Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2020) Volume 10, Issue 1
Removal of Polyphenols from Olive Mill Wastewater by FPX 66 Resin: Part II. Adsorption Kinetics and Equilibrium Studies
Aikaterini Vavouraki*Received: 03-Feb-2020 Published: 20-Feb-2020, DOI: 10.35248/2252-5211.20.10.373
Abstract
Adsorption experiments of polyphenols from olive mill wastewater (OMW) using a cross-linked styrene– divinylbenzene polymer namely FPX 66 as a sorbent have been conducted. In particular the process of adsorption of phenolic compounds and carbohydrate derived from OMW by FPX 66 was rapid. A 68 and 60% reduction of polyphenols and carbohydrates, respectively was observed within the first 1 h. FPX 66 resin adsorption capacity increased by increasing polyphenol concentration values. At OMW-effluent-pH below 7.5 polyphenol removal from FPX 66 was high (77%) and for pH greater than 9.0 decreased (40%). Adsorption kinetics and equilibrium studies were carried out, fitting the equilibrium data to both Langmuir and Freundlich models. Batch adsorption models, based on the assumption of the pseudo-first-order, pseudo-second-order and intraparticle diffusion mechanism showed that kinetic data of the adsorption of polyphenols derived from OMW on FPX 66 resin followed the pseudosecond- order than the pseudo-first-order and intraparticle diffusion. Regeneration studies showed that low pH value was efficient for the recovery of phenolic compounds implying that the main mechanism of regeneration might be the chemisorption. These results of adsorption kinetics and equilibrium studies indicate the efficiency of FPX 66 resin as polyphenol sorbent from OMW effluent.
Keywords
Adsorption; Polyphenols; Olive mill wastewaters; OMW; Polymeric resins; FPX 66
Statement of Novelty
The scope of this study was to perform adsorption kinetics and equilibrium studies of polyphenol compounds derived from OMW effluent using FPX 66 sorbent resin. The effects of different parameters on adsorption were monitored and optimal experimental conditions were determined. Adsorption isotherms and kinetic models were examined to describe the experimental findings. Regeneration experiments were also conducted. The novelty of this study lies in suggesting a promising resin of FPX 66 for the adsorption of polyphenols from OMW.
Introduction
Olive mill effluents constitute a serious environmental problem in the Mediterranean Sea region due to high organic load associated with this type of agricultural waste [1]. Polyphenolic compounds contained in OMW effluents are considered to be extremely toxic thus biological degradation of such wastes is difficult [2]. Anaerobic co-digestion of OMW with different agro-industrial wastes could decrease phenolic compound loading [3]. However valorization of OMW may lead to high added value products such as the recovery of phenolic compounds and residual oil [4,5]. Biological, chemical and physical treatment methods have been proposed for the removal of phenolic compounds from OMW effluent [6-8].
In a recent literature review the current status and recent developments in the recovery and removal of phenolic compounds from OMW have been critically examined [8]. Physical adsorption method is generally considered to be the best, effective, low-cost and most frequently used method for the removal of phenolic compounds [9,10].
Limited studies focus on the physical adsorption method of polyphenol removal from OMW. The zeolite, compared to different mineral substrates i.e. clay soil and bentonite proved to be efficient in reducing the organic load, (polyphenol and COD) from OMW. The Low Temperature Ashing technique appears to be a very interesting eco-friendly regeneration technique, capable of reducing polyphenol and COD contents in OMW [11]. Previous studies investigated the efficiency of low cost bio-sorbent such as banana peel for the removal of phenolic compounds from OMW. The banana peel showed a high adsorption capacity of phenolic compounds (688.9 mg/g) [12]. Pretreatment of OMW by sedimentation and filtration processes showed an effective reduction of COD, phenols, and total solids content [13,14] investigated the efficiency of two matrices (i.e. dry-Azolla, granular activated carbon) in adsorbing and desorbing polyphenols from OMW. Zagklis et al. [14] studied the combination of membrane filtration of olive mill wastewater with further treated with resin adsorption/desorption with the non-ionic XAD4, XAD16, and XAD7HP resins.
Limited studies however examine FPX 66 adsorption capacity of polyphenols. FPX 66 resin exhibited optimal decolorization behavior of the sunflower crude extracts and deodorization of cashew apple juice by adsorption of polyphenols on the tested FPX 66 resin [15-17]. FPX 66 showed high adsorption and desorption capacity for polyphenols i.e. theaflavins present in black tea and also, anthocyanins from muscadine juice pomace and blueberry extracts [18-20]. The aim of this work is to perform adsorption kinetics and equilibrium studies of polyphenol compounds derived from OMW effluent using FPX 66 sorbent resin. The effects of operating parameters i.e. OMW effluent pH, sorbent dosage and contact time on adsorption were monitored and optimal experimental conditions were determined. Langmuir and Freundlich adsorption isotherms and kinetic models (pseudo-first, pseudo-second-order kinetics and intraparticle diffusion) were examined to describe these study experimental findings. Finally regeneration experiments were also conducted.
