth. These are also identified active ingredients in the herbs that we have screened for in preparation VIPDERVIR. The "versatile" properties of flavonoids in our selected herbs need to be further investigated to evaluate their effects on the SARS CoV- 2 virus [11]. Our initial testing on the H5N1 virus showed that VIPDERVIR has a strong inhibitory effect on concentrationdependent virus growth. The preparation at 100 μg/mL was not cytotoxic, even with the HTC116 cell line proliferation at a concentration of 200 μg/mL. With a dose of 3 mg or more, the virus in the test unit was completely eliminated.
Conclusion
We used biochemical technology and nanotechnology to obtain active ingredients from a number of Vietnamese herbs to create VIPDERVIR, which inhibits the development of viral RNA. VIRDERVIR is nanometer in size, and is not cytotoxic at a concentration of 100 μg/mL, even 200 μg/mL for HCT cell line 1116. VIPDERVIR product inhibits H5N1 virus growth depending on concentration. With a dose of 3 mg or more, the virus in the test unit was completely eliminated.
REFERENCES
- Cao B, Li XW, Mao Y, Wang J, Lu HZ. Clinical features of the initialcases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507–2517.
- Choe JY, Kim SK. Quercetin and ascorbic acid suppress fructose-induced NLRP3 inflammasome activation by blocking intracellular shuttling of TXNIP in human macrophage cell lines, Inflammation. 2017; 40:980–994
- Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S. Potential Inhibitor ofCOVID-19 Main Protease ( M pro ) from Several Medicinal Plant Compounds byMolecular Docking Study. Preprints, March, 2020:1–14.
- Lim H, Min DS, Park H, Kim HP, Flavonoids interfere with NLRP3 inflammasome activation. Toxicol Appl Pharmacol 2018;355:93–102.
- Manrrique M, Garcia A. P2Et Extract in the Symptomatic Treatment of Subjectswith COVID-19. 2020:NCT04410510.
- Onal H, Semerci SY. Effect of Quercetin on Prophylaxis and Treatment of COVID-19. 2020. NCT04377789.
- Piskorz MM, Tannin Specific Natural Extract for COVID-19 Infection (TaCOVID).2020. NCT04403646.
- Qamar MT, Alqahtani SM, Alamri MA, Chen LL. Structural basis ofSARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharma Analysis.2020.
- World Health Organization. Influenza (seasonal). Fact sheet No. 211; March2014.
- Yin W, Mao C, Luan X. Structural basis for inhibition of theRNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir, Science 2020;368:1499–11504.
- Yu R, Chen L, Lan R, Shen R, Li P. Computational screening of antagonistsagainst the SARS-CoV-2 (COVID-19) coronavirus by molecular docking. Int J Antimicrob Agents 2020:106012.
Citation: Le QH, Nguyen TQ, Duc HM, Doan TTQ, Chu THT, et al. (2021) Study on Screening Vietnamese Herbs with Antiviral Activity to Create Products to Support Treatment of Some Diseases Caused By RNA-Virus. J Nanomed Nanotech. 12: 560.
Copyright: © 2021 Le QH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


