Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 13, Issue 5
Pyrolysis of Different Biomass Wastes for the Production of Biochar: Evaluation of Yield and Physiochemical Characteristics
Farooque Ahmed1*, Mahmood Laghari1, Moazam Ali1, Liaquat Ali1, Ali Raza1 and Azharuddin Chachar22Department of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai, China
Received: 09-Jul-2023, Manuscript No. IJWR-23-22084; Editor assigned: 11-Jul-2023, Pre QC No. IJWR-23-22084 (PQ); Reviewed: 01-Aug-2023, QC No. IJWR-23-22084; Revised: 08-Aug-2023, Manuscript No. IJWR-23-22084 (R); Published: 15-Aug-2023, DOI: 10.35248/2252-5211.23.13.549
Abstract
Today, one of the biggest issues facing climate change is carbon emissions from biomass burning. However, if biomass can be converted into biochar through the pyrolysis process and stored in the ground, it will reduce carbon emissions from the atmosphere and allow carbon to be sequestered from the environment. It could be a sustainable solution to global challenges such as climate change, waste management, and soil improvement. This experiment was conducted for the preparation of biochar from different waste biomass, i.e., cow dung, poultry manure, municipal sewage sludge, and waste wood pieces, which were pyrolyzed in a slow pyrolysis reactor. The waste material was collected, then sun- and oven-dried before being placed in the reactor using an aluminum box. Afterwards, different biomass samples were subjected to slow pyrolysis at 600°C for one hour under limited oxygen conditions. After one hour, the reactor was opened, and the biochar was taken out of it and stored in airtight sample bags for testing. Results showed that different yields of biochar were obtained with different types of biomass materials. Municipal sewage sludge produced the highest biochar yield (66.23%), and Russian waste wood produced the lowest biochar yield (23.34%). The basic properties of the biochar, such as pH, EC, WHC, and ash content, also varied with the type of biomass used. Different biochars were alkaline in nature, with a maximum water holding capacity of 9.6 g/g in Aak (Calotropis gigantea) wood biochar. The biochars were also rich in some plant nutrients, with maximum N and P contents of 2.6% and 3.51%, respectively, in poultry manure biochar.
Keywords
Wood biochar; Slow pyrolysis; Biochar characterization; Plant nutrients
Abbreviations
pH:Power of hydrogen; EC:Electrical Conductivity; WHC:Water Holding Capacity; AC:Ash Content; N:Nitrogen; P:Phosphorus; K:Potassium; Ca:Calcium; Na:Sodium
Highlights
• Biochar produced from different types of biomass, such as cow dung, poultry manure, municipal sewage sludge, and waste wood, can effectively sequester carbon and reduce carbon emissions.
• Municipal sewage sludge resulted in the highest biochar yield (66.23%), while Russian waste wood had the lowest yield (23.34%).
• The biochar derived from various biomass sources exhibited different properties, including alkaline nature, water holding capacity, and nutrient content, making it a potentially valuable resource for soil improvement and waste management.
