Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 6
"Preparation of CuO-NPs Coated Cotton, Starched Cotton and its CuO-Ag Nanocomposite, Cu(II) Curcumin Complex Coated Cotton and their Antimicrobial Activities".
Issa M. El-Nahhal1*, Jamil Salem1, Fawzi S. Kodeh1, Rawan Anbar1 and Abdelraouf Elmanama22Department of Medical laboratory sciences, Islamic University of Gaza, P.O Box 108, Gaza, Palestine
Received: 03-Jun-2021 Published: 23-Jun-2021, DOI: 10.35248/2157-7439.21.12.568
Abstract
Copper oxide nanoparticles (CuO-NPs) coated cotton, starched cotton and their functionalized CuO-Ag nanocomposites and Cu(II) curcumin complex coated cotton have been prepared. The use of starched cotton materials instead of pristine cotton is to minimize the leaching of CuO-NPs. The use of none-toxic biocompatible starch has improved the adhesion properties of the cotton fibers and enhanced its durability towards CuO-NPs. The antimicrobial activities of these coated cotton materials have been examined. The results have showed excellent antimicrobial activity against E. coli and S. aureus for all coated materials. Since that these materials have strong antimicrobial activity therefore, they could also have excellent antiviral activity and can be used to combat the spread of COVID-19 Corona Virus and attempts for minimizing corona virus outbreak. The use of textile fabrics in health facilities such as medical cloths for doctors, nurses, cleaning personnel and patients, could contribute to combat the spread of COVID-19 Corona Virus.
Introduction
Pathogenic microorganisms such as bacteria, viruses, parasites, and fungi and their caused infection are of great interest for the public health nowadays. Therefore, the world has recently encountered a great efforts for potential research to meet the essential demands in the field of public health [1,2]. Part of this effort is due to the resistances of microbial activity against classical antimicrobial therapies increased due to increase in the ability of these organisms to develop resistance to virtually all antimicrobial systems [3]. New strategies are recently developed and directed towards the use various types of metals or metal oxides nanoparticles coated textiles to impart their antimicrobial activity. The new materials showed high stability and antibacterial effectiveness even after intensive laundry regimes are employed in hospitals [4,5]. More recently inorganic metals and metal oxides nanoparticles coated cotton composites have attracted attention, due to their significant antimicrobial activities [6-13]. Several types of masks including cotton masks, surgical masks with nanofibers and new medical masks and effective cloth masks were used for protection [14-18]. Special attention has been directed toward the use of antimicrobial coated cotton fabrics e.g. medical facilities including cloth face masks to minimize the chance or slow down the spread of Covide-19 Corona Virus [19-25]. The Covid-19 is the largest global public health outbreak nowadays based on WHO reports, the main source of the infection comes throughout transmission basically from infected person to a new host by several means. In order to prevent the spread of viral particles is to use functionalized antimicrobial and antiviral materials for protection from bacterial or viruses. There is an increasingly demand for safe and stable antimicrobial textile materials with less leachable, and more effective from transmitted bacterial or viruses through the use of metal oxides modified medical cloths, face masks and other forms of cleaning surfaces materials. Therefore, protective clothing, such as uniforms for health-care workers made with functionalized materials with antimicrobial finishing agents, could be an alternative to reduce and prevent COVID-19 and Healthcare-Associated Infections (HAIs) [26,27]. Copper-based antimicrobial agents were employed to develop antimicrobial and antiviral material [28]. There is no any firmed claim that the natural biodegradable and biocompatible biopolymers chemically linked with metal oxides have been recently used to enhance the nanoparticles stability. The reported work of using the enzymes, chemicals or binding agents as tools for enhancement the activation of textiles may result in changes in the nature character of the cotton fibers [29,30]. Instead of that, our research group are the first people, who have recommended the use of surfactants for preparation of stable antimicrobial metal oxides coated cotton [6,31]. The purpose of using surfactants is to stabilize metal oxides nanoparticles by controlling their shape and size as coated species [6,31]. In our previous article, we have modified our research methodology and prepared of very stable and efficient antimicrobial active material by using starch material as none toxic material and compatible with cotton cellulose to enhance the stability, adhesion properties of the surface of cotton fiber surface towards immobilization of ZnO nanoparticles [32]. In this context, in an analogous method, we have also used starched cotton fabrics for coating copper oxide nanoparticles on the cotton fabrics. Figure 1a presented the synthetic pathway of CuO-NPs coated cotton and starched cotton and their antimicrobial activity against pathogenic bacteria. The starched cotton fibers (1-3w% concentrations of starch) were used to deposit copper oxide nanoparticles. The durability results showed that starched cotton samples exhibit much higher deposition of copper oxide nanoparticles than the corresponding samples prepared without using starch. Minimum leaching of CuO-NPs from the cotton fabrics was observed even after 10 washings cycles. Figure 1b presented some protected medical facilities which may actively can prevent the spread of Covide-19. The functionalized CuO-Ag nanocomposite and Cu(II) curcumin complex materials exhibit significant antimicrobial activity against E. coli and S. aureus. Several methods were employed for investigation the morphology and their chemical structure properties of the cotton coated materials including XRD, SEM and TEM analysis.
