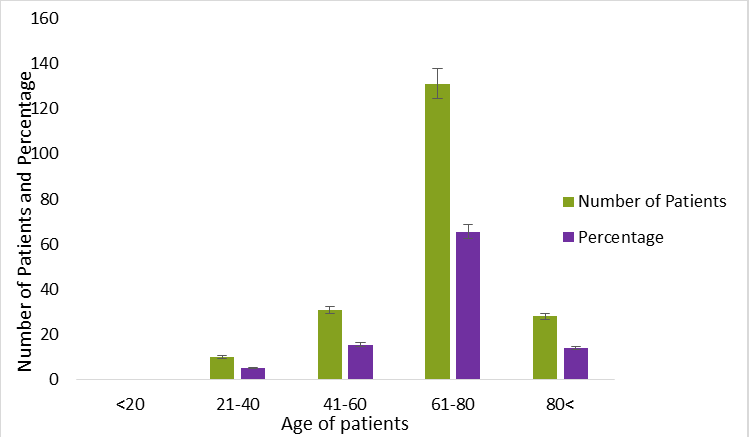Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 17, Issue 1
Prognostic Values of Enzyme Changes in Pre and Post-Treated Pancreatic Cancer Patients
N. Sivakumar1, B. Prabasheela2*, R. Arivazhagan3 and R. Vijayalakshmi42Department of Biotechnology, Aarupadai Veedu Institute of Technology, Vinayaka Mission’s Research Foundation, Paiyanoor, Kancheepuram, Tamilnadu, India
3Department of Biochemistry, Cancer Institute (WIA), Gandhi Nagar Adyar, Chennai-20, India
4Department of Zoology, A.D.M. College for Women, Nagapattinam-611001, Tamilnadu, India
Received: 07-Nov-2023, Manuscript No. BLM-23-23822; Editor assigned: 10-Nov-2023, Pre QC No. BLM-23-23822 (PQ); Reviewed: 24-Dec-2023, QC No. BLM-23-23822; Revised: 09-Jan-2025, Manuscript No. BLM-23-23822 (R); Published: 16-Jan-2025, DOI: 10.35248/0974-8369.24.17.764
Abstract
Introduction: The extensive desmoplastic microenvironment, essential for tumor growth, invasion, metastasis, angiogenesis, immunosuppression, and chemoresistance, is thought to be the cause of PDAC's high aggressiveness. Since tumor cells' energy metabolism differs from that of normal cells and is mostly based on aerobic glycolysis, fatty acid oxidation, and glutaminolysis, it is regarded as one of the distinguishing characteristics of cancer. As a result, a deeper comprehension of the intricate molecular traits of pancreatic cancer is required. There is mounting evidence that alterations in serum enzyme levels are crucial for the initiation and spread of cancer. Before and after chemotherapy, cancer patients have a variety of biochemical serum enzyme indicators, including Lactate Dehydrogenase (LDH), Adenosine Deaminase (ADA), Gamma-Glutamyl Transferase (GGT), amylase, and lipase imbalance. The meticulous monitoring of variations in serum enzyme marker levels greatly influenced the prognosis of the disease.
Materials and methods: We have included 500 pancreatic cancer patients (test group), regardless of age or gender, who were enrolled at "The Cancer Institute" in Adyar, Chennai, Tamil Nadu between 2016 and 2020. Hospital staff, age-matched, non-alcoholic, non-diabetic, voluntary blood donors, and a total of 100 healthy volunteers served as the study's control group.
Result and discussion: Males are more likely than females to contract the sickness (3:2), and the risk rises with age (>61-80). Based on current research, it appears that aberrant metabolism leading to changes in serum enzyme levels is a significant factor in PDAC patients. Serum enzyme panel levels have shown severe abnormalities in PDAC patients (P<0.05), indicating a compromised physiological state. The level of these enzyme biomarkers significantly decreased following three consecutive rounds of chemotherapy (1st, 2nd, and 3rd), demonstrating the potential of the chemotherapy medications. According to our research, combination chemotherapy the administration of many medications is a promising treatment option for pancreatic cancer.
