Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- The Global Impact Factor (GIF)
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 3
Simultaneous Determination of Paracetamol, Propyphenazone, Aspirin and Caffeine in White Wine Samples by Liquid Chromatography-Triple Quadrupole Tandem Mass Spectrometry
Dan Han1, Yongliang Ding1, Kun Peng1, Bobing Tang2, Cunxian Xi2 and Anping Deng3*2Department of Pharmaceutical Sciences, The Inspection Technical Centre of Chongqing Customs, Chongqing, China
3Department of Chemical Engineering and Materials Science, Soochow University, Suzhou, China
Received: 02-May-2023, Manuscript No. PAA-23-21188; Editor assigned: 04-May-2023, Pre QC No. PAA-23-21188(PQ); Reviewed: 18-May-2023, QC No. PAA-23-21188; Revised: 25-May-2023, Manuscript No. PAA-23-21188(R); Published: 01-Jun-2023, DOI: 10.35248/2153-2435.23.14.731
Abstract
A highly sensitive Liquid Chromatography-Triple Quadrupole Tandem Mass Spectrometry (LC-MS/MS) for simultaneous determination of analgesic-antipyretics drugs, e.g. Paracetamol (PC), Propyphenazone (PZ), Aspirin (AS) and Caffeine (CF), in white wine samples was presented. The chromatographic separation was carried out on a narrow C18 column by a gradient elution using Acetonitrile (A) and 10 mM ammonium acetate with 0.1% formic acid (B) as the solvents at a flow rate of 0.3 ml/min. Qualitative and quantitative analysis of PC, PZ and CF were achieved by Multiple Reaction Monitoring (MRM) in positive mode, while the analysis of AS was completed by MRM in negative mode. Under optimal conditions, good linearity with high correlation coefficient (>0.9981) were obtained between 0.05-50 ng mL-1 for PC, 0.005-20 g mL-1 for PZ, 0.3-200 g mL-1 for AS, and 1.0-50 g mL-1 for CF. The Limit Of Detection (LOD) values of the method for PC, PZ, AS and CF were found to be 0.015, 0.0015, 0.08 and 0.25 g mL-1, respectively. Spiking experiments revealed good recoveries in range of 87.6~111.0% with Relative Standard Deviations (RSD) of 2.1~9.4%. Total 1006 white wine samples were collected and analysed by the proposed method. PC was found in three inferior white wines samples, and the residual levels of PC, PZ, AS and CF in other samples were undetectable. It was demonstrated that the proposed LC–MS/MS is a powerful analytical tool for sensitive determination of PC, PZ, AS and CF in white wine samples.
Keywords
Analgesic-antipyretics drugs; Paracetamol; Propyphenazone; Aspirin; Caffeine; White wine samples
Introduction
Paracetamol (PC), Propyphenazone (PZ) and Aspirin (AS) are three kinds of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) which exhibit the effective analgesic and antipyretic activities [1,2]. As they can inhibit on pain and thermoregulatory centres in Central Nervous System (CNS), they are the most frequently prescribed drugs worldwide for treatment of pain and fever including headache, toothache, rethum and pyrexia, arthralgia, neuralgia, migraine, cancerous pain and postoperative pain, etc.[3-5]. Caffeine (CF) is one kind of CNS stimulants that occurs naturally in some plants (Figure 1) [6-8]. Products containing caffeine such as coffee, tea, soft drinks and chocolate are enjoyed by many people throughout the world. CF can be also used as an active ingredient in some cold remedies and various pain killers. When CF is combined with PC, PZ and AS, the analgesic and antipyretic effects can be greatly improved [9,10]. However, over intake of PC, PZ, AS and CF can be harmful to human health such as suppressing prostaglandin production, decreasing the subsequent gastric mucosa layer, reducing gastric acid neutralization that causes stomach ulcers, stomach bleeding and many other intestinal disorders [11-13].
Figure 1: Molecular structures of (a) Paracetamol, (b) Propyphenazone, (c) Aspirin and (d) Caffeine.