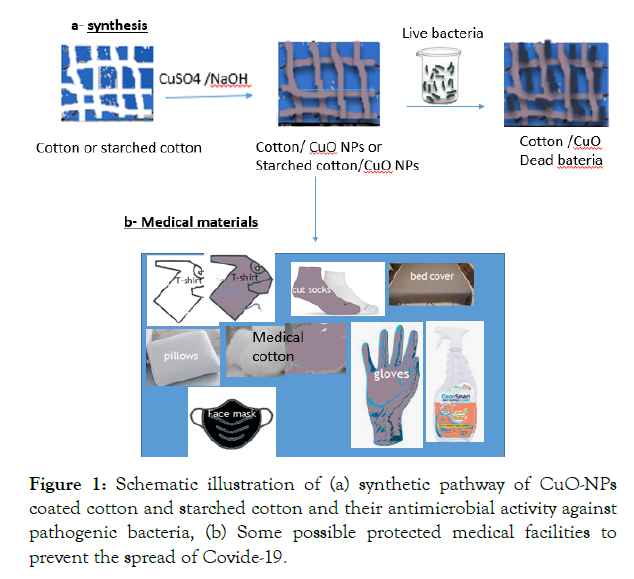
Figure 1: Schematic illustration of (a) synthetic pathway of CuO-NPs coated cotton and starched cotton and their antimicrobial activity against pathogenic bacteria, (b) Some possible protected medical facilities to prevent the spread of Covide-19.
Materials and Methods
Chemical and Reagents: Copper acetate monohydrates (Cu(CH3COOH)2.H2O) and sodium hydroxide (NaOH) were purchased from Hi Media, India. Starch (from corn) was purchased from Merck. A Turkish cotton (100% cotton) was purchased from local market and pretreated before used. Dehydrated nutrient agar powder, nutrient broth powder, Sabouraud Dextrose Agar (SDA), Di-Chloran Rose Bengal Chloramphenicol (DRBC) agar and Potato Dextrose Agar (PDA), were purchased from (HiMedia, India) and used to prepare culture media.
Analytical methods: Chemical analysis of the CuO-NPs coated cotton, coated starched cotton and their functionalized CuO-Ag nanocomposite and Cu(II) curcumin complex materials were investigated using various techniques: scanning electron microscopy (SEM) using Carl Zeiss AG - EVO® 60), transmission electron microscope (TEM) JEM2010 (JEOL), X-ray diffraction (XRD) using EQ uniox 3000, INEL, France.
Preparation of starched cotton fibers: Cotton fibers were firstly washed with 1% solution of sodium dodecyl sulfate (SDS) at 60°C for 2 hrs., rinsed out the excess of SDS with distilled water several times and dried under vacuum (0.1 torr) at 80°C for 24 hrs. The dried cotton fibers were starched using three different concentrations (1-3 starch wt. %) by treating cotton fibers (3.0 g) with 30 mL of different concentrations (1-3 starch wt. %) of corn starch at 70°C for 3 hrs. The starched cotton fibers were washed with distilled water and dried under vacuum (0.1torr) at 60°C for 48 hrs [32].