Introduction
The production of biochar primarily involves thermochemical conversion procedures with a range of process variables, such as slow pyrolysis, quick pyrolysis, Torre faction, and gasification [1]. The physical condition and chemical structure of biomass are permanently altered by these methods to create biochar. They occur at certain temperatures and pressures in the absence or with a constrained supply of oxygen. When the chemical components of renewable biomass are severely cross-linked, broken down, and depolymerized, biochar forms as a solid carbon residue. In addition, depending on the reaction conditions, bio-oil or tar, an organic liquid that may condense, and a non-condensable combustible gas composed of hydrogen, carbon dioxides, light hydrocarbons, and other chemicals may also be produced [2]. Biochar has been proposed as a strategy to generate energy, improve soil quality, increase Carbon sequestration (C), and improve the quality of the environment [3-5]. The various benefits of biochar demonstrate how it may enhance the economic sustainability of creating systems for producing cellulosic bioenergy [6-8]. On the other hand, applying biochar to the ground can reduce net emissions of greenhouse gases and permanently sequester carbon in the soil [9-10]. It can also increase crop production and reduce nutrient, sediment, and pollutant loss through improved nutrient availability and the physical, chemical, and biological features of the soil [11-15]. Utilizing biochar can help replace essential organic matter that was lost when biomass from forestry or agricultural systems was removed for energy production, in addition to assisting the soil in retaining carbon. Consequently, biochar may offer two simultaneous financial benefits. First, it may enhance the biomass generation process's capacity for sustainable and agronomic expansion. Second, balancing material purchases with money from charcoal earnings may increase the economic viability of bioenergy businesses. The impact of biochar on agronomic, environmental, and soil qualities is yet largely unknown. The agricultural and bioenergy sectors won't be willing to pay for biochar until its exact effects on soil characteristics and crop production are demonstrated, despite the fact that it has the potential to create income and improve sustainability in agriculture and the environment. Biochar must first demonstrate particular advantages to crop productivity and soil characteristics, and then tie these advantages to the features of biochar, as well as to its proper application and financial worth, in order to be fully developed as a commercial commodity. One of the most important components in making this a reality is the capacity to understand how this product is made and how the production method affects its performance. Its benefits for soil health, agricultural productivity, and the environment will only matter if it can be copied and used consistently. However, Pakistan generates a significant amount of organic waste, including food waste, human waste, manure waste, and many more types. The environment becomes contaminated when garbage is not disposed of in an appropriate manner for each type of waste. In order to protect the environment, biomass waste needs to be carefully handled and put to use. Therefore, this study focused on making biochar from various forms of biomass, and its goal was to investigate the possible applications for biochar, such as enhancing soil fertility, producing biofuel, making bio-adsorbents, etc. based on their fundamental characteristics.
Material and Methods
Collection of material
The waste material cow dung, poultry manure, municipal sewage sludge, mango waste wood, conocarpus waste wood, Russian Diyar waste wood, and two desert plant Aak (Calotropis gigantea) and Thoohar (Euphorbia neriifolia L.) wood were chosen to be utilized as the feedstock for making biochar. The desert plant (Aak and Thoohar wood) was collected from Tharparkar desert and all other waste materials were collected from nearby farm of Sindh Agriculture University Tandojam.
Pre-treatment of feedstock
The pieces of wood were cut down into small pieces according to the size of our pyrolysis reactor. The wood pieces were sun-dried for many days and then oven-dried at 105°C before the pyrolysis.
Pyrolysis
A certain amount of oven-dried sample was placed in an aluminum tray. The tray was housed in the pyrolysis reactor, and then it was packed using the top plate with nuts and bolts. The seal was placed between the top plate and the surface of the walls to create an oxygen-limited internal environment. The reactor was placed in the locally modified kiln at 600°C for one hour. Subsequently, the kiln was heated up at 600°C, and the reactor was placed inside the kiln for one hour. After one hour, the reactor rapidly cooled to temperatures below 100°C. Afterward, the reactor was opened, and the biochar was removed.
Testing of prepared biochars
The prepared biochar was tested for different physicochemical properties using standard laboratory procedures are as follows:
Characterization of produced biochars:
The pH and EC of biochars: The pH and EC of the produced biochars were tested in a 1:20 (w:v) suspension of biochar in distilled water. The biochar was physically crushed into a fine powder in a ceramic pot, then put in a conical flask with distilled water. The sample was shaken for one hour on a reciprocating shaker at 100 RPM. Afterward, the pH and EC were measured using high-precision glass electrode pH and EC meters. Three random samples from each type of biochar were tested, and the average results were entrained.
Ash content of biochar: The ash content of the biochar was tested by burning one gram of oven-dried sample in a muffle furnace at 750°C in an air atmosphere for one hour. The ash content was calculated by dividing the ash mass by the oven-dried sample’s mass.
Water holding capacity: Without further size reduction, the ovendried biochar samples were soaked in distilled water for one hour. The samples were allowed to drain freely on the filter paper, and the Water Holding Capacity (WHC) of the biochar was calculated by the amount of water stored by the biochar while adjusting the weight of the filter paper.
