Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 16, Issue 9
Propylene Fibers Infused with Silver Nanoparticles Effectively Combat Saprolegnia Fungi and Boost the Survival Rates of Fertilized Caspian Salmon Salmo trutta caspius Eggs
Amin Azimzadeh1, Mohammad Reza Kalbassi2*, Mehdi Soltani3 and Omid Safari42Department of Aquaculture, Faculty of Marine Sciences, Tarbiat Modares University, Noor, Iran
3Department of Aquatic Animal Health, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
4Department of Environment, Faculty of Natural Resources and Environment, Ferdowsi University of Mashhad, Mashhad, Iran
Received: 06-Aug-2024, Manuscript No. JARD-24-26707; Editor assigned: 08-Aug-2024, Pre QC No. JARD-24-26707 (PQ); Reviewed: 11-Aug-2024, QC No. JARD-24-26707; Revised: 07-Sep-2025, Manuscript No. JARD-24-26707 (R); Published: 14-Sep-2025, DOI: 10.35248/2155-9546.25.16.949
Abstract
In order to examine the antifungal properties of nanoparticles for managing saprolegnia fungal infections in endangered Caspian salmon hatcheries, we conducted a study using filters containing three different concentrations of Silver Nanoparticles (SNP) (0.7, 1.5, and 2.5 g SNP) infused on polypropylene fibers (PSNP). These PSNPs were integrated into polyethylene-cylinder filters within Semi-Recirculation Incubators (SRI) containing 150 fertilized eggs (n=3). Additionally, absorbent materials such as active carbon and zeolite (200 g each in SRIs) were placed in the filters, alongside a positive control treatment without any active carbon, zeolite, and PSNPs. SEM images showed silver nanoparticles confused on fiber surfaces, and ICP analysis confirmed polypropylene fiber's effectiveness in retaining SNP. We measured the Survival Rates (SR) at three stages: From fertilized eggs to eyed eggs (P1), from eyed eggs to hatching (P2), and from hatching to the swim-up larval stage (P3). The survival rates at the eyed-egg stage in SRIs with 2.5% PSNP (96.44%) were significantly higher (P<0.05) than those in the control treatment (81.11%). We also observed a significant increasing trend in SRs (average 94.12-96.26%) during the P2 stage as the PSNP concentration increased from 1.5% to 2.5%, compared to the control treatment (83.64%). Furthermore, at higher PSNP concentrations, the larval survival rates (P<0.05) significantly improved (71.33-87.56%) compared to the control treatment (40.89%) during the P3 stage. Also, the decreased fungal density of the incubation water from 3 to 2 (log fungi spore density), revealed the antifungal properties of Ag-nano fibers in 2.5% PSNP. In conclusion, filters containing PSNP effectively managed the density of fungal spores in the hatchery incubator and improved the survival rate of Caspian salmon.
Keywords
Caspian salmon; Semi-recirculation incubator; Polypropylene; Silver nanoparticle
Introduction
Aquaculture is rapidly expanding worldwide due to the decline in marine fisheries caused by food stock shortages in recent years. Captive fish reproduction, especially for species like salmon, has made significant progress. However, it's essential to prioritize aquatic health management for sustainable development, as the Caspian salmon (Salmo trutta caspius) is endangered. Artificial propagation and releasing of this rare species into natural habitats are crucial for conservation efforts. To protect the health of salmon eggs, maintaining the quality of the incubation water is vital to prevent mortality from diseases, particularly fungal infections, with species from the Saprolegnia family being the most common culprits. Different antifungal drugs have been developed to combat pathogenic fungal species. However, these compounds have several drawbacks, such as being toxic to fish and eggs, insoluble in water, carcinogenic, and having limited antifungal activity. As a result, their use is restricted in some cases. Due to the spread of bacterial and fungal resistance to antibiotics and other fungicides, it is important to explore alternative materials [1-5].