Coating Process: CuO-NPs coated cotton and starched cotton materials were prepared as previously described method [32] by treating 1.00 g of cotton or starched cotton with copper (II) acetate (0.01mol) in presence of NaOH as base (0.02 mol) in 100 mL deionized water. The mixture was irradiated using Ultrasonicator (Model US-150 Ti-horn, 20 kHz, output 10 Turning 7) for 60 minutes, while rotating the cotton sample at speed 250 rps to ensure that cotton or starched cotton fabrics were homogeneously coated with CuO-NPs. CuO-NPs coated cotton or coated starched cotton materials were then washed thoroughly several times with distilled water and dried at 80 °C overnight. These coated materials are labeled as CuO/cotton and CuO/starched cotton (1-3 starch wt.%).
Preparation of CuO-Ag nanocomposite coated cotton: The nanocomposite CuO-Ag coated cotton was prepared as previously reported [32] by treatment of 200 mg of CuO-coated cotton with 15 ml of Ag-NPs sol (0.10 mM) (Figure2 a). The mixture was vigorously shaken for 10 minutes to ensure complete interaction with Ag-NPs sol. Dark brown of CuO-Ag nanocomposite coated cotton material (Figure 2 b) was separated, washed with two successive portions of 20 ml distilled water to remove the unreacted Ag-NPs and dried at 70°C under vacuum (0.1torr) for 24 hrs.
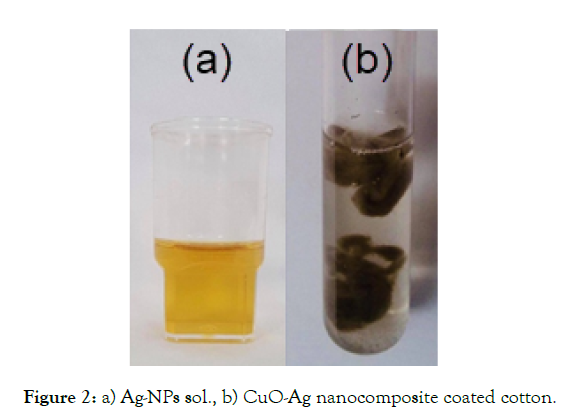
Figure 2: a) Ag-NPs sol., b) CuO-Ag nanocomposite coated cotton.
Functionalized cotton and CuO/cotton with curcumin [32]: 200 mg of pristine cotton and CuO coated cotton samples were separately treated with 10 ml of ethanol solution of curcumin (2.71*10-3M). An brownish yellow of copper(II) curcumin complex coated cotton was formed (Figure 3a & b). The curcumin Cu(II) complex coated cotton and curcumin/cotton were washed with three successive portions of 20 ml ethanol to remove free curcumin and dried at 70 °C under vacuum (0.1torr) for 24 hrs.
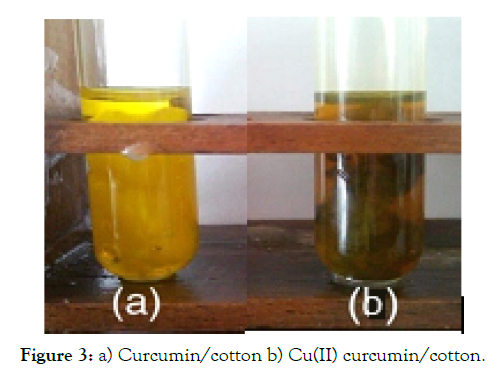
Figure 3: a) Curcumin/cotton b) Cu(II) curcumin/cotton.
Antimicrobial activity tests: The antimicrobial activities of CuO/ cotton, CuO/starched cotton, CuO-Ag/cotton nanocomposite and Cu(II) curcumin complex materials were examined against the Gram-negative Escherichia coli and the Gram-positive Staphylococcus aureus. The antimicrobial activities were tested according to the standard quantitative test (AATCC 100, 2004) with some modifications [32]. Two pieces were examined for each sample, a piece of the coated cotton was tested against a known concentration of bacterial suspension and the reduction in the viable cell was calculated. A dry piece of uncoated cotton material (100 mg) was used for the antimicrobial test. The two pieces were sterilized by autoclaving and by UV-sterilization before testing the antimicrobial activity tests were done. The cotton materials “Test and Control “were soaked in sterile normal saline and incubated for a standard time without shaking. A preliminary antibacterial test was carried out for CuO/starched cotton of different concentrations of starch (1-3 wt.% starch), where the cotton materials were washed with water very well and then allowed to dryness.