Plant nutrients content:
Nitrogen: The N content of prepared biochar was determined by the mixture of 1 gram of oven-dried biochar and 12.5 ml of concentrated sulphuric acid (H2SO4). The mixture was placed in sample tubes and a block digester for 30 minutes from 350°C to 400°C. After the complete dilution, 50 ml of distilled water was added to cool the sample. Afterward, the 8 to 10 drops of Tashiro indicator were added, and the samples were placed for distillation at the Kjeltec distillation unit for 7 minutes. The steam was adjusted to 7 ml-8 ml of distillate per minute. The sample was titrated with 0.1 N hydrochloric acid. Then the nitrogen content was calculated through a formula.
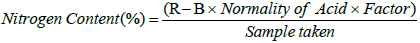
Where,
R=Reading of sample
B=Reading of blank sample
N=Normality of acid
F=Factor
S=Sample taken
Phosphorous, Calcium, Potassium, and Sodium: The plant nutrients such as phosphorous, calcium, potassium, and sodium of prepared biochars were tested by the mixture of 0.5 g of ovendried biochar and two different acids, namely Nitric acid and Perchloric acid, which were mixed at different volumes of 70 ml and 15 ml for the dilution process. The sample tubes were placed in a block digester set at 380°C for 2 hours. After the complete digestion, the tubes were removed for cooling for 20 minutes. 100 ml of distilled water was added to set the volume of the volumetric flask. Afterward, 10 ml of diluted sample and 10 ml of Color Development Reagent (CDR) were mixed, and 50 ml of distilled water was added to set the volume of the volumetric flask.
A few milliliters of the diluted sample were taken and tested using a spectrometer for phosphorus. A spectroscope was used to determine the phosphorus test. Then a calibration curve was created for the standards by plotting the absorbance against the respective P concentration. Therefore, the P concentration was noted from the calibration curve. Then all readings were calculated through the following formula:
A flame photometer determined the potassium, calcium, and sodium concentrations. A calibration curve was constructed, and then the concentrations were calculated using the calibration curve.
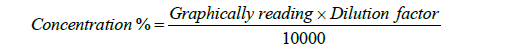
Results and Discussion
Yield of biochar
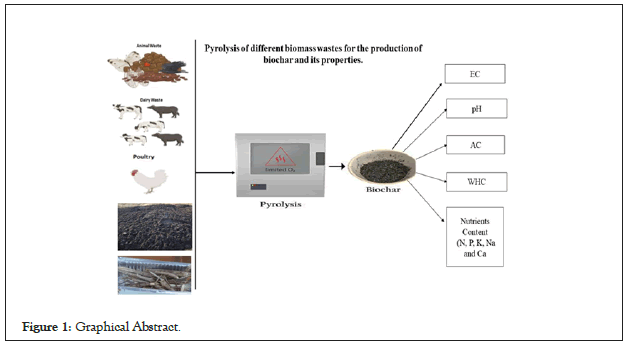
The components of biomass, cellulose, hemicellulose, and lignin, have a significant impact on the devolatilization, product yield, and form of the biochar created during the pyrolysis procedure [16-17]. The biochar yields are given in Figure 1.The biochar yield from all the pyrolyzed feedstock varied from 22.34% to 66.23%, as shown in Figure 2, the pyrolyzing temperature and feedstock type were important variables in regulating the biochar output. The eight types of feedstocks CD=Cow Dung, PM=Poultry Manure, MS=Municipal Sewage Sludge, MW=Mango Wood, CW=Conocarpus Wood, RW=Russian Wood, AW=Aak Wood, TW=Thoohar Wood (CD, PM, MS, MW, CW, RW, AW, and TW) were used for biochar preparation and their yield was 32.52%, 38.30%, 66.23%, 28.67%, 28.71%, 22.34%, 26.79%, and 29.21% respectively. The highest biochar yield of 66.23% was obtained from municipal sewage sludge, while the lowest yield of 22.34% was from Russian waste wood. Due to variations in the content and characteristics of their feedstocks, each feedstock's yield of biochar generated from it at the same temperature varies from the others. Many researchers have found that the results of biochar yield under the same conditions are consistent with their observations. However, produced biochar from CD, PM, and MS, respectively, and their findings are in line with our study [18-20]. The biochar produced from wood sources yield was consistent with revealed that compared to agricultural residues and wood biomass, the output of biochar from animal litter and municipal waste was greater (Figure 2) [21-25].