Silver, as an inorganic compound, has been used since ancient times to treat burns, wounds, and certain bacterial infections in the form of metal or salt (silver nitrate). After the discovery of antibiotics, less attention was given to the antimicrobial properties of silver. However, its potential has been reconsidered due to limitations on antibiotic use. Various studies have shown the bactericidal effects of silver, both as a metal and as an ion.
Silver Nanoparticles (SNP) have been found to exhibit effective antimicrobial properties against a wide range of microorganisms. This effect is attributed to the increased contact area of silver with microorganisms when the metal is broken down into smaller particles. Additionally, SNP forms a stable and nonwashable bond with polypropylene, causing no change in the properties of the fiber matrix. When antibacterial metals such as silver, copper, and zinc are placed on inorganic carriers, they are slowly released and act as inorganic disinfectants. The use of these materials offers many advantages, including immunity, durability, and resistance to heat compared to common organic materials [6-8].
This study aimed to evaluate the antifungal properties of silver nanoparticles on polypropylene fibers in a safer method using semi-recirculation incubators for Caspian salmon.
Materials and Methods
Preparation of polypropylene fibers containing SNPs
Polypropylene fibers containing polypropylene granules were prepared by the National Polymer Institute in Iran. PSNP powders for creating different concentrations (0.7%, 1.5%, and 2.5% of PSNPs) were purchased from Xuzhou Hongwu Nanometer Co, China. The process of producing PSNPs was carried out in two stages. In the first stage, the polypropylene granules were weighed (0.0001 g), melted, and mixed with varying concentrations of PSNP using an internal mixer (HBI system 90, 60 cc, United States and Germany) at 180°C for 10 minutes. The resulting product was then cleaned, pressed into a thin layer, and cut into small cubes for the next stage. In the second stage, the production of PSNP fibers was accomplished using a spinner (LMD model, manufactured in the United States and Germany) at 235°C. The extruder rotation degree was adjusted to a low range to minimize the exit of melted materials and produce thinner fibers (unpublished data). To verify the covering of SNP on polypropylene fibers, an electronic microscope SEM was utilized. The release of SNPs into water content was measured using ICP in a graphite oven.
Designing the SRIs and executing the treatments
This research was conducted at the Shahid Bahonar Center of Reproduction and Production of Cold Water Fishes in Kelardasht, Iran. The study compared three different concentrations of PSNPs (polyethylene filter) in SRIs with a control treatment. The experimental units consisted of fiberglass troughs and cylinder polyethylene filters in SRIs. A total of 1800 fertilized eggs from healthy wild Caspian salmons (3 females to 1 male, n=160) were distributed into twelve troughs, each containing three baskets with 50 eggs in each basket. To maintain consistent environmental conditions, all troughs were covered with nylon [9].
The three treatments were a combination (w/w) of 11g polypropylene with 0.7%, 1.5%, and 2.5% PSNP, in addition to active carbon and zeolite (200 g each). The position of these materials in the cylindrical filters from top to bottom was PSNPs, active carbon, and zeolite, respectively. The control treatment did not include active carbon and zeolite. The water collected at the end of the troughs was pumped onto the filters placed beside the troughs, and then the drained water was returned to the entry section of the troughs. The water in the entry section of the troughs was aerated to maintain dissolved oxygen levels.
The following observations were made daily: Physicochemical properties of water, the inflow and outflow of pumped water, the condition of materials formed in the filters (including biofilm formation, coagulation, and sedimentation), and egg morphology. The temperature, pH, and dissolved oxygen levels were maintained within optimal ranges at 8.5 ± 0.5â??, 7.3 ± 0.4, and 8.1 ± 0.3 ppm, respectively. The daily water exchange rate in the SRI was kept at a low 5%. Before introducing fertilized eggs into the troughs, the SRIs were operated for 48 hours to ensure that the fish exhibited active swimming behavior. Three stages were analyzed: the transformation of fertilized eggs into eyed eggs (P1), eyed eggs into hatching (P2), and hatching into active swimming (P3). The rates of eyed, hatching, and overall survival of the experimental fish (including eggs, larvae, and fry) were computed. Fungal spore density was assessed using the pour plate method (n=40) and reported in a logarithmic scale.