There is a long history of drinking white wines in China, and every year more than thousands of tons of different Chinese white wines are produced and consumed. High quality white wines made from cereals are aroma and palatable without serious headache. But the inferior white wines made by mixing the alcohol with chemical flavours can easily make the consumers feel strong headache because inside the inferior white wines there is high content of fuel oils containing many longer carbon-chain alcohols which are difficult to decompose in human body. To reduce the headache symptom, some profiteers illegally added analgesic-antipyretics drugs such as PC, PZ, AS and CF into inferior white wines to pretend high quality liquors. From the medical point of view, PC, PZ, AS and CF are the drugs that are forbidden to be taken with alcohol together because drinking the liquors containing analgesic-antipyretics drugs will cause serious negative effects on the consumers such as anaphylaxis, nausea and vomiting, hepatic renal or stomach disease and even death. To protect food safety and safeguard human health, it is very important and urgent to develop sensitive and effective method for simultaneous determination of PC, PZ, AS and CF in white wines samples.
The main analytical methods for the determination of PC, PZ, AS or CF are chromatographic analyses with different detectors [14-17]. These chromatographic methods are usually used for the detection of single or two analgesic-antipyretics drugs in pharmaceuticals [18- 21]. Some chromatographic methods are found for the detection of AS or CF in different foods such as soft drinks, teas, fruits, vegetables, wines, spices, sauces and beverages [22-24]. There are few reports of chromatographic methods for the detection of PC in food samples. In 1997, R. Izquierdo-Hornillos developed a Liquid Chromatography UV detector (LC-UV) for the determination of PC in chewing gum samples [25]. Recently, another LC-UV was appeared for the determination of PC in milk-based samples [26]. However, up to now, the chromatographic methods for the detection of PZ in food samples are not observed, only in 2015, a LC-MS/MS for simultaneous determination of aminopyrine and antipyrine, which are the pyrazolone derivatives similar to PZ, in porcine muscle, milk, and eggs was published [27].To the best of our knowledge, there is no report of the chromatographic methods for the simultaneous detection of PC, PZ, AS and CF in food samples.
In this paper, coupling with a simple reduced-pressure distillation sample pre-treatment method, a sensitive and selective Liquid Chromatography-Triple Quadrupole Tandem Mass Spectrometry (LC-MS/MS) for simultaneous determination of PC, PZ, AS and CF in Chinese white wine samples was developed. Experimental conditions in LC-MS/MS were carefully optimized. High sensitivities of the LC-MS/MS for PC, PZ, AS and CF with low values were obtained. Total 1006 white wine samples were collected and analysed by the proposed method. It was demonstrated that the developed LC–MS/MS is a powerful analytical tool for sensitive and accurate determination of PC, PZ, AS and CF in white wine samples. To the best of our knowledge, it is the first report of the analytical method for simultaneous detection of PC, PZ, AS and CF in white wine samples.
Materials and Methods
Chemicals and reagents
PC, PZ, AS and CF (≥ 99%) reference standards were purchased from National Institutes for Food and Drug Control (Beijing, China). Ammonium acetate, HPLC-grade formic acid, acetonitrile and methanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultra-high purity water was generated with a Milli-Q (Millipore, Bedford, MA, USA) water purification system.
Preparation of solutions
The individual stock solutions (1.0 mg/mL) of PC, PZ, AS and CF were prepared by dissolving each reference standard in methanol. Calibration standards of PC, PZ, AS and CF were prepared by diluting stock solutions with pure water. The standard concentrations of each analyte were prepared in range of: 0.05-50 ng/mL for PC, 0.005-20 ng/mL for PZ, 0.3-200 ng/mL for AS, and 1.0-50 ng/mL for CF. All solutions were stored at 4 ºC.
Sample pre-treatment methods
In this study, for the detection of targets analyte in spiked white wine samples, two pre-treatment methods were tested and compared.
• Low pressure distillation: 10.00 mL of the spiked sample was added to a pear-shaped bottle and distilled under vacuum at 50 ± 5 ºC. When the volume of sample in the bottle was about one third of the starting volume, the distillation was terminated. The sample in the bottle was transferred to another 10 mL of volumetric flask. The bottle was rinsed 3 times with small amount of pure water. All rinsing solutions were added to the volumetric flask and supplemented with pure water to the scale. The bottle was shaken about 30 seconds. After filtration, the sample solution was subjected to LC-MS/MS.