Keywords
Pancreatic ductal adenocarcinoma; Serum enzyme markers; Lactate dehydrogenase; Adenosine deaminase; Gamma-glutamyl transferase; Chemotherapeutic drugs
Introduction
As the seventh most common cause of cancer related deaths worldwide, pancreatic cancer is an incurable disease [1]. In India, there are 0.5–2.4 cases of pancreatic cancer for every 100,000 men and 0.2–1.8 cases for every 100,000 women [2]. Pancreatic cancer is still a very aggressive malignancy with a high death rate, even with recent substantial advancements in treatment techniques. It is predicted that PDAC rates will rise soon because of changes in lifestyle both internationally and in India [3]. Aberrant serum enzyme synthesis is always the result of normal cells turning into cancer cells and abnormal cancer cell proliferation; this phenomenon can even be identified before changes in tumor morphology or the onset of clinical symptoms [4]. Compared to non-transformed cells, cancer cells have a different metabolism that produces enough biomaterials and energy for endless growth. Reprogrammed metabolism also plays a role in other malignant features such as resistant cell death and metastasis. According to Warburg's 1956 paper, one crucial component of cancer metabolism is accelerated glycolysis. Aberrant serum enzyme synthesis is always the result of normal cells turning into cancer cells and abnormal cancer cell proliferation. This phenomenon can even be detected before changes in tumor morphology or the onset of clinical manifestations, which is why the roles of abnormal serum enzymes in cancers are recently generating increasing interest and concern.
Common blood enzyme Lactate Dehydrogenase (LDH) is involved in the process of converting pyruvate into lactate and is essential for sustaining glycolysis, which is intimately linked to the development and spread of tumors. Due to its necessity for tumor maintenance, LDH has been thought to function as an indication of tumor burden and aggressiveness [5]. In the purine salvage route, adenosine is hydrolytically converted to inosine by the enzyme Adenosine Deaminase (ADA). This enzyme is crucial to preventing the buildup of harmful compounds during the fast cell division. It has been noted that ADA activity, a marker of accelerated DNA synthesis, is elevated in malignant tissue. Cancer patients had higher serum levels of ADA. Purine metabolism is facilitated by the polymorphic enzyme ADA, which is extensively dispersed throughout bodily fluids and tissues. Since ADA is necessary for lymphocyte maturation, proliferation, and differentiation, the most significant biological activity is therefore associated with lymphoid tissue and is specifically observed in T cells [6]. The role of GGT enzyme in hepatic and pancreatic diseases is well established. GGT-specific activities significantly alter in precancerous and cancerous phases of pancreatic tissues. GGT is released into the bloodstream as a result of pancreatic cell injury, which causes an increase in the cells' activity during the initial stages of cancerization [7]. Tissue specific expression of GGT is altered under a variety of pathologic and physiological circumstances, such as development and carcinogenesis. The pathophysiology and prognosis of pancreatic cancer may be significantly influenced by pancreatic enzymes. One often used standard test that can be achieved as a biological marker for acute pancreatitis is the detection of amylase in plasma [8].
Materials and Methods
100 healthy volunteers, including non-alcoholic, non-diabetic, age-matched voluntary blood donors, and hospital staff, were used as material for a study group and control group in this study, out of the 500 patients (of which 200 were female and 300 were male) who were diagnosed with pancreatic carcinoma and who visited the OP department of the Cancer Institute, Adyar. Under the standards outlined in the declaration of cancer institute, venous blood samples from patients with pancreatic cancer were collected from the portal venous. All patients participating in this work have family members who have given their consent. The Cancer Institute's Ethics Committee gave its approval to the study (Reference no.: IEC/ 2021/Aug 07). Prior to any action, each participant provided signed, informed permission.