Two cotton pieces “Test and Control “were inoculated with 500 μL of the bacterial suspension and then each piece was inserted in a vial containing 20 ml of sterile physiologic saline solution (NaCl 0.9%). The vials were tightly closed and allowed to vigorous shaking for about 1 min. and then incubated at 37 °C for 24 hr. After incubation, 1000 μL of each sample was taken and serially diluted with 0.9% NaCl solution and 100 μL of each dilution was transferred onto nutrient agar plates. The plates were allowed to grow overnight at 37°C and the viable bacteria were counted.
Results and Discussion
Durable Results: In comparison of the durable results of CuO-NPs onto cotton and starched cotton fabrics with that of ZnO-NPs [32]. It is found that less wash durable with lower content of CuO-NPs were deposited onto the cotton or starched cotton fabrics at same conditions than that of ZnO-NPs [32]. This probably refers to the difference in shape and size of nanoparticle of the two materials.
Washing durability tests: Wash durability results for CuO-NPs coated cotton at different washing cycles (5 and 10 washing cycles) and compared with the corresponding CuO-coated starched cotton (1-3 starch wt. %) are shown in Figure 4. There is a significant reduction of leaching of CuO-NPs from cotton materials after 5 and 10 washing cycles in presence of starch (Figure 4), this reduction of leaching of CuO coated species occurs upon using starch as natural adhesive material. The reduction of leaching has decreased in absence or using low concentration of starch (Figure 4).
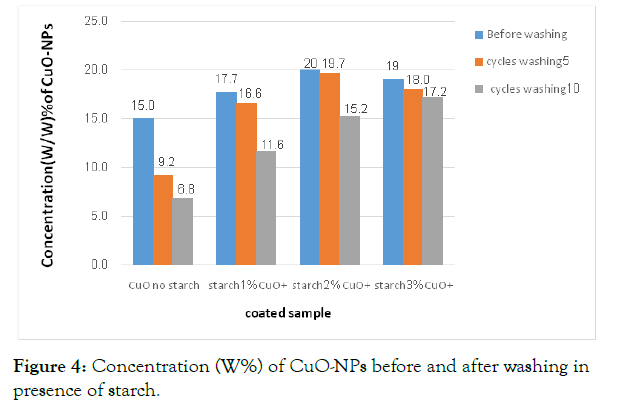
Figure 4: Concentration (W%) of CuO-NPs before and after washing in presence of starch.
The results starched cotton fibers showed higher coating CuO content than in the case of pristine cotton. The leaching percentage decreases upon increasing the percentage of starch from 1 to 3%. The least leaching of CuO-NPs was observed when 3% of starch was used. The presence of starch has great effect in minimizing on leaching of metal oxide from cotton fabrics. Similar behavior was reported in using ZnO-NPs coated cotton and starched cotton, but with presence of high content of ZnO-NPs deposited onto starched cotton even after application of 10 washing cycles [32].
The morphological analysis before and after deposition of CuONPs onto cotton fibers was examined by SEM and the images are presented in Figure 5a-d. SEM of the cotton blank (image a) isclearly showed grooves and fibrils on the surface of the fiber. The morphology of CuO-NPs coated cotton fibers in the absence of starch, small agglomerates of CuO-NPs as nano wire pieces of different lengths are well adhered to the surface of cotton fabrics (Figure 5, image b). The morphology of CuO-NPs coated starched cotton is different from that coated onto pristine cotton, this is probably that the presence of starch onto the surface of cotton fabrics has changed the morphology of CuO-NPs. It showed large agglomerates of nano fibers or flakes of CuO-NPs, that well adhered onto the surface of cotton fabrics. (Figure 5, image c). Upon formation of CuO-Ag nanocomposite by treatment of CuONPs coated cotton material with Ag-NPs, a new morphology of CuO-Ag particles which is fully covered the cotton fabrics surface with dense nano shells or flakes fully covered and adhered onto the surface of the cotton fabrics (Figure 5, image d).