Materials and Methods
Olive mill wastewater (OMW) samples were obtained from a local olive oil mill using a three-phase centrifugation decanter located in Patras (Western Greece). Prior to experiments OMW samples were centrifuged twice (4 000 rpm HERMLE Labortechnik). OMW samples were stored immediately after sampling in the freezer at -18°C until further experimental use. The physicochemical composition analysis of raw OMW is summarized in Table 1 [21]. Adsorbent resin of FPX 66 (Rohm and Haas Co.) consist of a crosslinked styrene–divinylbenzene polymer with BET surface area, as determined by N2 adsorption (BET Micromeritics Gemini III) 759.26 ( ± 5.63) m2/g.
| Parameters | OMW |
|---|---|
| pH (25°C) | 5.24 |
| TS (g/L) | 82.53 (±0.02) |
| VS (g/L) | 65.84 (±0.02) |
| TKN (g/L) | 0.81 (±0.02) |
| Ammonium nitrogen (g/L) | 0.12 (±0.04) |
| Total carbohydrates (g eq. glucose/L) | 30.33 (±0.04) |
| Soluble carbohydrates (g eq. glucose/L) | 24.88 (±0.02) |
| Oil & Grease (g/L) | 12.75 (±0.05) |
| Total phosphorus (g/L) | 0.48 (±0.04) |
| Soluble phosphorus (g/L) | 0.31 (±0.02) |
| Total COD (g O2/L) | 140.0 (±2.0) |
| Dissolved COD (g O2/L) | 66.3 (±1.0) |
| Total phenols (g eq. phenol/L) | 4.3 (±0.1) |
Table 1: Physicochemical characteristics of raw OMW samples.
For the adsorption kinetic experiments, pre-weighted amounts of FPX 66 resin (1% ratio of solid: solution) were placed into centrifuged OMW. Prior to adsorption experiments tested resin were wetted in acetone for 8 h and then dried for 2 h under vacuum at room temperature (~25°C) [22]. All solutions were sealed and shaken in an orbital shaking water bath (Grant OLS200) at 125 rpm at constant temperature (25°C) for 24 h. The time required to reach equilibrium was ascertained when phenolic compound (adsorbate) concentration (equivalent phenol/L) in the solution remained constant. During the course of the adsorption/ desorption, samples were withdrawn, filtered through syringe filters (0.2 μm Nylon Whatman) in order to separate the liquid from the solid (resin).
The amount of resin collected on the filters was kept for further regeneration tests whereas the phenolic content in the filtered samples was determined spectrophotometrically at 760 nm (Cary 50 Varian UV/Vis) according to the Folin–Ciocalteu method [21]. Additionally, soluble carbohydrate content (equivalent glucose/L) were determined by forming a colored sugar derivative, through the addition of L-tryptophan, sulphuric and boric acid solution that was then measured spectrophotometrically at 520 nm [23].
Adsorption Experiments
The background theory of both Langmuir and Freundlich adsorption isotherms which were applied to describe the adsorption of polyphenol molecules (adsorbate) on the resin surface (adsorbent) was describe in previous study [24]. Initially the effects of different parameters i.e. contact time, adsorbent dosage, pH on equilibrium adsorption experiments of phenolic compounds from raw OMW were defined. Both important isotherm equations of Langmuir and Freundlich were adjusted to this study experimental values. The adsorbent used for the adsorption experiments of OMW was separated from the solution by filtration (membrane filtres 0.45 μm Whatman) and washed gently with water to remove any unabsorbed phenolic compounds. Regeneration experiments were also conducted right after adsorption experimental OMW samples with 20% FPX 66 resin. Prior to performing regeneration experiments with a solvent mixture of 50/50 (v/v) ethanol/ isopropanol FPX 66 resin was washed firstly with distilled water. Batch regeneration experiments were subjected to agitation for 24 h.
Results and Discussion
Effects of different parameters on equilibrium adsorption experiments of phenolic compounds from raw OMW
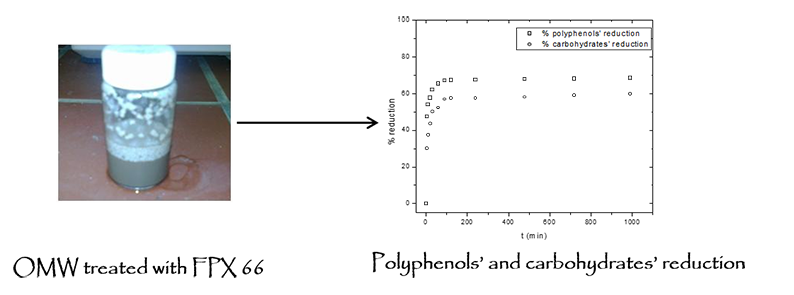
Effect of contact time: The adsorption rate of phenolic compound from OMW effluent by different adsorbents is very rapid and the adsorption equilibrium can reach in less than 24 h. Achak et al. [10] observed that the adsorption equilibrium of phenolic compounds derived from OMW effluent was obtained after 3 h with an adsorption of approximately 96% of the phenolic compounds. Azzam et al. [10] also noticed that after 2 h, no significant reduction in phenols took place after a series of adsorption steps with activated carbon as adsorbent. Figure 1 presents the equilibrium time needed for the adsorption of both phenolic compounds’ and carbohydrate from OMW effluent by FPX 66 resin. The adsorption of phenolic compounds and carbohydrate derived from OMW by FPX 66 was very rapid due to the fact that the largest amount of phenol and carbohydrate (68 and 60% reduction, respectively) was removed with in the first 1 h. The rapid adsorption of phenolic compounds to FPX 66 ensures that sufficient time is available for adsorption equilibration. Initially, the adsorption process of both phenolic compounds and carbohydrate is very fast due to the available vacant surface sites of FPX 66 within the first hour of adsorption therefore the amount of both phenolic compound and carbohydrate concentration increases rapidly. Consequently the remaining vacant surface sites are difficult to be occupied due to formation of repulsive forces between both the phenolic compounds and carbohydrate on the solid surface and also with the bulk phase. Both phenolic compounds and carbohydrate have to penetrate deeper into the pores encountering much higher resistance. Subsequently a plateau of the adsorption is observed [12]. Additionally to the phenolic compounds and carbohydrate contained in OMW effluent competition of other types of compounds such as aminoacids and proteins may occur during adsorption on specific sites contributing to the decrease of the adsorption rate of the targeted phenolic compounds from OMW. Adsorption rate of phenolic compounds on FPX 66 was found to be relatively equal with those reported for some other normal adsorbents (banana peel, activated carbon). However, for subsequent experiment, the samples were left for 12 h to ensure the adsorption equilibrium.