Sample collection
Following an overnight fast, 10 ml of venous blood was drawn from the pancreatic cancer patients' portal veins at the time of diagnosis (before chemotherapy) and once within 30 days of treatment (post-chemotherapy). The samples were placed in a plain vacuum sealed tube without the use of an anticoagulant, and they were repeated twice after chemotherapy, each time for 30 minutes. The serum was separated by cooling centrifugation at 3,000 rpm for 10 minutes after clotting at room temperature. Without delay, the transparent supernatant was moved to a different test tube and utilized for the examination of enzyme parameters.
Serum Lactate Dehydrogenase (LDH) assay
The fully automated approach for calculating serum Lactate Dehydrogenase (LDH) is provided, using the Biosystem BA400 (Spain) system. Pyruvate is reduced by NADH to produce lactate and NAD+, and this process is catalyzed by Lactate Dehydrogenase (LDH). The catalytic concentration is measured calorimetrically and is derived from the rate at which NADH is reduced. The procedure is less costly than the majority of reported automated procedures, and the chemicals remain stable for a minimum of four weeks. A kinetic spectrophotometric approach and the results show good correlation [9].
Serum Adenosine Deaminase (ADA) assay
Giusti and Galanti, 1984 described a colorimetric approach for estimating the ADA assay: The Biosystem BA400 (Spain) completely automated method. The measurement of ammonia in a Berthelot reaction serves as its foundation [10]. When ADA and adenosine (the substrate) interact, ammonia is produced. The quantity of ADA required to release one μmol of ammonia per minute from the substrate under typical experimental conditions is the chemical definition of one unit of total ADA. To reduce ammonia residue, the in house kit's solutions were made with sterile bi-distilled water. The creation of blue color by indophenol was measured at 628 nm in the final chemical process. There were samples of both positive and negative controls.
Serum Gamma Glutamyl Transferase (GGT) assay
The GGT activity in serum or plasma is measured using an enzymatic rate approach by the fully automated analyzer Biosystem BA400 (Spain). The reaction produces colored pnitroaniline when the GGT catalyzes the transfer of a gammaglutamyl group from the colorless substrate, gamma-glutamyl-pnitroaniline, to the acceptor, glycylglycine. Over a predetermined period, the system tracks the rate of change in absorbance at 410 nm. The amount of GGT present in the sample is directly correlated with the rate at which absorbance changes. GGT measures are employed in cancer illness detection and treatment.
Serum direct amylase assay
Utilizing a chromogenic substrate called CNPG3 (2-chloro-4- nitrophenyl linked with Galacto-maltoside), the direct amylase assay allows for the release of over 95% of 2-chloro-4-nitrophenol (CNP) by -α Amylase, resulting in the formation of 2-chloro-4- nitrophenyl-α-D-maltoside (CNPG2), maltotriose (G3), and glucose (G). The rate at which 2-chloro-4-nitrophenol is formed is directly correlated with the sample's α-amylase activity, which may be measured using a kinetic test at 405 nm.
Serum lipase assay
In the kinetic colorimetric approach, a 1,2-diglyceride, a naturally occurring long chain fatty acid, is used to measure lipase activity in serum. Pancreatic lipase hydrolyzes the clear substrate solution in the presence of colipase, deoxycholate and calcium ions to form a 2-monoglyceride, which is then converted by a 2-monoglyceride lipase into glycerol. Next, glycerol is measured by glycerol kinase, glycerol phosphate oxidase and peroxidase are the subsequent enzyme reactions that breakdown glycerol and yield a violet quinone monoamine dye, which peaks in absorbance at 550 nm.
Statistical analysis
The mean ± Standard Deviation (SD) was used to express the results. To compare the group mean values, a one-way ANOVAwas employed. Next, to ascertain the difference between the groups, the student-t test, Mann-Whitney U test, Kruskal-Wallis test and Shapiro-Wilk test were employed; a P-value <0.05 was deemed statistically significant. The statistical software program SPSS® was used to perform the analyses (SPSS for windows version 12.0, SPSS Inc., Chicago, Illinois, USA).
Results and Discussion
The sequels of the present experimental strategies are summarized below.