• Boiling evaporation: 10.00 mL of the spiked sample was added to a pear-shaped bottle and evaporated at an evaporating dish until about one third of the volume was left. The sample in the bottle, combing with rinsing solutions, was transferred to another 10 mL of volumetric flask. After adding pure water to the scale and mixing sufficiently, the sample solution was detected by LC-MS/MS.
LC–MS/MS analysis
A Shimadzu HPLC system consists of a DGU-20A3 degasser, two LC-20AD pumps, a SIL-20A auto sampler, a CTO-20A column temperature oven, a CBM-20A communications bus module (Shimadzu Corporation, Tokyo, Japan) for solvent and sample delivery. Chromatographic separation was achieved on a Waters Narrow Bore C18 analytical column (150 mm × 2.1 mm, 5 μm particle sizes, Milford, USA) at 25 ºC. The mobile phase consisted of solvent A (acetonitrile) and solvent B (10 mmol L-1 ammonium acetate with 0.1% formic acid). The gradient elution program of two solvents on the column (Table S1). The injection volume of 20 μL was delivered at a flow rate of 0.3 mL/min.
Mass spectrometric detection was conducted on an API 4000 (AB Sciex, Foster City, CA, USA) triple quadrupole mass spectrometer equipped with an Electro Spray Ionization (ESI) source. For PC, PZ and CF, the identification and quantification was performed by Multiple Reaction Monitoring (MRM) in positive mode with the parameters as follows: 550 °C ion source temperature; 5500 V ion spray voltage; 15 psi nebulizer gas (GS1); 15 psi turbo gas (GS2). While for quantification of AS, MRM in negative mode was used due to AS belonging to an acidic compound. The conditions of MS/MS for AS were: 400 °C ion source temperature; 4500 V ion spray voltage; 15 psi nebulizer gas (GS1); 15 psi turbo gas (GS2). Ultra-pure nitrogen was used as the drying and collision gas. The data acquisition and processing in positive or negative mode were performed with Analyst version 1.5.2 software (AB Sciex, Foster City, CA, USA). The precursor ion, product ion, corresponding De-clustering Potential (DP) and Collision Energy (CE) were shown in Table S2.
Spiking experiment
Three blank white wine samples with brands of JX, NX and MX containing no any target compounds were used for spiking experiment. Different amount of stock solutions was added to blank samples to form three spiked levels, e.g. 4, 20, 40 ng mL-1 for PC; 0.5, 4, 8 ng mL-1 for PZ; 10, 100, 180 ng mL-1 for AS, and 5, 60, 90 ng mL-1 for CF, respectively. After sample pre-treatment, the spiked samples were analysed by LC–MS/MS.
Real sample analysis
To investigate the possibly illegal actives of analgesic-antipyretics drugs addition in white wine samples, total 1006 white wine samples with were collected from different supermarkets as many as possible. After sample pre-treatment (low pressure distillation), the samples were analysed by the proposed LC–MS/MS.
Results and Discussion
LC separation
The mobile phase has an important effect on the detection and separation signals of the tested compounds. To get high resolution and sensitivity, in this study, methanol and acetonitril were tested as solvent A, and four different buffers (0.1% formic acid in pure water, 0.1% acetic acid in pure water, 0.1% formic acid with 10 mmol L-1 ammonium acetate, 0.1% acetic acid with 10 mmol L-1 ammonium acetate) were investigated as solvent B. We found that the best results was obtained when acetonitrile instead of methanol was chosen as the organic phase, and 0.1% formic acid together with 0.1 mmol L-1 ammonium acetate instead of acid solution or water was selected the aqueous phase. In addition, to improve resolution and reduce separation time, a gradient elution program of solvent A and solvent B was utilized (Table S1). It was observed that all analyte were able to be separated completely within 6.2 min, and the retention times of PC, PZ, AS and CF were found to be 3.03, 5.03, 4.01 and 3.36 min, respectively (Table S2).