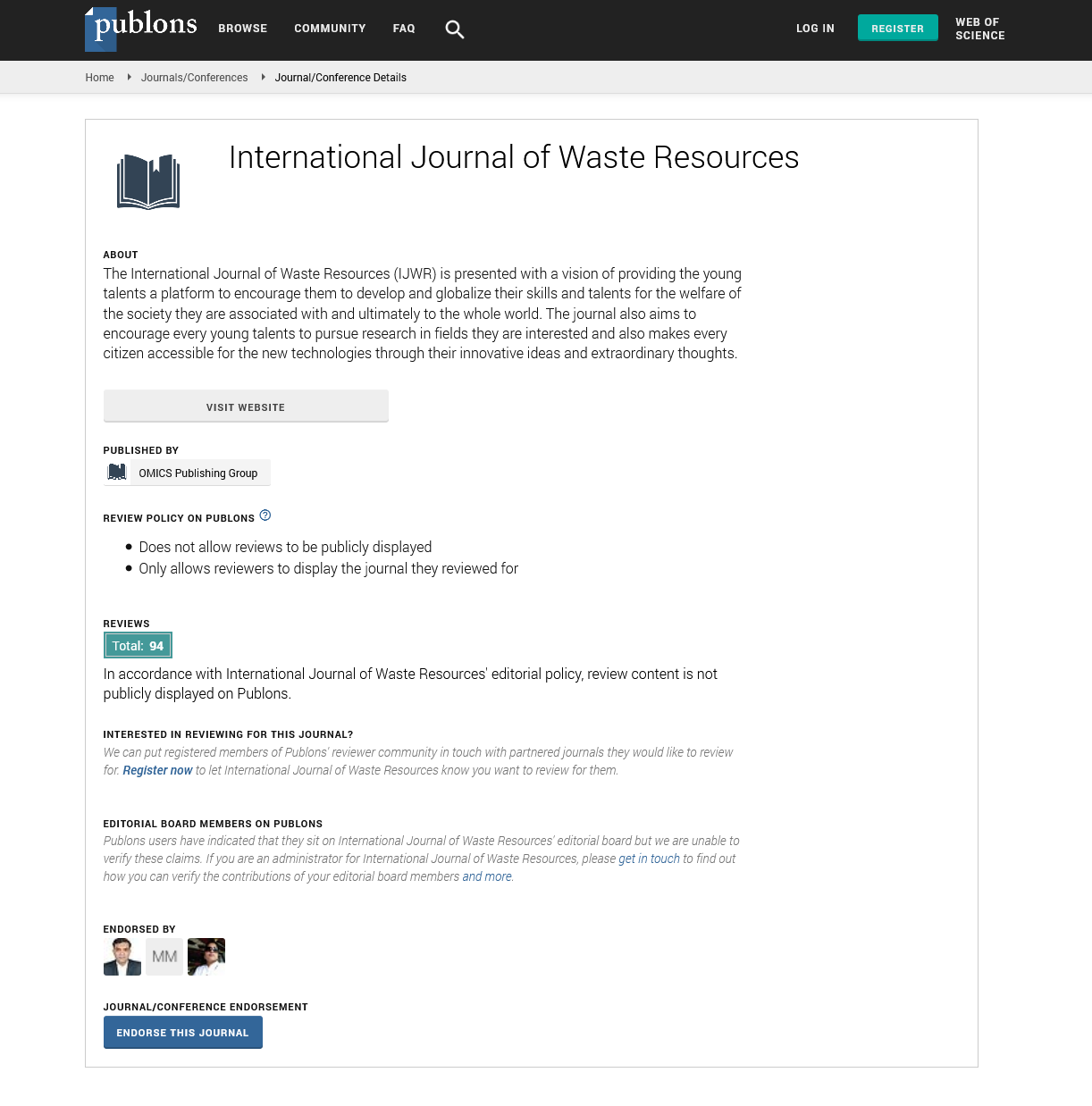
Figure 1: Graphical Abstract.