Pancreatic cancer: demographic and clinical features
Table 1 presents the general clinical and demographic aspects of patients with pancreatic cancer. The pancreatic cancer unit treated 500 patients from 2016 to 2020 with definitive chemotherapy or postoperative adjuvant chemotherapy. With a 3:1 male to female sex ratio, there were 500 patients in total 300 men (60%) and 200 women (40%). It would seem from this that men are more likely than women to get cancer. This may be because internationally, men are more likely than women to be addicted to alcohol and tobacco, which is a significant risk factor for pancreatic cancer [11,12]. The average diagnostic age, as mentioned above (Figure 1), was 68.86 years, with at least a maximum of 83.6 years and a minimum of 25.5 years. The majority of our patients range in age from 61 to 80. This study made it abundantly evident that a patient's age (more than 60) is a significant factor in cancer. This may be due to preclinical histories such as high blood pressure and diabetes linked to a certain diet, all of which are known to be risk factors for pancreatic cancer and were further supported by American Cancer Society [12].
| Characteristics | Number of patients | Percentage |
|---|---|---|
| Total persons (n=500) | - | - |
| Control (Normal person) (100) | 100 | - |
| PC Patients (Male) | 300 | 60 |
| PC Patients (Female) | 200 | 40 |
| Sex ratio | - | 03:02 |
| Age | ||
| (<20) | Nil | Nil |
| (21-40) | 37.5 ± 1.50 | 7.37 ± 0.10 |
| (41-60) | 74 ± 2.34 | 14.88 ± 0.22 |
| (61-80) | 310.45 ± 5.46 | 62.5 ± 1.45 |
| (80<) | 75 ± 1.56 | 15.14 ± 0.34 |
| Symptoms | ||
| Jaundice | 325 ± 6.89 | 65.2 ± 0.00 |
| Epigastric pain | 193.75 ± 3.56 | 38.75 ± 1.56 |
| Right hypocondrium pain | 156.25 ± 2.67 | 31.25 ± 0.40 |
| Vomiting and nausea | 144.25 ± 1.54 | 28.5 ± 1.34 |
| Dark urine | 257.5 ± 5.67 | 51.5 ± 0.55 |
| Pruritus | 115.5 ± 1.10 | 31 ± 1.44 |
| Discolored saddle | 188.5 ± 4.45 | 37.75 ± 0.55 |
| Chronic diarrhea | 38.75 ± 0.00 | 7.75 ± 0.00 |
| Fever | 32.5 ± 0.50 | 6.5 ± 0.00 |
| Weight loss | 358.2 ± 5.89 | 71.6 ± 3.56 |
| Loss of appetite | 327.5 ± 4.67 | 65.5 ± 3.87 |
| Tiredness | 467.5 ± 11.67 | 93.5 ± 1.50 |
| Medical histories | ||
| Diabetes | 227.5 ± 2.56 | 45.5 ± 1.45 |
| High blood pressure | 151.2 ± 3.56 | 30.25 ± 0.20 |
| Rheumatism | 17.5 ± 0.10 | 3.5 ± 0.00 |
| Hypothyroidism | 23.75 ± 0.40 | 4.75 ± 0.10 |
Table 1: Demographic, clinical characteristics, and medical history of pancreatic cancer patients.
Figure 1: Demographic and clinical characteristics of pancreatic cancer patient.