MS/MS detection
To select the appropriate MS/MS Multiple Reaction Monitoring (MRM) transitions, a compromise between sensitivity and selectivity had to be sought. Suitable precursor-to-product MS/MS transitions were selected through the optimized procedure. The final MS/ MS conditions are detailed in Table S2. Under positive ESI-MS conditions, the Extract Ion Chromatograms (EIC) of PC, PZ and CF were illustrated in Figure 2. It can be seen from Figure 2(A) that in blank sample, there was no interference peaks appeared at the retention times of PC (3.03 min) and PZ (5.03 min), however, a noticeable interference peak appeared at the retention time of CF (3.36 min), which will significantly reduce the sensitivity of the LC-MS/MS for CF. While under negative ESI-MS conditions, the EIC of AS was shown in Figure 3. Apparently from Figure 3(A), there was no interference peak appeared at the retention times of AS (4.01 min). Besides EIC, the mass spectra of PC, PZ, AS and CF were presented (Fig. S1).
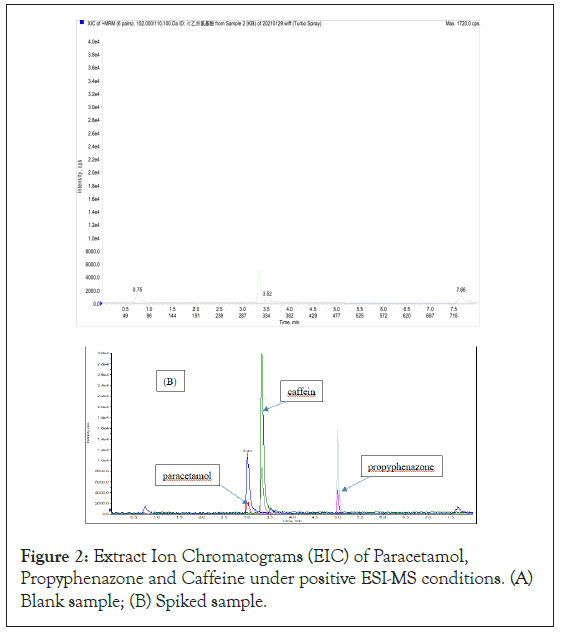
Figure 2: Extract Ion Chromatograms (EIC) of Paracetamol, Propyphenazone and Caffeine under positive ESI-MS conditions. (A) Blank sample; (B) Spiked sample.
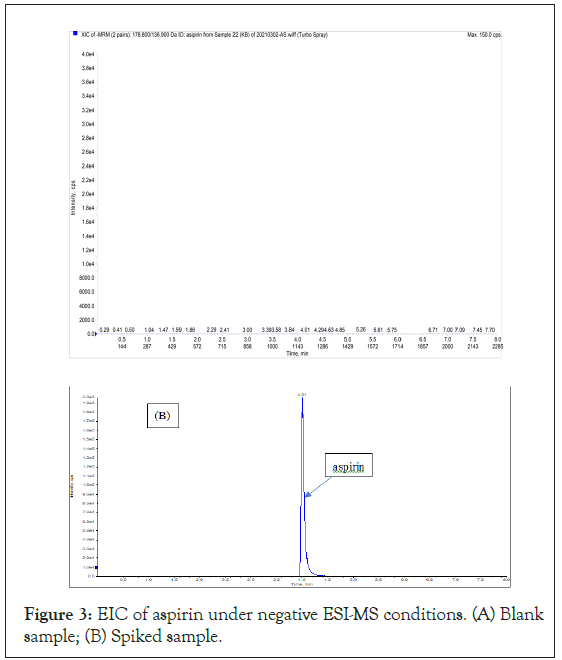
Figure 3: EIC of aspirin under negative ESI-MS conditions. (A) Blank sample; (B) Spiked sample.
In positive mode of MRM for PC, PZ and CF, two product ions derived from precursor ion were observed (Table S2, Fig. S1). Considering the parameters of signal responses, stability and linearity, etc., in this study, ion pairs of 152/110, 231/189 and 195/138 were selected for quantitative detection of PC, PZ and CF, respectively; while ion pairs of 152/93, 231/201 and 195/110 were used for qualitative identification of these three compounds. In contrast, in negative mode of MRM for AS, only one product ion (m/z 137) derived from precursor ion (m/z 179) was observed, thus the ion pair of 179/137 had to be for both quantitative detection and qualitative identification of AS.