During the experiment, the efficiency of filters was evaluated by inoculating the water content in SRIs four times with one ml of homogenized fungal biomass from white salmon eggs (n=20). Specifically, it was inoculated once in P1 during the first week before the eyed stage, and thrice in P2. Once the eye spots appeared and the egg sensitivity reduced concerning manipulation and transportation, the water content was sampled 48 hours after the fungi inoculation to measure the fungi spore density. Furthermore, the eggs containing fungi spots were removed 72 hours after the inoculation.
Statistical analysis
All percentage data was transformed using the arcsine method. After confirming the homogeneity of variance and normality of the data using Leaven and Kolmogorov-Smirnov tests, respectively, one-way ANOVA was used to compare the treatments. Duncan's test was applied to spot significant differences among the treatments (P<0.05).
Results
The characteristics of the produced PNSPs
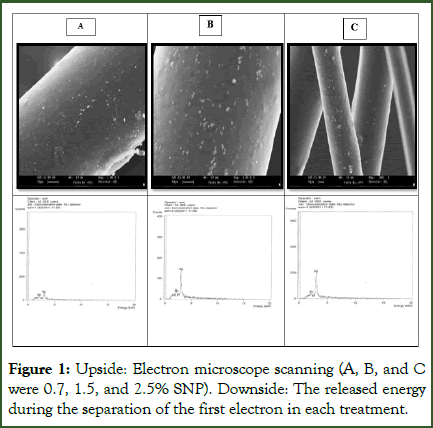
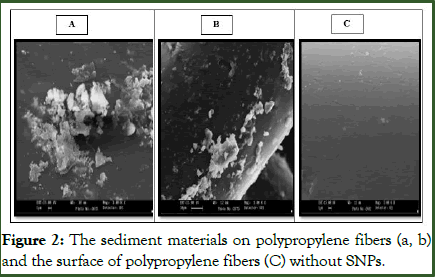
Confirming SNP covers on polypropylene fibers: The images captured using a Scanning Electronic Microscope (SEM) revealed the presence of SNPs (Silver Nanoparticles) on polypropylene fibers, with the substrates being entirely covered by SNPs. This observation was corroborated by both the images taken and the ICP analysis of the SNPs on the fibers. As depicted in Figure 1, the SNP concentrations varied in treatments containing 0.7 (A), 1.5 (B), and 2.5 (C) g of PSNP. Comparing the SNP contents to the gold ion, which served as an internal indicator in the ICP spectrum, revealed measurements of 0.7%, 1.4%, and 2.1%, respectively. These measurements align with the amount of PSNPs utilized in each treatment (Figure 1). Furthermore, Figure 2 (a,b) confirmed the accumulation of diverse sediments on the surface of polypropylene fibers, with the surface SNPs being obscured beneath the sediments. Consequently, this may diminish the antifungal properties of PSNPs.
Figure 1: Upside: Electron microscope scanning (A, B, and C were 0.7, 1.5, and 2.5% SNP). Downside: The released energy during the separation of the first electron in each treatment.
Figure 2: The sediment materials on polypropylene fibers (a, b) and the surface of polypropylene fibers (C) without SNPs.
ICP analysis
Atomic absorption results of ICP show that the water content of the SRIs in control and treatments with 0.7%, 1.5%, and 2.5% PSNP was measured and its average amount in 3 replicates was 0, 3, 5.5, and 11.5 parts per billion (ppb) respectively. The treatment with 2.5% PSNP showed the highest quantity of Ag ions on the surface of polypropylene fibers.