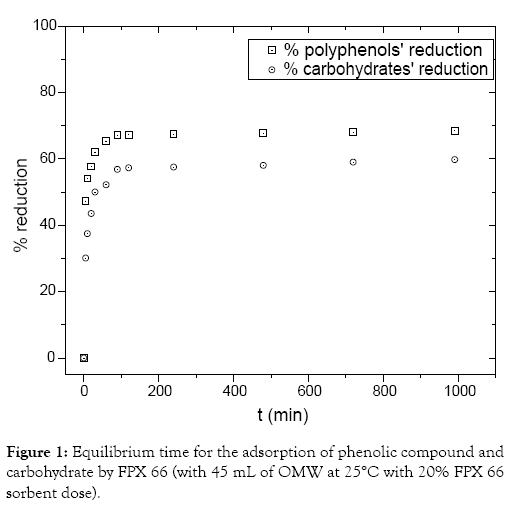
Figure 1: Equilibrium time for the adsorption of phenolic compound and carbohydrate by FPX 66 (with 45 mL of OMW at 25°C with 20% FPX 66 sorbent dose).
Effect of adsorbent dosage: The effect of adsorbent FPX 66 dosage is investigated at initial OMW phenol concentration (4.15 g eq. phenol/L). The experiments of the adsorbent dosage effect were conducted on 65 mL OMW. Agitation was maintained for 24 h (125 rpm) at (25 ± 2°C) and initial pH -5.24 at different adsorbent dosage (5 to 40%). Figure 2 shows that the increase in adsorbent dosage from 5 to 40% resulted in a increase from 20 to 70% OMW phenolic and/or carbohydrate reduction. In general the increase in the adsorbent dosage increases the number of available adsorption sites thus the amount of adsorbed both phenolic and carbohydrate compound increases. However this is not always the case. The augmentation of adsorbent dosage over 20% gave ~70 and 60% of phenolic and carbohydrate reduction, respectively. The limitation of the adsorption capacity at high adsorbent dosage may be attributed to the complicated molecular structure of polyphenols including long chains blocking adsorbent active sites. Consequently all our subsequent experiments were performed at an adsorbent dosage of 20% of FPX 66 resin.
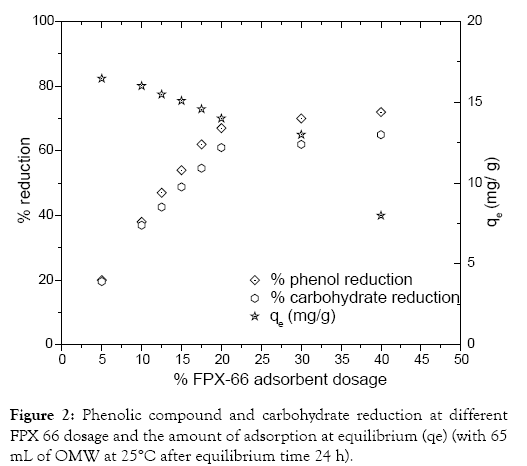
Figure 2: Phenolic compound and carbohydrate reduction at different FPX 66 dosage and the amount of adsorption at equilibrium (qe) (with 65 mL of OMW at 25°C after equilibrium time 24 h).
The results concerning the influence of initial polyphenol concentration (g equiv. phenol/L) are presented in Figure 3. When polyphenol concentration increases, the adsorption capacity also increases for the same FPX 66 dosage (20%). The amount of phenolic compounds adsorbed at equilibrium (qe) increased from 5 to 14 mg/g when the phenol concentration changed from 1.4 to 4 g/L. In previous adsorption assay of the removal of phenols from coke wastewater with granular activated carbon as adsorbent [25] indicated that the increase of phenol induces the augmentation of the adsorption capacity also increases for the same dosage of adsorbent.