Comparison of two sample pre-treatment methods
White wine samples spiked with four analyte at different levels (PC: 4, 20, 40 ng/mL; PZ: 0.5, 4, 8 ng/mL; AS: 10, 100, 180 ng/mL CF: 5, 60, 90 ng/mL) were subjected to two pre-treatment methods and detected by LC-MS/MS. The results of recovery and Relative Standard Deviation (RSD) of the LC-MS/MS for four analytes were presented in Table S3. It was seen that for two pre-treatment methods, although RSD values in two pre-treatment methods were very small (<6.8%), the recoveries from boiling evaporation were a little lesser than that from low pressure distillation, which is probably relative to the fact that small amount of analyte may be lost during boiling evaporation. Thus low pressure distillation was adopted as the sample pre-treatment method in this study.
Standard curves of LC-MS/MS for four drugs
Under optimal experimental conditions, the standard concentrations of each analyte in ranges of 0.05-50 ng/mL for PC, 0.005-20 ng/mL for PZ, 0.3-200 ng/mL for AS, and 1.0-50 ng/ mL for CF were applied to LC-MS/MS performance. The standard curves of LC-MS/MS for PC, PZ, AS and CF were presented in Figure 4, and the corresponding linear equations, correlation coefficients (r), linear ranges, Limits of Detection (LOD), and Limits of Quantification (LOQ) were shown in Table 1 (Figure 4). The response function of the four drugs was found to be linear with a correlation coefficient (r) higher than 0.9981 in the tested range. The LOD and LOQ which were calculated as the lowest analyte concentration yielding a signal-to-noise (S/N) ratios of 3 and 10, were in the range of 0.0015–0.25 ng mL-1 and 0.005– 1.0 ng mL-1, respectively. From Table 1, it was observed that the LOD and LOQ values of the LC-MS/MS for CF were the highest compared to those for other three drugs, which is close relative to the high signal noise in CF detection.
| Drug | Linear equation | Correlation coefficient (r) | Linear range(ng mL-1) | LOD (ng mL-1) | LOQ (ng mL-1) |
|---|---|---|---|---|---|
| paracetamol | Y=23052X+211 | 0.9981 | 0.05-50 | 0.015 | 0.05 |
| propyphenazone | Y=84386X+354 | 0.9992 | 0.005-20 | 0.0015 | 0.005 |
| aspirin | Y=308.84X+2.67 | 0.9991 | 0.3-200 | 0.08 | 0.3 |
| caffein | Y=10947X+98.3 | 0.9989 | 1.0-50 | 0.25 | 1 |
Table 1: Linear equations, correlation coefficients (r), linear ranges, limits of detection (LOD) and limits of quantification (LOQ) of the LC-MS/MS for four drugs
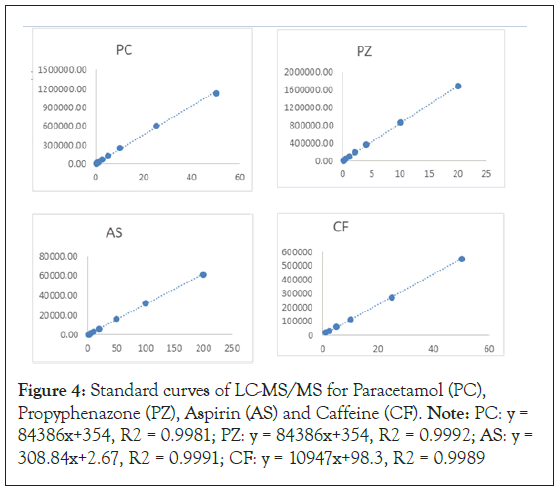
Figure 4: Standard curves of LC-MS/MS for Paracetamol (PC), Propyphenazone (PZ), Aspirin (AS) and Caffeine (CF). Note: PC: y = 84386x+354, R2 = 0.9981; PZ: y = 84386x+354, R2 = 0.9992; AS: y = 308.84x+2.67, R2 = 0.9991; CF: y = 10947x+98.3, R2 = 0.9989
Accuracy and precision of LC-MS/MS
Different amount of analytes was added to three blank white wine samples to form the spiked samples with the concentration of 4, 20, 40 ng/mL for PC; 0.5, 4, 8 ng/mL for PZ; 10, 100, 180 ng/ mL for AS, and 5, 60,90 ng/mL for CF. The spiked samples were subjected to low pressure distillation sample pre-treatment, and analysed by LC-MS/MS Figure 2(B), 3(B). The results of spiking experiment are summarized in Table 2. It can be seen that the recoveries for four analytes in spiked samples were found in range of 87.0~111.3%, and the RSD values of the LC-MS/MS for four analytes were with 0.8~6.2% (n=6). On the other hand, it should be mentioned that the inter-day RSD values were also tested, which was in range of 4.8–9.7%. These results demonstrated the high accuracy and precision of LC-MS/MS for PC, PZ, AS and CF in white wine samples.