Effects of PSNPs on the Caspian salmon life cycle
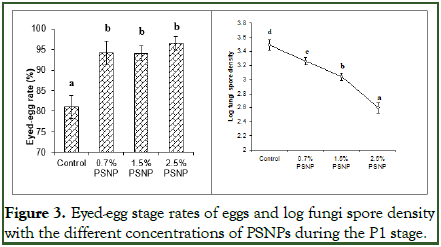
Eyed rates and the density of fungi spore during the P1 stage: The effects of different concentrations of PSNPs on the lifecycle of Caspian salmon were analyzed from the fertilized eggs to active swimming in SRIs. The rates of eyed-eggs stage of the fertilized eggs in SRIs with different concentrations of PSNPs were illustrated in Figure 3. The eyed-eggs rates in the treatments with different concentrations of PSNPs significantly increased (P<0.05) compared to the control treatment. Conversely, a decreasing trend was observed between the fungi spore density and PSNP concentrations (Figure 3). Notably, the treatment containing 2.5% PSNPs exhibited the highest eying rate at 96.4% (Figure 3). While there were no significant differences in the eying rates among treatments containing PSNPs (ranging from 94% to 96.22%), these rates were significantly (P<0.05) higher than that of the control treatment at 81.1% (Figure 3). Significantly, using PSNPs in filters in SRIs reduced the log fungi spore density from 3.49 in the control treatment to 2.60 in the treatment containing 2.5% PSNP (Figure 3).
Figure 3. Eyed-egg stage rates of eggs and log fungi spore density with the different concentrations of PSNPs during the P1 stage.
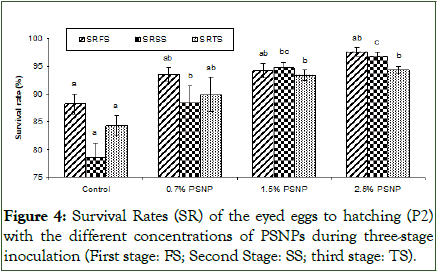
Hatching rates and the density of fungi spore during P2 stage: The incubator water content of SRIs was inoculated three times during the P2 stage. The water was sampled 48 hours after the inoculation to count the fungi spore density, and then the eggs with fungi spots were removed 72 hours after the inoculation. The results were recorded separately for each stage (1, 2, and 3) (Figure 4). With an increase in the concentration of PSNP, the survival rates of the treated eggs in SRIs at each stage increased. Specifically, the treatment containing 2.5% PSNP showed a significant (P<0.05) increasing trend compared to the control during all stages (Figure 4).
Figure 4: Survival Rates (SR) of the eyed eggs to hatching (P2) with the different concentrations of PSNPs during three-stage inoculation (First stage: FS; Second Stage: SS; third stage: TS).
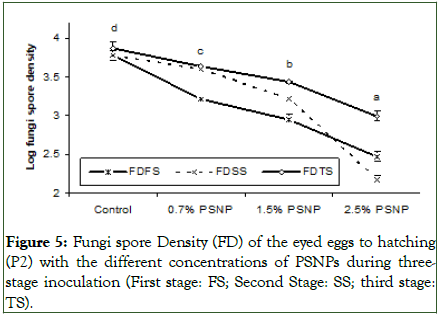
Similarly, trends in survival rate were observed in the log fungal spore density during the three-stage inoculation (Figure 5). The treatment containing 2.5% PSNP recorded significantly (P<0.05) lower density of fungal spores (2.17-2.99) during the three-stage inoculation (Figure 5). As depicted in Figure 5, the highest fungal spore count was observed in the third stage, resulting in the lowest survival rate of the treated eggs during this stage (Figure 5).
Figure 5: Fungi spore Density (FD) of the eyed eggs to hatching (P2) with the different concentrations of PSNPs during threestage inoculation (First stage: FS; Second Stage: SS; third stage: TS).