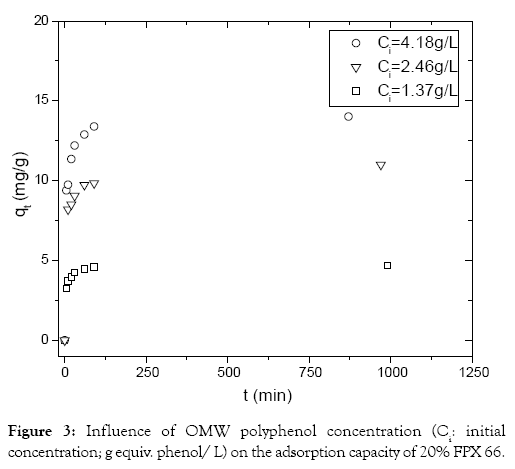
Figure 3: Influence of OMW polyphenol concentration (Ci: initial concentration; g equiv. phenol/ L) on the adsorption capacity of 20% FPX 66.
Effect of pH on phenolic compounds biosorption: The most important parameter influencing the adsorption capacity is the pH of adsorption medium. pH solution of an adsorption medium affects the adsorption mechanisms on the adsorbent surface and influences the nature of the physicochemical interactions of the species in solution and the adsorptive sites of adsorbents [12]. The experiments of the pH effect were conducted on 45 mL OMW. Agitation was maintained for 18 h (125 rpm) at (25 ± 2°C) at the predetermined optimal adsorbent dosage (20%).
The adsorption behaviour of phenolic compounds from OMW was studied under various OMW effluent pH conditions (pH 3 to 11; Figure 4). At initial OMW pH 5.24 phenolic compounds’ and carbohydrates’ reduction reached 68 and 60%, respectively whereas the adsorption capacity of phenols (qe,ph, qe,ch) and carbohydrates on FPX 66 tends to decrease. Phenol removal from FPX 66 was high for OMW solution pH below 7.5, and decreased (40%) for pH greater than 9.0. This is due to phenol solubility increasing at higher pH values [26]. Thus high elimination (77%) of phenolic compounds derived from OMW was achieved in low pH values (pH 3 to 7.5). Considering that there is no necessity to very accurate adjustment of OMW effluent pH for the removal of phenolic compounds it is of great interest from a practical point of view. Similar experimental findings were reported in other studies considering phenols’ adsorption by Amberlite resins (namely XAD- 4 and XAD-7, respectively) from alkaline solution conditions [27-29]. Phenols act as weak acids in aqueous solution thus potential pH change in the solution has an impact on the dissociation of phenol’s hydrogen ion. In acidic conditions the molecular form dominates whereas in alkaline solution the anionic form predominates. Experimental results show that OMW-phenols are removed effectively (77%) by FPX 66 resin at acidic medium and reduce at alkaline solutions but still significant (40%). Therefore, OMW phenols’ adsorption may be considered mainly as non-polar phenols- resin interaction inducing van der Waals forces [29,30]. This behavior provides potential for recovery of the adsorbate as well as regeneration of adsorbent by simple non-destructive methods, such as solvent washing [30].
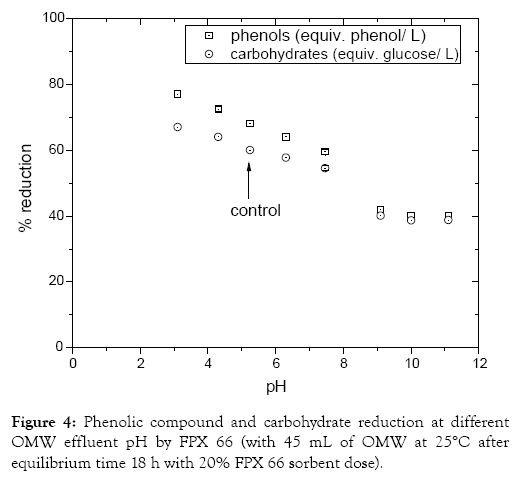
Figure 4: Phenolic compound and carbohydrate reduction at different OMW effluent pH by FPX 66 (with 45 mL of OMW at 25°C after equilibrium time 18 h with 20% FPX 66 sorbent dose).
The effect of solution pH on the removal of various phenols from aqueous solutions by adsorption can be discussed by considering the theoretical distribution of various phenol species in aqueous solutions. Since most phenols (POHs) act like weak acids in aqueous solution, the dissociation of hydrogen ion from the phenol molecules strongly depends on the pH level of solution (Eq. 1):
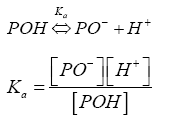 (1)
(1)
where [POH] and [PO] represents the concentrations of the molecular and dissociated ionic phenol species, respectively, and Ka is the dissociation constant of phenols. Thus, the distribution of POH and PO species can be calculated as a function of solution pH. The molecular species, POH, is the predominant in acidic solutions, whereas the ionic species, PO, predominates in alkaline solutions [28]. Aksu et al. [31] studied the adsorption of phenols on dried sludge reporting similar increase of adsorption at lower pH values, and a decrease at higher pH values. Due to pH increase adsorbent surface charge is being modified thus the number of negatively charged sites increases. A negatively charged surface site on the adsorbent does not favour the adsorption of phenolate ions due to the electrostatic repulsion. Additionally lower adsorption of phenolic compounds derived from OMW at alkaline pH is due to the presence of excess OH¯ ions competing with the phenolate ions (anions) for the adsorption sites. At alkaline pH significant adsorption (40 and 55% phenolic and carbohydrate removal) of the anionic compounds on the adsorbent FPX 66 still occurred.