| Drug | Added (μg/L) | Found (μg/L) | Recovery (%) | RSD (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| JX | NX | MX | JX | NX | MX | JX | NX | MX | ||
| Paracetamol | 4 | 3.84±0.27 | 3.82±0.21 | 3.79±0.28 | 95.5 | 97.9 | 95.9 | 6 | 6.1 | 6.2 |
| 20 | 22.0±1.15 | 18.25±1.05 | 18.35±1.05 | 111.3 | 90.8 | 93.3 | 3.8 | 5.2 | 5.6 | |
| 40 | 40.75±2.55 | 34.85±1.35 | 36.1±1.52 | 101.8 | 87 | 89.8 | 4.6 | 3.4 | 3.5 | |
| 0.5 | 0.52±0.02 | 0.46±0.01 | 0.48±0.01 | 104.7 | 91.7 | 97.9 | 3 | 1.8 | 3.3 | |
| Propyphenazone | 4 | 4.71±0.21 | 3.88±0.15 | 3.96±0.16 | 101.4 | 96.3 | 98.5 | 3.7 | 4.8 | 3.9 |
| 8 | 8.17±0.22 | 7.525±0.315 | 7.72±0.31 | 101.8 | 94.2 | 96.8 | 2.2 | 2.9 | 3.2 | |
| 10 | 0.92±0.05 | 1.10±0.04 | 1.07±0.05 | 90.5 | 109.8 | 107.2 | 3.5 | 2.4 | 3 | |
| Aspirin | 100 | 87.55±0.65 | 97.45±1.45 | 99.95±1.05 | 87.4 | 97.7 | 100.1 | 1.6 | 1.3 | 0.8 |
| 180 | 182.05±1.01 | 183.06±2.22 | 182.13±2.45 | 101.2 | 101.7 | 100.8 | 2.4 | 1 | 1 | |
| 5 | 4.59±0.27 | 4.69±0.04 | 4.69±0.18 | 92.2 | 93.3 | 95.4 | 3.9 | 2.7 | 2.8 | |
| Caffeine | 20 | 55.51±1.62 | 55.55±0.85 | 56.42±1.71 | 93 | 92.9 | 93.7 | 2 | 1.1 | 2.4 |
| 40 | 88.25±2.05 | 87.45±1.85 | 88.15±1.34 | 97.4 | 96.7 | 97.9 | 1.7 | 1.4 | 1.5 | |
Table 2: Validation parameters obtained for four drugs at three spiked levels in three kinds of white wine matrix (n=6, x ± s)
Method application
To prove the applicability of the developed method, total 1006 white wine samples were collected from many supermarkets. After sample pre-treatment, the collected samples were analysed by the proposed LC–MS/MS. Among 1006 samples, only in three inferior white wines samples, one kind of illegal drug (PC) was found with the concentrations of 6.02, 18.1 and 11.7 μg mL-1, respectively. However, in these 3 samples, PZ, AS and CF were undetectable. In other 1003 samples, the residual levels of PC, PZ, AS and CF were also no detectable. These results demonstrated that although the illegal addition of analgesic and antipyretic drugs in white wine is uncommon (<0.3%), the occurrence of this illegal active is real existence. To safeguard human health, it is still necessary monitor the illegal drugs like PC, PZ, AS and CF in white wines samples frequently.
Conclusion
Combining with a simple sample pre-treatment method (low pressure distillation), a sensitive LC-MS/MS for simultaneous determination of PC, PZ, AS and CF in white wine samples was developed. The analytes were separated on a narrow C18 column by a gradient elution program using Acetonitrile (A) and 10 mM ammonium acetate with 0.1% formic acid (B) as the solvents. PC, PZ and CF were detected by MRM in positive mode, while AS was measured by MRM in negative mode. Under optimal conditions, good linearity with high correlation coefficient, and low LOD values were achieved. Spiking experiments demonstrated high accurate and precision of the method. Total 1006 white wine samples were collected and analysed by the proposed method. In three inferior white wines samples, PC was found with the concentration more than 6.0 μg mL-1. In other 1003 samples, no detectable PC, PZ, AS and CF were found. It was proven that the proposed LC–MS/ MS was able to sensitive detect PC, PZ, AS and CF in white wine samples simultaneously, providing an effective toll for food-safety supervision.