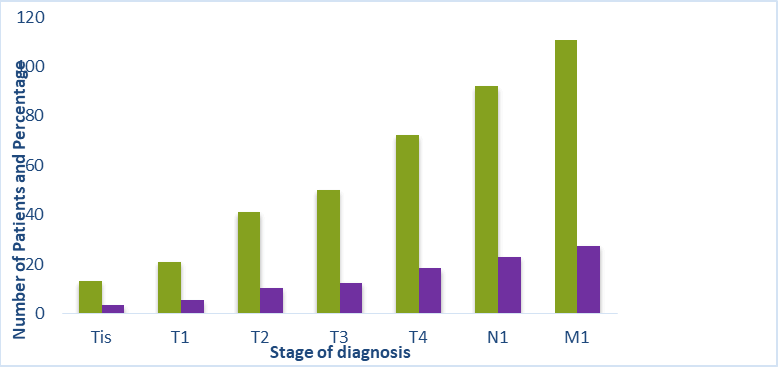
The symptoms and indicators of pancreatic cancer are listed in Table 1. Early pancreatic cancer symptoms are typically nonspecific, and both the patient and the physician frequently overlook them. These include flatulence, diarrhea, vomiting, constipation, and epigastric bloating. Patients have painless jaundice and weight loss as the condition worsens. The location and size of the tumor affect the frequency of symptoms. Jaundice and a tumor in the pancreatic body or tail are always linked to delayed presentation and inoperability because of metastases to the liver or hilar nodes. In this study, several symptoms were noted, including jaundice, nausea and vomiting, epigastric discomfort, right hypochondriac pain, dark urine, itching, discolored saddle, chronic diarrhea, fever, and reduced weight, loss fatigue, and appetite loss. Diabetes accounted for 44.0% of all medical histories, with high blood pressure coming in second at 28%. A few specific cases of rheumatism and hypothyroidism were also noted. In terms of surgical histories, 2.5% had an appendectomy, 13.5% had a hernia, and 27.5% had a cholecystectomy. As indicated in Table 2 and Figure 2, we discovered that the majority of our patients had metastatic stage (M1) 27% (138 patients), followed by node and tumor stages N1–23% (115 patients), T4–18% (91 patients), T3–12% (62 patients), T2-10% (51 patients), and T1-3%, respectively. Additionally, [13,14] revealed that because there are no symptoms specific to PDAC, patients often appear at an inoperable metastatic stage at the time of diagnosis, making early detection challenging. The majority of patients received a diagnosis of Metastatic stage-1 (M1), which may have been caused by the extremely subpar diagnostic methods used in the 1990’s, the absence of medical personnel, the misdiagnosed symptoms of that a significant role also played by cancer, and the pancreatic anatomic deeper site [15]. Due to the cancer's deep location, the American cancer society reported that more than half of individuals with pancreatic cancer receive a diagnosis at an advanced metastatic disease stage [12].
| Tis (Pre-cancer stage) | 16.25 ± 0.00 | 3.25 ± 0.00 |
| T1 (Tumor stage 1) | 26.25 ± 0.10 | 5.25 ± 0.00 |
| T2 (Tumor stage 2) | 51.25 ± 0.00 | 10.25 ± 0.35 |
| T3 (Tumor stage 3) | 62.5 ± 0.55 | 12.5 ± 0.67 |
| T4 (Tumor stage 4) | 91.0 ± 5.89 | 18.2 ± 0.00 |
| N1 (Node stage 1) | 115.0 ± 6.65 | 23.0 ± 0.10 |
| M1 (Metastatic stage) | 137.5 ± 8.12 | 27.5 ± 0.30 |
Table 2: Pre-clinical stage of diagnosis.
Figure 2: Pre-clinical stage of diagnosis.