However other studies showed that phenol biosorption capacity was strongly infuenced by the pH of the aqueous solution with an observed maximum phenol removal at pH around 6 [30,32,33]. Lin et al. [33] reported that adsorption capacity of phenol onto hydroxyapatite nanopowders was decreased with increasing pH up to 8 and then increased with further increasing the pH to alkaline value (maximal adsorption capacity at pH 2). The adsorption efficiency of phenolic compounds from OMW by banana peel increased at high pH solution (pH 7) [12].
Adsorption isotherms
Vavouraki et al. [23] studied adsorption equilibrium by describing both important isotherm equations of Langmuir and Freundlich. Table 2 presents the isotherm constants and the correlation coefficients of both Langmuir and Freundlich isotherms. Adsorption experiments of phenolic compounds on FPX 66 were conducted to determine the equilibrium isotherm(s). Standard volume of 100 mL of OMW effluent was used at known concentration of phenolic compounds (4 g/L). The system was agitated for 12 h (125 rpm) at constant temperature (25°C) at initial pH 5 at five adsorbent dosage (5, 10, 12.5, 15, 17.5, 20%). Figure 5 demonstrates the adsorption isotherms of Langmuir (Figure 5a) and Freundlich (Figure 5b) of phenolic compounds’ adsorption using FPX 66 resin. Both Langmuir and Freundlich isotherm constants and the correlation coefficients were calculated (Table 2). Derived correlation coefficient values (R2) prove that the adsorption of phenolic compounds from OMW is well fitted to both Langmuir and Freundlich models. The calculated parameters for phenols compare very well to reported parameters in previous preliminary study [24]. Buran et al. [19] studied the adsorption of anthocyanins and polyphenols from blueberries using macroporous adsorbent resins including FPX 66. Equation constants and correlation coefficients of both Langmuir and Freundlich isotherms were defined and lower compared to our study.
| Effluent | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qm | b | RL | R2 | KF | N | R2 | |
| mg/g | L/mg | mg1-n.Ln/g | |||||
| OMW | 19 | 0.002 | 0.1 | 0.9999 | 3.74 | 5.4 | 0.9797 |
aAdsorption is at controlled temperature (25°C) and is based on total phenolic compounds (in equivalent phenol/L).
Table 2: Langmuir and Freundlich isotherm constants and correlation coefficients (R2) for the adsorption of phenolic compounds in OMW samples by FPX 66a.
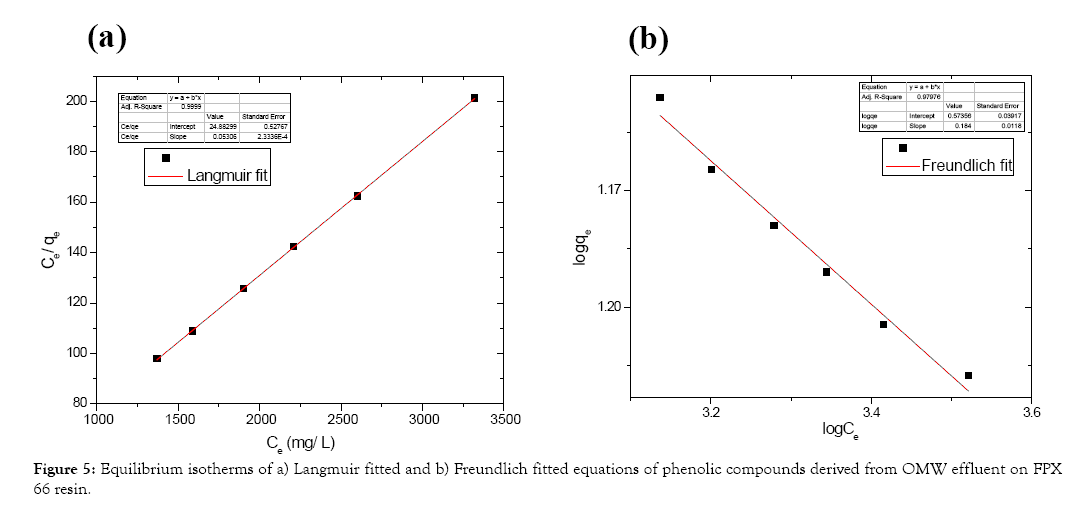
Figure 5: Equilibrium isotherms of a) Langmuir fitted and b) Freundlich fitted equations of phenolic compounds derived from OMW effluent on FPX 66 resin.