Figure 2: Yield of biochar from different types of biomasses.
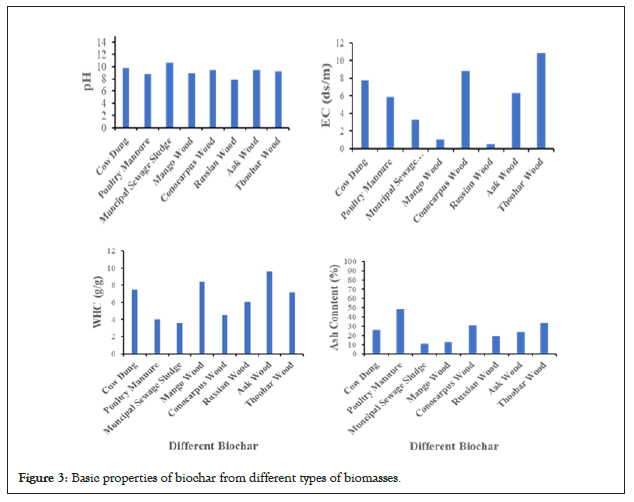
Basic properties of biochar
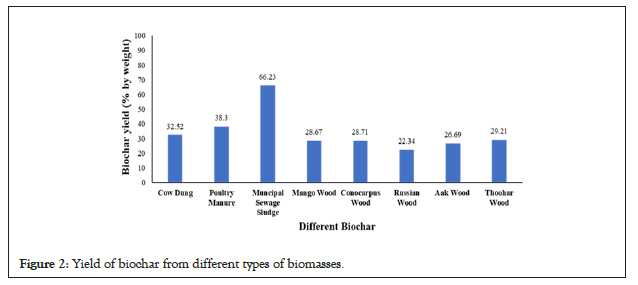
The results of the basic properties of different biochars are shown in Figure 2. The biochar samples in this research reported alkaline pH values ranging from 7.9 to 10.61, which is consistent with the fact that many biochar products have alkaline pH [26-29]. On the other hand, it is discovered that fundamental qualities such as pH increases with increasing temperature [30-32]. As the degree of acidity decreased and the basicity of the biochar increased, increasing the thermal decomposition temperature resulted in an increase in pH content [33-34]. The electrical conductivity of the biochar is responsible for the exchange of ions, and the electrical conductivity in our experiment increased from 0.52 dS m−1 to 10.88 dS m−1. The properties of biochar greatly depended on the production procedure and type of raw material [35-37]. The water holding capacity of the biochar was recorded between 3.6 g g−1to 9.6 g g−1. The weight fractions of the different-sized particles in the biochar varied with different types of biomasses. The porous structure and interconnectedness of pores in a substance determine its ability to hold water or capacity to maintain water [35-38]. This phenomenon reveals why high temperature biochars have a high porosity that can hold more water. Due to fewer pores, decreased interconnection of pores, and residual tar compounds that obstruct biochar pores, low-temperature biochar varieties with porous structures may be less available for water [39]. Moreover, the ash content increased from 11% to 48.38% [40-42]. A low silica concentration of forest biomass could indicate poor ash content. The ash content of biochar made from woody sources was lower than that of grasses and straws [43]. Moreover, this might be due to the concentration of non-pyrolyzed inorganic elements in the basic biomasses and increased ash content at elevated temperatures (Figure 3) [44].
Figure 3: Basic properties of biochar from different types of biomasses.