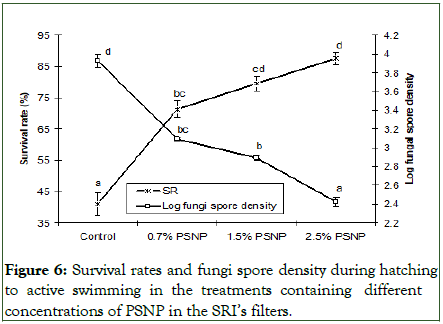
Survival rates and the density of fungi spore during the P3 stage: The survival rate increased significantly (P<0.05) from 71.23% to 87.56% as the concentration of PSNP rose from 0.7% to 2.5%, compared to the control rate of 40.89%. Eggs with 2.5% PSNP exhibited a significantly (P<0.05) higher survival rate than those with 0.7% PSNP (Figure 6). Despite no fungal biomass being added during P3 (hatching to active swimming), the filters with 2.5% PSNP demonstrated the best performance in significantly reducing the density of fungal spores (2.42) compared to filters with other PSNP concentrations (2.89-3.09). The control treatment had the highest fungal spore density in the water content (3.93) of all treatments (Figure 6). Throughout P3, larval behavior and morphology were regularly monitored, and no mortality or abnormal behavior was observed until the end of the experimental period.
Figure 6: Survival rates and fungi spore density during hatching to active swimming in the treatments containing different concentrations of PSNP in the SRI’s filters.
Discussion
Extending the incubation period of Caspian salmon eggs, from fertilization to the active swimming stage, requires a significant amount of water with stable physicochemical properties. However, there are various environmental challenges, such as seasonal floods, diseases, and climate changes that make it difficult to supply high-quality cold water (8.5 ± 1.5°C). One significant challenge is the outbreak of Saprolegnia, an opportunistic fungal species that thrives in cold water and has a high rate of spore production. One proposed solution to address these issues is the utilization of advanced technology, including nanoparticles, to develop Semi-Recirculation Incubators (SRI). Silver nanoparticles embedded in a propylene matrix (PSNP) have been suggested as a viable option. The primary objective of the current study is to assess the effectiveness of PSNP in SRI filters under the most challenging conditions, with a constant presence of fungal spores (manually inoculated), and to evaluate the impact of this treatment on the health of the fertilized eggs housed in the incubators with partial water replacement.
In a recent study, the significant effects of PSNPs in controlling infectious fungal activity in SRIs were verified. These findings were based on results obtained by previous researchers. Additional studies involved taking SEM pictures of PSNPs to confirm the binding, size, and content of SNPs on the propylene matrix. Evaluation of the images showed that the propylene fibers had a spherical form, and the surface of the SNP was smooth. Furthermore, the concentrations of SNPs (ranging from 0.7 to 2.5%) differed notably from each other. The higher weight percentage of PSNP led to a greater likelihood of adherence to the fibers. Efforts were made to produce the thinnest fibers through maximum rotation in the spinner. The treatment containing 2.5% PSNP exhibited the densest distribution of SNPs on the propylene fibers. All spin engineering processes in the study prioritized exposing PSNPs more to water flow in the filters' SRIs, thereby increasing the likelihood of fungal spores coming into contact with the nanoparticle surfaces. Evaluation of the images obtained from PSNPs after their use in the filters' SRIs confirmed the adhesion of sediments to the surface of fibers, which consequently diminished the efficacy of PSNPs in controlling fungal species. These findings aligned with the reports of Marcucci et al., who concluded that sedimentation on the filtering layers of water, specifically organic matters and bio-sediments, acted as significant barriers on the layers and reduced their efficiency in treating water content.