Adsorption kinetics
Kinetic model of phenolic compounds adsorption: Adsorption kinetics has been proposed to elucidate the adsorption mechanism. The mechanism of adsorption depends on the physical and chemical characteristics of the adsorbent as well as on the mass transport process. The mechanism of the adsorption of phenolic compounds derived from OMW was investigated and the potential rate-controlling step, i.e., mass transfer or chemical reaction was examined. In this study the capability of pseudo-first-order and pseudo second- order kinetic models was examined. The equations for the pseudo-first-order and pseudo-second-order kinetics are presented (Eq. 2 and Eq. 3, respectively):
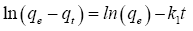 (2)
(2)
 (3)
(3)
where qe and qt refer to the amount of phenolic compounds adsorbed (mg/g) at equilibrium and at any time, t (h), respectively; k1 (h−1) is the equilibrium rate constant of pseudo-first-order sorption and k2 (g/g h) is the rate constant for pseudo-second-order kinetics. Table 3 presents the measured kinetic model values thus theoretical qe values, rate constants (k1, k2), correlation coefficients (R1, R2). The correlation coefficient of pseudo second-order equation 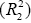 is
higher than that of pseudo-first-order
is
higher than that of pseudo-first-order 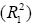 . Adsorption experiments of OMW polyphenols on different adsorbent FPX 66 dosages (5 to 20%) showed that when the dosage is increased, the adsorption capacity decreased in both kinetic models. For pseudo-first-order adsorption kinetic the adsorption capacity decreased from 11.3 to 6.4 mg/g for 5 to 20% FPX 66, respectively. For pseudo-secondorder adsorption kinetic the adsorption capacity decreased from 20 to 13.3 mg/g for 5 to 20% FPX 66, respectively. The theoretical qe value calculated from pseudo-second-order was more close to the experimental qe value (17 mg/g) than from pseudo-firstorder. Therefore the pseudo-second-order kinetic model fits for the adsorption of polyphenols derived from OMW on the FPX 66 resin. Similar findings were observed by Achak et al. [10] and proposed that possible chemical sorption in the adsorption process may occur indicating the rate-limiting step for the process. Similar finding to our study Buran et al. [19] suggested that the pseudo second order model appeared to be better than pseudo first order in describing the adsorption kinetics of total phenolics from blueberry water extracts on macroporous adsorbent resins i.e. FPX 66.
. Adsorption experiments of OMW polyphenols on different adsorbent FPX 66 dosages (5 to 20%) showed that when the dosage is increased, the adsorption capacity decreased in both kinetic models. For pseudo-first-order adsorption kinetic the adsorption capacity decreased from 11.3 to 6.4 mg/g for 5 to 20% FPX 66, respectively. For pseudo-secondorder adsorption kinetic the adsorption capacity decreased from 20 to 13.3 mg/g for 5 to 20% FPX 66, respectively. The theoretical qe value calculated from pseudo-second-order was more close to the experimental qe value (17 mg/g) than from pseudo-firstorder. Therefore the pseudo-second-order kinetic model fits for the adsorption of polyphenols derived from OMW on the FPX 66 resin. Similar findings were observed by Achak et al. [10] and proposed that possible chemical sorption in the adsorption process may occur indicating the rate-limiting step for the process. Similar finding to our study Buran et al. [19] suggested that the pseudo second order model appeared to be better than pseudo first order in describing the adsorption kinetics of total phenolics from blueberry water extracts on macroporous adsorbent resins i.e. FPX 66.
| OMW Effluent | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (h-1) |  |
qe (mg/g) | k2 (g/g h) |  |
|
| FPX-66 dosage | ||||||
| 5% | 11.3 | 1.20 | 0.83 | 20 | 0.15 | 0.95 |
| 10 | 11.5 | 1.06 | 0.89 | 14.5 | 0.43 | 0.97 |
| 15 | 8.9 | 1.43 | 0.87 | 14 | 1.01 | 0.96 |
| 20 | 6.4 | 1.72 | 0.82 | 13.3 | 1.88 | 0.94 |
Table 3: Kinetics constants and the correlation coefficients for pseudo-first and pseudo-second order kinetic models regarding OMW effluent adsorption on FPX 66 resin.
Diffusion model: Achak et al. [9] described the intraparticle diffusion model (Eq. 4):
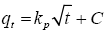 (4)
(4)
where qt is the amount of phenolic compounds adsorbed at equilibrium (mg/g) at time t, kp is the intraparticle diffusion rate constant (g/gh1/2) and C is the intercept (mg/g).
The correlation coefficients were calculated for different phenol concentration values for 20% FPX 66 (Table 4). All correlation coefficient values are by far lower than that of the pseudo-secondorder model. Therefore, the pseudo-second order model prevails among the three kinetic models to describe the adsorption behavior of phenolic compounds onto FPX 66 resin. The pseudosecond- order adsorption mechanism seems to be predominant and the overall rate of the adsorption process appears to be controlled by the chemical reaction.
| Polyphenol concentration | Kp | C | R2 |
|---|---|---|---|
| g eq. phenol/L | mg/gh1/2 | mg/g | |
| 1.37 | 0.60 | 2.98 | 0.13 |
| 2.46 | 1.58 | 6.26 | 0.19 |
| 4.18 | 2.12 | 8.24 | 0.21 |
Table 4: The parameter of the intraparticle diffusion model for the adsorption capacity of 20% FPX 66.