Plant nutrients content of biochar
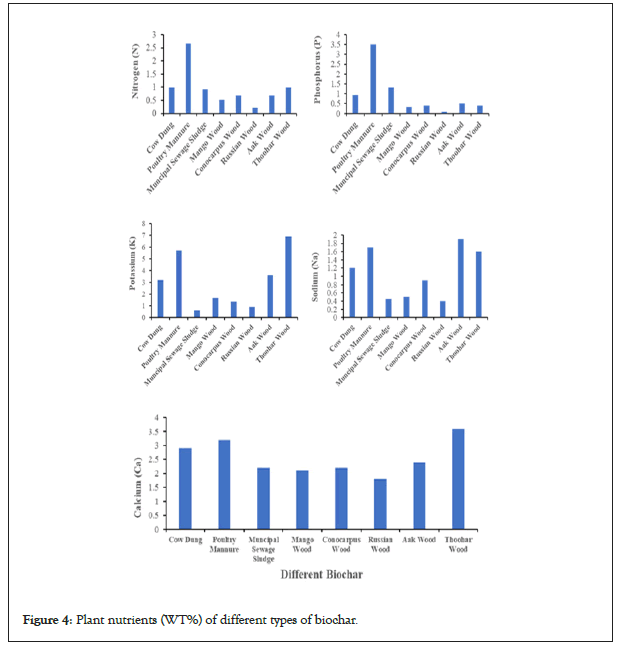
An overview of the complete relative nutrient content of woody and non-woody biochars produced at the same temperatures can be seen in Figure 4. The lower Nitrogen (N) content was found for Russian waste wood (0.21%), while a higher value was found for poultry manure (2.67%). The highest Phosphorus (P) content was obtained for poultry manure (3.51%), while the lowest value was found in Russian wood (0.09%). The maximum Potassium (K) content was recorded in Thoohar wood (6.9%); however, the minimum value was obtained in municipal sludge (0.6%). The minimum sodium (Na) content was obtained in Russian wood (0.4%), while the maximum value obtained in Aak wood (1.9%). The lower calcium (Ca) content was found for Russian wood (1.8%), while a higher value was recorded in Thoohar wood (3.6%) [20-35]. Moreover, another researcher discussed the plant nutrient content of various biochars and found that the delivery of accessible nutrients by biochar may be fairly varied and that biochars can contain a lot of inorganic components [40-45]. The feedstock source and the pyrolysis temperature impacted the nutrients in biochar [46]. Both the biochar products and their different feedstocks have a wide range of total mineral element concentrations. Other researchers have discovered significant differences in mineral element concentrations across biochar products generated from various biomass resources [47]. According to most studies, the total mineral nutrients in biochar compounds were greater than in the biomass used to make biochar products (Figure 4) [48].
Figure 4: Plant nutrients (WT%) of different types of biochar.
Conclusion
From the results of this study, it is concluded that manure and waste wood may be used for biochar preparation. The slow pyrolysis of these feedstocks at 600°C revealed that the poultry manure biochar possesses 3.51% phosphorus. At the same time, the wood biochars are rich in potassium and possess a good water-holding capacity. Hence, such biochar could be utilized for soil amendment.
Conflict of Interest
The authors declare that they have no competing interests.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Funding Statement
This study was supported by Department of Energy and Environment, Faculty of Agricultural Engineering, Sindh Agricultural University Tando Jam, Sindh, Pakistan.
References
- Leng L, Huang H, Li H, Li J, Zhou W. Biochar stability assessment methods: A review. Sci Total Environ. 2019;647:210-222.
[Crossref] [Google Scholar] [PubMed]
- Giudicianni P, Cardone G, Ragucci R. Cellulose, hemicellulose and lignin slow steam pyrolysis: Thermal decomposition of biomass components mixtures. J Anal Appl Pyrolysis. 2013;100:213-222.
- Clough TJ, Condron LM. Biochar and the nitrogen cycle: Introduction. J Environ Qual. 2010;39(4):1218-1223.
[Crossref] [Google Scholar] [PubMed]
- Qian K, Kumar A, Zhang H, Bellmer D, Huhnke R. Recent advances in utilization of biochar. Renew Sust Energ Rev. 2015;42:1055-1064.