Furthermore, the biological effects of different concentrations of PSNPs on the lifecycle of Caspian salmon were investigated, from fertilized eggs to active swimming. A reverse trend was observed between the quantity of SNP on the propylene matrix and the counted fungal spore density with an increase in log fungal spore density over time and through inoculation at four stages. In the first stage, the fungal spore density was in the lowest content, but in the fourth stage of inoculation, the highest content of the fungi spore density existed The control treatment exhibited the lowest survival rate. While the filters were notably effective at removing fungal spores, various factors, including the flow rate of pumped water, temperature, pH, and other nuisance factors, made it impossible to eliminate fungal spores. Handy et al. reported that some supplementary compounds including active carbon and zeolite could bind with silver ions and reduce their reactive characteristics, enclose the SNPs in their pores, make sediments, and cause them not to be available. Therefore, one of the reasons for the relative difference may be related to these compounds. Discrepancies between the present results and other studies on drinking water treatment, attributing these differences to the low flow rate in drinking water treatment filters compared to the high flow rate in SRIs. For example, flow rates of 0.5 Lmin-1 by Jain and Pradeep and 0.01 Lmin-1 by LV et al. were reported, whereas the flow rate of the present study was 6.5 Lmin-1. The increase in flow rate shortened the duration of contact between water containing fungal spores and the antiseptic agents (SNPs).
The dimension of silver ion (about 2.23 Å) was the main reason for removing the fungi spore. Gradual release could directly eliminate the microorganisms. The more the dimension of silver compounds reduces, the more antimicrobial properties increase Consequently decreasing the effect of SNPs.
The study highlighted the importance of complementary research to optimize the production process of PSNP, construct filters in SRIs more efficiently, and increase biomass loading to enhance the efficacy of these systems. Among the propylene fibers containing SNPs, fibers containing 2.5% SNP were considered based on the highest survival rates and the lowest fungi spore density as an efficient concentration (which may not be optimum) in semi-recirculation incubators. However, complementary studies must be done on optimizing the production process of PSNP, efficiently constructing filters in SRIs increasing the biomass loading, etc.
Conclusion
The propylene fibers containing 2.5% SNP showed significant results. They resulted in a 96.44% hatching rate in P1 and an average of 96.26% in P2, as well as survival rates of 87.56% in P2 and P3, compared to 81.11%, 83.64%, and 40.89% respectively in the control treatment. The important finding of this study was the use of SNPs on propylene fibers to preserve them on the surface and minimize their release, which was confirmed with ICP analysis. The use of nanoparticles in aquaculture systems to produce safer fungicides and ensure food safety requires further in-depth studies in the future.
References
- Bailey TA. Effects of twenty-five compounds on four species of aquatic fungi (Saprolegniales) pathogenic to fish. Aquaculture. 1984; 38:97–104.
- Bailey TA, Jeffrey SM. Evaluation of 215 candidate fungicides for use in fish culture. U.S. Fish and Wildlife Service, Investigations in Fish Control. 1989;(99):1–99.
- Bruno DW, Wood BP. Saprolegnia and other Oomycetes. 1999;599–659.
- Cho KH, Park JE, Osaka T, Park SG. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta. 2005;51:956–960.
- FAO. Food Outlook: Global Market Analysis. Rome: Food and Agriculture Organization of the United Nations; 2011.
- Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668.
[Crossref] [Google Scholar] [PubMed]
- Gong P, Li H, He X, Wang K, Hu J, Tan W, et al. Preparation and antibacterial activity of Feâ??Oâ??–Ag nanoparticles. Nanotechnology. 2007;18:604–11.
- Handy RD, von der Kammer F, Lead JR, Hassellov M, Owen R, Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17:287–314.
[Crossref] [Google Scholar] [PubMed]
- Jain P, Pradeep T. The potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol Bioeng. 2005;90:59–63.
[Crossref] [Google Scholar] [PubMed]
Citation: Azimzadeh A, Kalbassi MR, Soltani M, Safari O (2025) Propylene Fibers Infused with Silver Nanoparticles Effectively Combat Saprolegnia Fungi and Boost the Survival Rates of Fertilized Caspian Salmon Salmo trutta caspius Eggs. J Aquac Res Dev. 16:949.
Copyright: © 2025 Azimzadeh A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.