Acknowledgements
Dan Han: Conceptualization, Resources, Investigation, Data Curation, and Writing–original draft, Writing–review and editing, Yongliang Ding: Resources. Kun Peng: Resource, Investigation, Data Curation, Bobing Tang: Resources, Investigation, Methodology, Cunxian Xi: Investigation, Data Curation, Anping Deng: Conceptualization, Resources, Writing – original draft, Writing–review and editing.
Sources of Funding
The authors acknowledge the financial support provided by Chongqing Science and Technology Bureau, grant number cstc2019jcyj-msxmX0499.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Gurpinar E, Grizzle WE, Piazza GA. NSAIDs Inhibit Tumorigenesis, but How? COX-Independent Mechanisms of NSAID Chemoprevention. Clin Cancer Res. 2014;20(5):1104-1113.
[Crossref] [Google Scholar] [PubMed]
- Medve RA, Wang J, Karim R. Tramadol and acetaminophen tablets for dental pain. Anesth Prog. 2001;48(3): 79–81.
[Crossref] [Google Scholar] [PubMed]
- Diamond S, Balm TK, Freitag FG. Ibuprofen plus caffeine in the treatment of tension-type headache. Clin Pharmacol Ther. 2000;68(3):312-319.
[Crossref] [Google Scholar] [PubMed]
- Ibrahim H, Boyer A, Bouajila J, Couderc F, Nepveu F. Determination of non-steroidal anti-inflammatory drugs in pharmaceuticals and human serum by dual-mode gradient HPLC and fluorescence detection. J Chromatogr B. 2007;857(1):59-66.
[Crossref] [Google Scholar] [PubMed]
- Martin FL, McLean AE. Comparison of paracetamol-induced hepatotoxicity in the rat in vivo with progression of cell injury in vitro in rat liver slices. Drug Chem Toxicol. 1998;21(4):477-494.
[Crossref] [Google Scholar] [PubMed]
- Sweetman SC. Martindale: the complete drug reference. Pharmaceutical press; 2009.
- Cappelletti S, Daria P, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2015;13(1):71-88.
[Crossref] [Google Scholar] [PubMed]
- Okuno T, Sugiyama T, Tominaga M, Kojima S, Ikeda T. Effects of caffeine on microcirculation of the human ocular fundus. J Ophthalmol. 2002;46(2):170-176.
[Crossref] [Google Scholar] [PubMed]
- Ibrahim H, Hamdy AM, Merey HA, Saad AS. Simultaneous determination of paracetamol, propyphenazone and caffeine in presence of paracetamol impurities using dual-mode gradient HPLC and TLC densitometry methods. J Chromatogr Sci. 2021 ;59(2):140-147.
[Crossref] [Google Scholar] [PubMed]
- Palmer H, Graham G, Williams K, Day R. A risk-benefit assessment of paracetamol (acetaminophen) combined with caffeine. Pain Med. 2010;11(6):951-965.
[Crossref] [Google Scholar] [PubMed]
- Graham GG, Scott KF. Mechanisms of action of paracetamol and related analgesics. Pharmacol. 2003;11:401-413.
[Crossref] [Google Scholar] [PubMed]
- Iancu I, Strous RD. Caffeine intoxication: history, clinical features, diagnosis and treatment. Harefuah. 2006;145(2):147-151.
[Crossref] [Google Scholar] [PubMed]
- Jiang W, Wu Y, Jiang X. Coffee and caffeine intake and breast cancer risk: an updated dose–response meta-analysis of 37 published studies. Gynecol Oncol. 2013;129(3):620-629.
[Crossref] [Google Scholar] [PubMed]
- Kulikov AU, Verushkin AG. Simultaneous determination of paracetamol, caffeine, guaifenesin and preservatives in syrups by micellar LC. Chromatographia. 2008;67(5-6):347-355.