- Hua L, Wu W, Liu Y, McBride MB, Chen Y. Reduction of nitrogen loss and Cu and Zn mobility during sludge composting with bamboo charcoal amendment. Environ Sci Pollut. 2009;16:1-9.
[Crossref] [Google Scholar] [PubMed]
- Lehmann J. A handful of carbon. Nature. 2007;447(7141):143-144.
[Cross Ref] [Google Scholar] [PubMed]
- Laird DA, Brown RC, Amonette JE, Lehmann J. Review of the pyrolysis platform for coproducing bio‐oil and biochar. Biofuels, Biofuels Bioprod Biorefining. 2009;3(5):547-562.
- Sohi SP, Krull E, Lopez-Capel E, Bol R. A review of biochar and its use and function in soil. Adv Agron. 2010;105:47-82.
- Lehmann J, Rondon M. Bio-char soil management on highly weathered soils in the humid tropics. Biological Approaches to Sustainable Soil Systems. 2006;113(517):e530.
- Laird DA. The charcoal vision: A win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. J Agron. 2008;100(1):178-181.
- Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. J Soil Sci Plant Nutr. 2006;52(4):489-495.
- Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, Kiyono Y, et al. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009;111(1-2):81-84.
- Major J, Steiner C, Downie A, Lehmann J. Biochar effects on nutrient leaching. In Biochar for Environ. Manage. 2012:303-320.
- Cao X, Ma L, Gao B, Harris W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol. 2009;43(9):3285-3291.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Lin K, Hou Z, Richardson B, Gan J. Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J Soils Sediments. 2010;10:283-289.
[Cross Ref ] [Google Scholar]
- Ghysels S, Acosta N, Estrada A, Pala M, De Vrieze J, Ronsse F, et al. Integrating anaerobic digestion and slow pyrolysis improves the product portfolio of a cocoa waste biorefinery. Sustain Energy Fuels. 2020;4(7):3712-3725.
- Tomczyk A, Sokołowska Z, Boguta P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol. 2020;19:191-215.
- Cao H, Xin Y, Yuan Q. Prediction of biochar yield from cattle manure pyrolysis via least squares support vector machine intelligent approach. Bioresour Technol. 2016;202:158-164.
[Crossref] [Google Scholar] [PubMed]
- Jin J, Li Y, Zhang J, Wu S, Cao Y, Liang P, et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J Hazard Mater. 2016;320:417-426.
[Crossref] [Google Scholar] [PubMed]
- Omotade İ, Momoh S, Oluwafemi B, Agboola E. Comparative analysis of nutrients composition in biochar produced from different feedstocks at varying pyrolysis temperature. Environ Res Technol. 2020;3(2):64-70.
- Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, Yang JE, et al. Effects of pyrolysis temperature on soybean stover-and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol. 2012;118:536-544.
[Crossref] [Google Scholar] [PubMed]
- Al-Wabel MI, Al-Omran A, El-Naggar AH, Nadeem M, Usman AR. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour Technol. 2013;131:374-379.
[Crossref] [Google Scholar] [PubMed]
- Gupta GK, Gupta PK, Mondal MK. Experimental process parameters optimization and in-depth product characterizations for teak sawdust pyrolysis. Waste Manage. 2019;87:499-511.
[Crossref] [Google Scholar] [PubMed]
- Wei S, Zhu M, Fan X, Song J, Li K, Jia W, et al. Influence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere. 2019;218:624-631.
[Cross Ref] [Google Scholar] [PubMed]
- Wallikhani AH, Asakereh A, Farrokhian Firouzi A. Biochar and bioenergy production by pyrolysis of Conocarpus and Eucalyptus wastes: a case study, Khuzestan province, Iran. Int J Environ Sci Technol. 2021:1.
- Gaskin J, Steiner C, Harris K, Das KC, Bibens B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE. 2008;51(6):2061-2069.
- Spokas KA, Cantrell KB, Novak JM, Archer DW, Ippolito JA, Collins HP, et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J Environ Qual. 2012;41(4):973-989.