Changes in serum enzymes biomarker profile
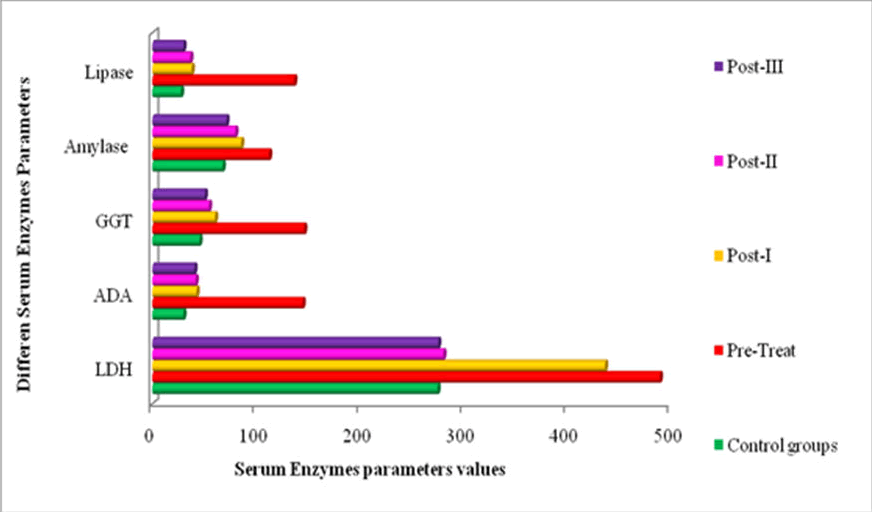
Changes in serum Lactate Dehydrogenase (LDH): Serum LDH levels were considerably higher in PDAC-pretreated patients (489.36.45; 436.58.56; 280.64.78; and 275.64.65 U/l) than in controls (27511.65 U/l), as shown in Table 3 and Figure 3. Following the three consecutive chemotherapies, I, II, and III, the LDH level decreased significantly (P<0.05) to 436.5 ± 8.56, 280.6 ± 4.78, and 275.6 ± 4.65 U/l. serum level of LDH. Because its gene is overexpressed, a rapid rise in the number of malignant cells affects the level of LDH in the cytoplasmic compartment of cells. Patients with pancreatic cancer see a rise in serum LDH levels as a result. These malignant cells' anaerobic glycolysis and ability to meet their metabolic needs are aided by the elevated LDH level [16]. It has been given that LDH is necessary for tumor maintenance, and it has been suggested that it could serve as an indication of tumor aggressiveness and burden [17]. Furthermore, there is proof that serum LDH levels rose in several malignancies and were linked to the prognosis of cancer patients, including PDAC. A common and fundamental feature of cancer metabolism, which is heavily dependent on dysregulated metabolic enzymes, is elevated glycolysis. Lactose Dehydrogenase (LDH) is an enzyme that helps with glycolysis by changing pyruvate into lactate. Several studies show that LDH expression is abnormally elevated in a variety of malignancies, and this is linked to the advancement of the malignancy.
Changes in serum ADA level
Serum ADA levels in PDAC pre-treated patients were greater (144.82 ± 2.55) than in the healthy control group (29.90 ± 1.44 U/l). As indicated by Table 3 and Figure 3, the level of this enzyme tends to decline (42.65 ± 1.87, 41.67 ± 1.45, and 40.5 ± 2.34 U/l) significantly (P<0.05) following the I, II, and III cycles of treatment. ADA is present in most bodily tissues, but its activity is highest in lymphoid tissues. It has been discovered that pancreatic acinar cells are localized in animals and in human studies. Serum ADA activities haven't, however, previously been examined in pancreas problems, as far as we know. It is proposed that the salvage pathway is significantly impacted by elevated ADA activity. This well-known enzyme has been thoroughly investigated in the context of pancreatic carcinogenesis, which is intimately linked to the synthesis of DNA and the metabolism of nucleic acids. The primary causes of it are carcinogen induced pro-oncogene activation or tumor suppressor gene inactivation.
| Serum enzyme markers U/Lit | Patients | Reference level U/Lit | Healthy control groups (100) | Before chemotherapy (Pre-treatments) | After chemotherapy | ||
|---|---|---|---|---|---|---|---|
| (Post-treatments-I) | (Post-treatments-II) | (Post-treatments-III) | |||||
| Serum lactate dehydrogenase | 500 | 200-400 | 275 ± 11.65 | 489.3 ± 6.45 | 436.5 ± 8.56 | 280.6 ± 4.78 | 275.6 ± 4.65 |
| Serum adenosine deaminase | 500 | <43 | 29.90 ± 1.44 | 144.82 ± 2.55 | 42.65 ± 1.87 | 41.67 ± 1.45 | 40.5 ± 2.34 |
| Serum gamma glutamyl transferase | 500 | <55 | 45.5 ± 1.45 | 146.4 ± 4.54 | 60.4 ± 3.65 | 54.6 ± 1.45 | 50.6 ± 1.78 |
| Serum amylase | 500 | <80 | 67.8 ± 2.45 | 112.5 ± 76 | 85.6 ± 4.55 | 80.0 ± 3.45 | 71.4 ± 4.98 |
| Serum lipase | 500 | <38 | 27.6 ± 1.67 | 136.78 ± 2.67 | 37.90 ± 1.33 | 36.56 ± 1.45 | 29.90 ± 0.90 |
| Different superscripts in the same column are significantly different at P<0.05 level (Least Significance Difference) mean followed by +SD | |||||||
Table 3: Changes in serum enzyme markers profile in pre and post-chemotherapy.
