Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Short Commentary - (2020) Volume 11, Issue 2
Short Commentary on TP53 and PTEN Mutations were shared in Concurrent Germ Cell Tumor and Acute Megakaryoblastic Leukemia
Keiichi Akizuki, Takuro Kameda, Kotaro Shide and Kazuya Shimoda*Received: 07-Oct-2020 Published: 28-Oct-2020
Abstract
Concurrent mediastinal Germ Cell Tumors (mGCTs) and hematological malignancies in the same patient have been reported in 2-3% of extragonadal GCT cases. In most cases, the involved GCTs are non-seminomatous and mediastinal, while the Hematological Malignancies (HMs) are often acute megakaryoblastic leukemia. Isochromosome 12p has been frequently detected in both tumors. Recently, two cases of concurrent mGCT and acute myeloid leukemia harboring TP53 and PTEN mutations were reported. We published our research article about the case of a 37-years old male patient with concurrent GCT and acute megakaryoblastic leukemia. Similar to previous studies, TP53 and PTEN mutations were shared in both tumors, in addition to the other seven shared mutations. This suggests that the concurrent occurrence of GCTs and HMs are associated with a common founding clone with a characteristic coexistence of TP53 and PTEN mutations.
Keywords
Acute myeloid leukemia; Germ cell tumor; TP53; PTEN
Description
We provide a short commentary on our previously published article titled “TP53 and PTEN Mutations Were Shared in Concurrent Germ Cell Tumor and Acute Megakaryoblastic Leukemia,” which highlighted the presence of a founding clone with TP53 and PTEN mutations. This founding clone potentially developed two concurrent malignancies of the mediastinal Germ Cell Tumor (mGCT) and Acute Megakaryoblastic Leukemia (AMKL) in one patient [1].
GCTs are the most common malignancies among adolescent males with approximately 2-5% of GCTs occurring in the extragonadal site. Among them, mGCTs occur predominantly within the anterior mediastinum. Although clinical characteristics differ between mGCTs and testicular GCTs, the lack of cytogenetic differences suggests that both are derived from gonadal lesions [2]. Since 1985, approximately 60 cases have reported a possible association between mGCTs and Hematological Malignancies (HMs) [3,4]. In a large, international, multicenter database study including 635 patients with extragonadal GCT, HMs were observed in 17 extragonadal GCTs, with 6% incidence rate of concurrent mGCT and HM [5]. In most cases, the involved GCTs were non-seminomatous and mediastinal, while the HMs were predominantly AMKL. The prognosis of patients with mGCTs and associated HMs is extremely poor, with a median overall survival of 5 months [6]. Historically, chromosomal analysis used to analyze the pathogenesis of these concurrent tumors has elicited frequent presence of isochromosome 12p in both tumors. This finding suggests that HMs and mGCTs potentially arise from common progenitor cells because isochromosome 12p is the most common chromosomal abnormality in GCTs, but is rare in AML without mGCT association [5-8]. However, the mechanism of concomitant disease development was still largely unknown.
Recently, by utilizing whole-exome sequencing, two cases of concurrent mGCT and AML were found to have TP53 and PTEN mutations [9,10]. Interestingly, in our case, as the same result in previous two cases, loss-of-function mutations of TP53 and PTEN were shared in both tumors, in addition to the other seven shared mutations. These three cases suggest that the coexistence of GCTs and HMs may be a biologically unique entity arising from a common founding clone with characteristic concomitant genomic aberrations of TP53 and PTEN. TP53 mutations have been widely observed in a variety of tumors, including AML, although they are uncommon in GCT [11]. Similarly, PTEN mutations have mostly been reported in a number of tumors, except in AML [12]. Mice with heterozygous PTEN deletion demonstrated genomic instability and the development of multiple spontaneous tumors. The simultaneous deletion of TP53 and PTEN in mice promoted tumor genesis and metastasis [13], and its molecular mechanisms might reflect the pathology and dismal prognosis of the concurrent disease of mGCT and AML.
In our case, variant allele frequency values suggest that the initiator clone was formed by four mutations (TP53, PTEN, RLF, DLG2, and YY2) and developed by accumulation of the following four mutations (PCLO, GOLGA8J, EDRF1, and ASF1A) (Figure 1).