[Crossref] [Google Scholar] [PubMed]
- Ronsse F, Van Hecke S, Dickinson D, Prins W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Glob Change Biol Bioenergy. 2013;5(2):104-115.
- Cárdenas-Aguiar E, Gasco G, Paz-Ferreiro J, Mendez A. Thermogravimetric analysis and carbon stability of chars produced from slow pyrolysis and hydrothermal carbonization of manure waste. J Anal Appl Pyrolysis. 2019;140:434-443.
- Naeem MA, Khalid M, Arshad M, Ahmad R. Yield and nutrient composition of biochar produced from different feedstocks at varying pyrolytic temperatures. Pak J Agric Sci. 2014;51(1).
- Sarfraz R, Li S, Yang W, Zhou B, Xing S. Assessment of physicochemical and nutritional characteristics of waste mushroom substrate biochar under various pyrolysis temperatures and times. Sustainability. 2019;11(1):277.
- Jindo K, Mizumoto H, Sawada Y, Sanchez-Monedero MA, Sonoki T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences. 2014;11(23):6613-6621.
- Mukherjee A, Zimmerman AR, Harris W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma. 2011;163(3-4):247-255.
- Yuan JH, Xu RK, Zhang H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol. 2011;102(3):3488-3497.
[Crossref] [Google Scholar] [PubMed]
- Piash MI, Hossain MF, Parveen Z. Physico-chemical properties and nutrient content of some slow pyrolysis biochars produced from different feedstocks. Bangladesh j sci res. 2016;29(2):111-122.
- Kuryntseva P, Galitskaya P, Selivanovskaya S. Assessing the potential of using biochar from chicken manure as a soil amendment. InEGU General Assembly Conference Abstracts 2018(p. 741).
- Liu XH, Zhang XC. Effect of biochar on pH of alkaline soils in the loess plateau: results from incubation experiments. Int J Agric Biol. 2012;14(5).
- Yargicoglu EN, Sadasivam BY, Reddy KR, Spokas K. Physical and chemical characterization of waste wood derived biochars. J Waste Manag. 2015;36:256-268.
[Crossref] [Google Scholar] [PubMed]
- Weber K, Quicker P. Properties of biochar. Fuel. 2018;217:240-261.
- Ulusal A, Apaydın Varol E, Bruckman VJ, Uzun BB. Opportunity for sustainable biomass valorization to produce biochar for improving soil characteristics. Biomass Convers Biorefin. 2021;11:1041-1051.
- Santana KV, Apolônio FC, Wisniewski A. Valorization of cattle manure by thermoconversion process in a rotary kiln reactor to produce environmentally friendly products. Bioenergy Res. 2020;13:605-617.
- Kończak M, Oleszczuk P, Różyło K. Application of different carrying gases and ratio between sewage sludge and willow for engineered (smart) biochar production. J CO2 Util. 2019;29:20-28.
- Lehmann J, Joseph S. Biochar for Environmental Management: Science and Technology. Earthscan: London, UK. 2009.
- Novak JM, Lima I, Xing B, Gaskin JW, Steiner C, Das KC, et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. J Environ Sci. 2009.
- Enaime G, Lübken M. Agricultural waste-based biochar for agronomic applications. Appl Sci. 2021;11(19):8914.
- Ippolito JA, Spokas KA, Novak JM, Lentz RD, Cantrell KB. Biochar elemental composition and factors influencing nutrient retention. Biochar for Environ Manag. Sci Technol and implementation. 2015;139.
- Dumroese RK, Heiskanen J, Englund K, Tervahauta A. Pelleted biochar: Chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenergy. 2011;35(5):2018-2027.
- Judd LA. Physical and Chemical Analyses of Two Biochars Produced from Pine Wood Chips and Rice Hulls and Their Effects on Container Substrates. 2016.
Citation: Ahmed F, Laghari M, Ali M, Ali L, Raza A, Chachar A (2023) Pyrolysis of Different Biomass Wastes for the Production of Biochar: Evaluation of Yield and Physiochemical Characteristics. Int J Waste Resour. 13:549.
Copyright: © 2023 Ahmed F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.