Figure 3: Changes in serum enzyme markers profile in pre and post-chemotherapy.
Changes in Gama-Glutamyl Transferase (GGT)
Table 3 and Figure 3 showed that pre-chemotherapy patients exhibited an elevated level of the enzyme GGT (146.4 ± 4.54 U/l). The level of this enzyme was reported to significantly decline to 45 50.6 ± 1.78 U/l following the chemotherapy's third cycle. The role of this enzyme in hepatic and pancreatic diseases is well-established. GGT-specific activities significantly alter in precancerous and cancerous phases of pancreatic tissues. GGT is released into the bloodstream as a result of pancreatic cell injury, which causes an increase in the cells' activity during the initial stages of cancerization. Tissue-specific expression of GGT is altered under a variety of pathologic and physiological circumstances, such as development and carcinogenesis. GGT is a commonly found enzyme that has been thoroughly researched in pancreatic carcinogenesis, which has a tight relationship to the metabolism of nucleic acids and DNA synthesis. The primary causes of it are carcinogen-induced pro-oncogene activation or tumor suppressor gene inactivation.
Changes in amylase and lipase level
Serum amylase levels in PDAC patients who had received treatment were accurately represented as 112.5 ± 76 U/I in Table 3 and Figure 3. Following the III chemotherapy, the level of this enzyme steadily dropped (85.6 ± 4.55, 80.0 ± 3.45 U/l, and 71.4 ± 4.98 U/l). In contrast, the serum lipase levels for patients with prechemotherapy, post-treatment I, post-treatment II, and posttreatment III pancreatic cancer were 136.78 ± 2.67, 37.90 ± 1.33, 36.56 ± 1.45 U/l, and 29.90 ± 0.90 U/l, respectively. The blood levels of serum lipase and amylase in the healthy controls were 27.6 ± 1.67 U/l and 67.8 ± 2.45 U/l, respectively. Pre-treatment patients had considerably greater serum levels of lipase and amylase than the control and post-treatment groups. Because lipase has a longer half-life in serum than amylase due to renal tubular reabsorption, it is more selective and remains elevated longer. Lipose and amylase testing, as well as serial trending them, offer no benefits for tracking a patient's clinical development. Recently, studies on the potential predictive significance of lipase and amylase in intraductal papillary mucinous neoplasm, pancreatitis, and pancreatic cancer have been conducted. One potential new indicator to differentiate acute bouts of alcoholic pancreatitis from non-alcoholic cases is the lipase/amylase ratio.
Conclusion
Males are more likely than females to contract the sickness (3:2), and the risk rises with age (>61-80). Based on current research, it appears that aberrant metabolism leading to changes in serum enzyme levels is a significant factor in PDAC patients. Serum enzyme panel levels have shown severe abnormalities in PDAC patients (P<0.05), indicating a compromised physiological state. The level of these enzyme biomarkers significantly decreased following three consecutive rounds of chemotherapy (1st, 2nd, and 3rd), demonstrating the potential of the chemotherapy medications. According to our research, combination chemotherapy the administration of many medications is a promising treatment option for pancreatic cancer.