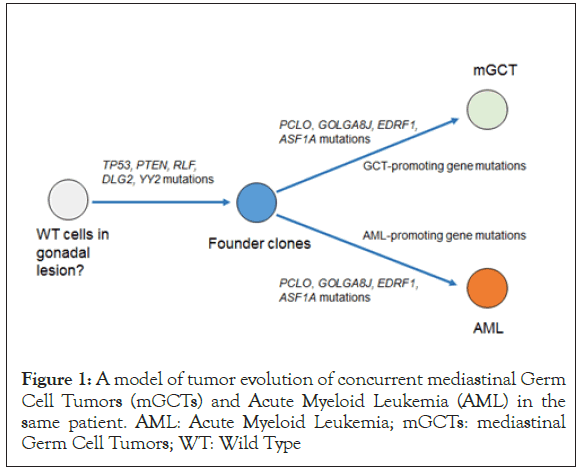
Figure 1. A model of tumor evolution of concurrent mediastinal Germ Cell Tumors (mGCTs) and Acute Myeloid Leukemia (AML) in the same patient. AML: Acute Myeloid Leukemia; mGCTs: mediastinal Germ Cell Tumors; WT: Wild Type
Subsequently, one subclone with GCT-promoting gene mutations and another subclone with HM-promoting gene mutations may develop from the initiator clone, resulting in the development of both tumors. Because the interval between the onset of mGCT and development of HM is usually less than 6 months, we suspect that mGCT-associated HMs develop by a mechanism different from that of general treatment-associated secondary AML or myelodysplastic syndromes which typically develop after a year of exposure to cytotoxic drugs. In these concomitant diseases, a preleukemic clone, with both TP53 and PTEN mutations, was possibly selected and expanded early following treatment with GCTs, resulting in rapid development of AML.
REFERENCES
- Akizuki K, Sekine M, Kogure Y, Kameda T, Shide K, Koya J, et al. TP53 and PTEN mutations were shared in concurrent germ cell tumor and acute megakaryoblastic leukemia. BMC Cancer. 2020; 20:5.
- Chaganti RSK, Rodriguez E, Mathew S. Origin of adult male mediastinal germ-cell tumours. The Lancet. 1994; 343:1130-2.
- DeMent SH, Eggleston JC, Spivak JL. Association between mediastinal germ cell tumors and hematologic malignancies. Report of two cases and review of the literature. Am J Surg Pathol. 1985; 9: 23-30.
- Nichols CR, Hoffman R, Einhorn LH, Williams SD, Wheeler LA, Garnick MB. Hematologic malignancies associated with primary mediastinal germ-cell tumors. Ann Intern Med. 1985; 102: 603-9.
- Hartmann JT, Nichols CR, Droz JP, Horwich A, Gerl A, Fossa SD, et al. Hematologic disorders associated with primary mediastinal nonseminomatous germ cell tumors. J Natl Cancer Inst. 2000; 92:54-61.
- Nichols CR, Roth BJ, Heerema N, Griep J, Tricot G. Hematologic neoplasia associated with primary mediastinal germ-cell tumors. N Engl J Med. 1990; 322:1425-9.
- Gibas Z, Prout GR, Pontes JE, Sandberg AA. Chromosome changes in germ cell tumors of the testis. Cancer Genetics and Cytogenetics. 1986; 19:245-52.
- Heinonen K, Rao PN, Slack JL, Cruz J, Bloomfield CD, Mrozek K. Isochromosome 12p in two cases of acute myeloid leukaemia without evidence of germ cell tumour. Br J Haematol. 1996; 93: 677-80.
- Oshrine BR, Olsen MN, Heneghan M, Wertheim G, Daber R, Wilmoth DM, et al. Acquired isochromosome 12p, somatic TP53 and PTEN mutations, and a germline ATM variant in an adolescent male with concurrent acute megakaryoblastic leukemia and mediastinal germ cell tumor. Cancer Genetics. 2014; 207:153-9.
- Lu C, Riedell P, Miller CA, Hagemann IS, Westervelt P, Ozenberger BA, et al. A common founding clone with TP53 and PTEN mutations gives rise to a concurrent germ cell tumor and acute megakaryoblastic leukemia. Molecular Case Studies. 2016; 2:a000687.
- Lutzker SG. P53 tumour suppressor gene and germ cell neoplasia. APMIS. 1998; 106:85-9.
- Liu TC, Lin PM, Chang JG, Lee JP, Chen TP, Lin SF. Mutation analysis of PTEN/MMAC1 in acute myeloid leukemia. Am J Hematol. 2000; 63:170-5.
- Sun Z, Huang C, He J, Lamb KL, Kang X, Gu T, et al. PTEN C-terminal deletion causes genomic instability and tumor development. Cell Rep. 2014; 6:844-54.
Citation: Akizuki K, Kameda T, Shide K, Shimoda K (2020) Short Commentary on “TP53 and PTEN Mutations were Shared in Concurrent Germ Cell Tumor and Acute Megakaryoblastic Leukemia”. J Data Mining Genomics Proteomics. 11:230. DOI: 10.35248/2153-0602.20.11.230.
Copyright: © Akizuki K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests: The authors have declared that no competing interests exist.