Regeneration studies: Regeneration experiments were performed in a water bath shaker at 125 rpm for 24 h at controlled temperature (25°C). The percentage of recovered polyphenolic compound and carbohydrate values were calculated using the Eq. 5:
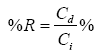 (5)
(5)
where Ci and Cd is the initial concentration of polyphenols (or carbohydrate) and concentrations of polyphenols (mg eq. phenol/L) (or carbohydrate; mg eq. glucose/L) in tested OMW effluent, respectively. The pH OMW effluent during regeneration experiments was corrected during adsorption experiments.
As the effluent pH was increased, the percentage of recovery of polyphenols during resin-regeneration decreased from 70 at pH 4 to 60 and 40% at alkaline pH values (Figure 6). Additionally the percentage of recovery of carbohydrate during resin regeneration decreased from 60 and 55% at pH 4 and 7.5, respectively to 40 and 35% at alkaline pH values. In agreement with previous study [9] the recovery of phenolic compounds increased in an acidic medium and decreased in an alkaline medium implying that the main mechanism of regeneration might be the chemisorption. Thus the recovery experiments of polyphenols and carbohydrate carried out with an organic mixture of 50/50 (v/v) ethanol/isopropanol suggested that the adsorption is held by the adsorbent through chemisorption. At low pH values the number of positive charged sites exceeds. A positively charged surface site on the adsorbent favours the recovery of phenolic compounds due to electrostatic repulsion. At pH 4, a significantly high electrostatic repulsion exists between the positively charged surface of the adsorbent and phenolic compounds.
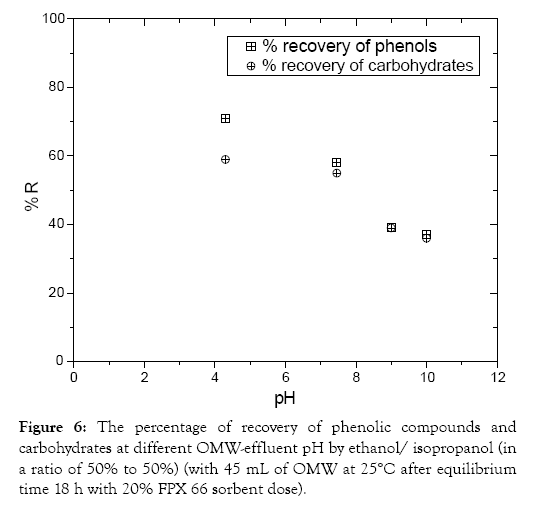
Figure 6: The percentage of recovery of phenolic compounds and carbohydrates at different OMW-effluent pH by ethanol/ isopropanol (in a ratio of 50% to 50%) (with 45 mL of OMW at 25°C after equilibrium time 18 h with 20% FPX 66 sorbent dose).
Conclusion
Langmuir and Freundlich isotherm models were very well fitted to describe the adsorption process of polyphenols derived from OMW on FPX 66 resin. The adsorption kinetic of phenolic compounds derived from OMW onto FPX 66 can be best fitted by the pseudosecond- order model. At initial pH 5.24 of OMW a 68% and 60% reduction of phenolic compounds and carbohydrates, respectively was observed. Regeneration of adsorbent resin with an organic mixture of ethanol/isopropanol (in a ratio of 50 to 50%) showed a 70% and 60% recovery of polyphenols and carbohydrates, respectively at pH 4.
REFERENCES
- Paraskeva P, Diamadopoulos E. Review technologies for olive mill wastewater (OMW) treatment: a review. J Chem Technol Biotechnol. 2006;81:1475-1485.
- Obied HK, Allen MS, Bedgood DR, Prenzler PD, Robards K. Bioactivity and analysis of biophenols recovered from olive mill waste. J Agric Food Chem. 2005; 53 :823-837.
- Vavouraki AI, Zakoura MA, Dareioti MA, Kornaros M. Biodegradation of polyphenolic compounds from olive mill wastewaters (OMW) during two-stage anaerobic co-digestion of agro-industrial mixtures. Waste Biomass Valor. 2019;1-9.
- Karakaya A, Kenar A, Laleli Y, Takaç S. A novel two-step process to co-valorize antioxidant rich by-products of olive and grape processing industries. Waste Biomass Valor. 2017; 8 :829-837.
- Tzathas K, Chrysagi E, Lyberatos G, Vlyssides A, Vlysidis A. Pretreatment of olive mill wastes for the extraction of residual oil and high added value compounds. Waste Biomass Valor. 2019.
- Mantzavinos D, Kalogerakis N. Treatment of olive mill effluents. Part I. Organic matter degradation by chemical and biological processes an overview. Environ Int. 2005;31:289-295.
- Kalogerakis N, Politi M, Foteinis S, Chatzisymeon E, Mantzavinos D. Recovery of antioxidants from olive mill wastewaters: A viable solution that promotes their overall sustainable management. J Environ Manag. 2013; 128 :749-758.
- Rahmanian N, Jafari SM, Galanakis CM. Recovery and removal of phenolic compounds from olive mill wastewaters. J Am Oil Chem Soc. 2014; 91 :1-18.
- Achak M, Hafidi A, Ouazzani N, Sayadi S, Mandi L. Low cost bio-sorbent “banana-peel” for the removal of phenolic compounds from olive mill wastewater. J Hazard Mater. 2009; 166 :117-125.