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of declaration
The authors would like to acknowledge the department of biochemistry, adyar cancer institute, Chennai for their support in carrying out the research work.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal, A et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
[Crossref] [Google Scholar] [PubMed]
- Dhir V, Mohandas KM. Epidemiology of digestive tract cancers in India IV. Gall bladder and pancreas. Indian J Gastroenterol. 1999;18(1):24-28. [Crossref]
[Google Scholar] [PubMed]
- Gaidhani RH, Balasubramaniam G. An epidemiological review of pancreatic cancer with special reference to India. IJMS. 2021;73(1):99-109. [Crossref]
[Google Scholar] [PubMed]
- Ferreira LM, Hebrant AJ, Dumont JE. Metabolic reprogramming of the tumor. Oncogene. 2012;31(36):3999-4011.
[Crossref] [Google Scholar] [PubMed]
- Xiao Y, Chen W, Xie Z, Shao Z, Xie H, Qin G, et al. Prognostic relevance of lactate dehydrogenase in advanced pancreatic ductal adenocarcinoma patients. BMC Cancer. 2017;17:1-7.
[Crossref] [Google Scholar] [PubMed]
- Sullivan JL, Osborne WR, Wedgwood RJ. Adenosine deaminase activity in lymphocytes. Br J Haematol. 1977;37(1):157-158. [Crossref]
[Google Scholar] [PubMed]
- Yao DF, Dong ZZ, Yao DB, Wu XH, Wu W, Qiu LW, et al. Abnormal expression of hepatoma-derived gamma-glutamyltransferase subtyping and its early alteration for carcinogenesis of hepatocytes. Hepatobiliary Pancreat Dis Int. 2004;3(4):564-570. [Crossref]
[Google Scholar] [PubMed]
- Chase CW, Barker DE. Serum amylase and lipase in the evaluation of acute abdominal pain. Am Surg.1996;62(12). [Crossref]
[Google Scholar] [PubMed]
- Kjeld M. An automated colorimetric method for the estimation of lactate dehydrogenase activity in serum. Scand J Clin Lab Invest. 1972;29(4):421-425.
[Crossref] [Google Scholar] [PubMed]
- Giusti G, Galanti B. Colorimetric method. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 1st edition. Weinheim, Germany: Verlag Chemie, 1984:315-323. [Crossref] [Google Scholar] [PubMed]
- Schiffman SC, Chu CK, Park J, Russell M, Keilin S, Kooby DA, et al. Is prior cholecystectomy associated with decreased survival in patients with resectable pancreatic adenocarcinoma following pancreaticoduodenectomy?. Am J Surg. 2011;201(4):519-524.
[Crossref] [Google Scholar] [PubMed]
- American Cancer Society. American Cancer Society’s publication, Pancreatic Cancer. 2016. [Crossref] [Google Scholar] [PubMed]
- Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B. Cancer Incidence in Five Continents. Vol X. Lyon: IARC Scientific Publication. 2014. [Crossref] [Google Scholar] [PubMed]
- Gillen S, Schuster T, Meyer zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS medicine. 2010;7(4):e1000267. [Crossref]
[Google Scholar] [PubMed]
- Brand RE, Greer JB, Zolotarevsky E, Brand R, Du H, Simeone D, et al. Pancreatic cancer patients who smoke and drink are diagnosed at younger ages. Clinical Gastroenterology and Hepatology. 2009;7(9):1007-1012. [Crossref]
[Google Scholar] [PubMed]
- Khan N, Tyagi SP, Salahuddin A. Diagnostic and prognostic significance of serum cholinesterase and lactate dehydrogenase in breast cancer. Indian J Pathol Microbiol. 199;34(2):126-30. [Crossref]
[Google Scholar] [PubMed]
- Xiao Y, Chen W, Xie Z, Shao Z, Xie H, Qin G, et al. Prognostic relevance of lactate dehydrogenase in advanced pancreatic ductal adenocarcinoma patients. BMC Cancer. 2017;17:1-7.
[Crossref] [Google Scholar] [PubMed]
Citation: Prabasheela B, Sivakumar N, Arivazhagan R, Vijayalakshmi R (2025) Prognostic Values of Enzyme Changes in Pre and Post- Treated Pancreatic Cancer Patients. Bio Med. 17:764.
Copyright: © 2025 Prabasheela B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.