- Qi ML, Wang P, Leng YX, Gu JL, Fu RN. Simple HPLC method for simultaneous determination of acetaminophen, caffeine and chlorpheniramine maleate in tablet formulations. Chromatographia. 2002;56:295-298.
- Cunha RR, Chaves SC, Ribeiro MM, Torres LM, Muñoz RA, Santos WT, et al. Simultaneous determination of caffeine, paracetamol, and ibuprofen in pharmaceutical formulations by high-performance liquid chromatography with UV detection and by capillary electrophoresis with conductivity detection. J Sep Sci.2015;38(10):1657-1662.
[Crossref] [Google Scholar] [PubMed]
- Gliszczynska-Swiglo A, Rybicka I. Simultaneous determination of caffeine and water-soluble vitamins in energy drinks by HPLC with photodiode array and fluorescence detection. Food Anal Methods. 2015;8:139-146.
- Ma M, Feng F, Sheng Y, Cui S, Liu H. Development and evaluation of an efficient HPLC/MS/MS method for the simultaneous determination of pseudoephedrine and cetirizine in human plasma: Application to Phase-I pharmacokinetic study. J Chromatogr B. 2007;846(1-2):105-111.
[Crossref] [Google Scholar] [PubMed]
- Pirker R, Huck CW, Popp M, Bonn GK. Simultaneous determination of gentisic, salicyluric and salicylic acid in human plasma using solid-phase extraction, liquid chromatography and electrospray ionization mass spectrometry. J Chromatogr B. 2004;809(2):257-264.
[Crossref] [Google Scholar] [PubMed]
- McEvoy E, Donegan S, Power J, Altria K. Optimisation and validation of a rapid and efficient microemulsion liquid chromatographic (MELC) method for the determination of paracetamol (acetaminophen) content in a suppository formulation. J Pharma Biomed Anal. 2007;44(1):137-143.
[Crossref] [Google Scholar] [PubMed]
- Havaldar FH, Vairal DL. Simultaneous determination of paracetamol, acetyl salicylic acid, mefenamic acid and cetirizine dihydrochloride in the pharmaceutical dosage form. E-J Chem. 2010;7(S1):S495-S503.
- Scotter MJ, Roberts DP, Wilson LA, Howard FA, Davis J, Mansell N. Free salicylic acid and acetyl salicylic acid content of foods using gas chromatography–mass spectrometry. Food Chem. 2007;105(1):273-279.
- J. C., Wu, W. Xie, J. Pawliszyn. Automated in-tube solid phase microextraction coupled with HPLC-ES-MS for the determination of catechins and caffeine in tea. Analyst. 2000;2216–2222.
[Crossref] [Google Scholar] [PubMed]
- Russo M, Dugo P, Fanali C, Dugo L, Zoccali M, Mondello L, et al. Use of an online extraction technique coupled to liquid chromatography for determination of caffeine in coffee, tea, and cocoa. Food Anal Methods. 2018;11:2637-2644.
- Gasco-Lopez AI, Izquierdo-Hornillos R, Jiminez A. Development and validation of a high-performance liquid chromatography method for the determination of cold relief ingredients in chewing gum. J Chromatogra A. 1997;775(1-2):179-185.
[Crossref] [Google Scholar] [PubMed]
- Fernandes TA, Aguiar JP, Fernandes AI, Pinto JF. Quantification of theophylline or paracetamol in milk matrices by high-performance liquid chromatography. J Pharmaceut Anal. 2017;7(6):401-405.
[Crossref] [Google Scholar] [PubMed]
- Park JA, Zhang D, Kim SK, Cho SH, Cho SM, Yi H, et al. Simultaneous determination of aminopyrine and antipyrine in porcine muscle, milk, and eggs using liquid chromatography with tandem mass spectrometry. J Sep Sci. 2015;38(23):4048-4054.
[Crossref] [Google Scholar] [PubMed]
Citation: Han D, Ding Y, Peng K, Tang B, Xi C, Deng A (2023) Simultaneous Determination of Paracetamol, Propyphenazone, Aspirin and Caffeine in White Wine Samples by Liquid Chromatography-Triple Quadrupole Tandem Mass Spectrometry. Pharm Anal Acta.14:731.
Copyright: © 2023 Han D, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