- Achak M, Mandi L, Ouazzani N. Removal of organic pollutants and nutrients from olive mill wastewater by a sand filter. J Environ Manage. 2009; 90 :2771-2779.
- Santi CA, Cortes S, D’Acqui LP, Sparvoli E, Pushparaj B. Reduction of organic pollutants in Olive Mill Wastewater by using different mineral substrates as adsorbents. Bioresour Technol. 2008; 99 :1945-1951.
- Buran Azzam MOJ, Al-Malah K, Al-Gazzawi Z, Al-Omari SA. Dynamic treatment response of olive mills wastewater using series of adsorption steps. Clean-Soil Air Water. 2010; 38 :822-830.
- Ena A, Pintucci C, Carlozzi P. The recovery of polyphenols from olive mill waste using two adsorbing vegetable matrices. J Biotechnol. 2012; 157 :573-577.
- Zagklis DP, Vavouraki AI, Kornaros ME, Paraskeva CA. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J Hazard Mater. 2015; 285 :69-76.
- Weisz GM, Schneider L, Schweiggert U, Kammerer DR, Reinhold C. Sustainable sunflower processing - I. Development of a process for the adsorptive decolorization of sunflower [Helianthus annuus L.] protein extracts. Innov Food Sci Emerg Technol. 2010; 11 :733-741.
- Sousa AD, de Brito ES. Effect of treatment with adsorbent resin on the volatile profile and physicochemical characteristics of clarified cashew apple juice. Food Sci Technol. 2013; 33 :619-623.
- Monsanto M, Mestrom R, Zondervan E, Bongers P, Meuldijk J. Solvent swing adsorption for the recovery of polyphenols from black tea. Ind Eng Chem Res. 2015; 54 :434-442.
- Sandhu AK, Gu L. Adsorption/ desorption characteristics and separation of anthocyanins from muscadine (Vitis rotundifolia) juice pomace by use of macroporous adsorbent resins. J Agric Food Chem. 2013; 61 :1441-1448.
- Buran TJ, Sandhu AK, Li Z, Rock CR, Yang WW. Adsorption/desorption characteristics and separation of anthocyanins and polyphenols from blueberries using macroporous adsorbent resins. J Food Eng. 2014; 128 :167-173.
- Dareioti MA, Dokianakis SN, Stamatelatou K, Zafiri C, Kornaros M. Biogas production from anaerobic co-digestion of agroindustrial wastewaters under mesophilic conditions in a two-stage process. Desalination. 2009; 248 :891-906.
- Agalias A, Magiatis P, Skaltsounis AL, Mikros E, Tsarbopoulos A. A new process for the management of olive oil mill waste water and recovery of natural antioxidants. J Agric Food Chem. 2007; 55 :2671-2676.
- Josefsson B, Uppström L, Östling G. Automatic spectrophotometric procedures for the determination of the total amount of dissolved carbohydrates in sea water. Deep-Sea Res. 1972;19:385-395.
- Vavouraki AI, Dareioti MA, Kornaros M. Olive Mill Wastewater (OMW) Polyphenols Adsorption onto Polymeric Resins: Part I. Batch Anaerobic Digestion of OMW. Waste Biomass Valor. 2019.
- Vazquez I, Rodrıguez-Iglesias J, Maranon E, Castrillon L, Alvarez M. Removal of residual phenols from coke wastewater by adsorption. J Hazard Mater. 2007; 147 :395-400.
- Aly AA, Hasan YNY, Al-Farraj A. Olive mill wastewater treatment using a simple zeolite-based low-cost method. J Environ Manage. 2014; 145 ;341-348.
- Young CC. Non-polar macroreticular resin to recover phenolic acids from a subtropical latosol. Soil Biol Biochem. 1984; 16 :377-380.
- Ku Y, Lee KC. Removal of phenols from aqueous solution by XAD-4 resin. J Hazard Mater. 2000; B80 :59-68.
- Pan B, Zhang W, Zhang Q, Zheng S. Adsorptive removal of phenol from aqueous phase by using a porous acrylic ester polymer. J Hazard Mater. 2008; 157 :293-299.
- Abburi K. Adsorption of phenol and p-chlorophenol from their single and bisolute aqueous solutions on Amberlite XAD-16 resin. J Hazard Mater B. 2003;105 :143-156.
- Aksu Z, Yener J. A comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents. Waste Manage. 2001; 21 :695-702.
- Dursun G, Çiçek H, Dursun AY. Adsorption of phenol from aqueous solution by using carbonised beet pulp. J Hazard Mater. 2005; B125 :175-182.
- Thawornchaisit U, Pakulanon K. Application of dried sewage sludge as phenol biosorbent. Bioresour Technol. 2005; 98 :140-144.
- Lin K, Pan J, Chen Y, Cheng R, Xu X. Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders. J Hazard Mater. 2009; 161 :231-240.
Citation: Vavouraki A (2020) Removal of Polyphenols from Olive Mill Wastewater by FPX 66 Resin: Part II. Adsorption Kinetics and Equilibrium Studies. Int J Waste Resour 10:373. doi: 10.35248/2252-5211.20.10.373
Copyright: © 2020 Vavouraki A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.