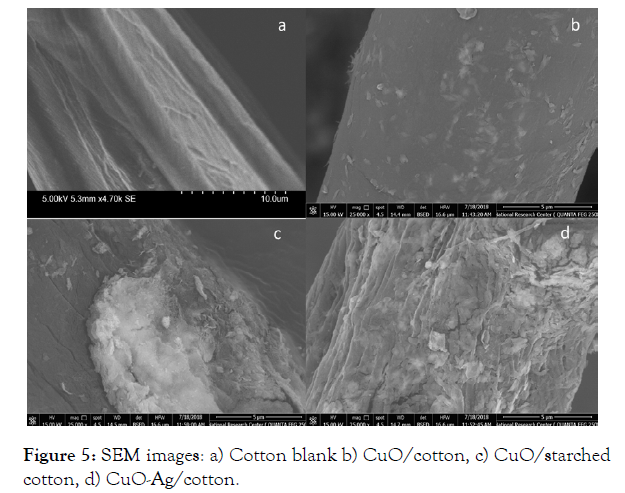
Figure 5: SEM images: a) Cotton blank b) CuO/cotton, c) CuO/starched cotton, d) CuO-Ag/cotton.
TEM Analysis
TEM analysis of sol Ag-NPs and its CuO-Ag nanocomposite coated cotton images are given in Figure 6a & b. The average particle size of Ag-NPs spheres has decreased from ca. 43 nm to ca. 29 nm upon interacted with CuO-NPs to form CuO-Ag nanocomposite (Figure 6b).

Figure 6: a) TEM image of Ag-NPs sol, b) TEM image of CuO-Ag nanocomposite.
XRD analysis
XRD patterns of CuO-NPs coated cotton, CuO-NPs starched cotton and CuO-Ag coated cotton are presented in Figure 7(a-c).
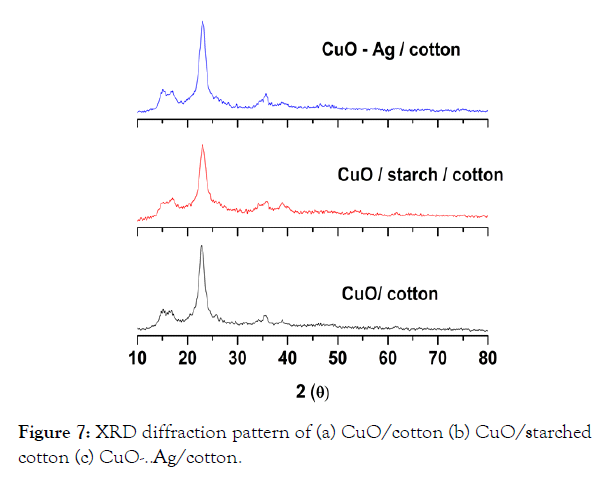
Figure 7: XRD diffraction pattern of (a) CuO/cotton (b) CuO/starched cotton (c) CuO-..Ag/cotton.
The diffraction pattern reveals a weak or broad diffraction peaks of CuO-NPs, which is well indexed with monoclinic structure formed onto the surface of cotton fibers [12,13,18,24]. The pattern corresponds to the diffraction peaks which centered at 2θ = 33.5, 35, 38.5, 48, 53 and 57° assigned to the 110, -111, 111, -202, 020 and 202 reflection lines of monoclinic CuO-NPs [12,13,18,31]. The crystallite sizes of the particles were not calculated by using Scherrer‘s equation due to low intensity diffraction peaks.
Antimicrobial activity of CuO-NPs coated cotton [32]
The antibacterial activity of theCuO-NPs coated cotton were applied according to the standard method AATCC 100. The reduction percentage (%) was calculated by the following formula:
R= 100 (B – A)/B, where R = reduction percentage (%), A = the number of microbial cells recovered from the inoculated coated cotton material incubated 24 hrs contact period, B = the number of microbial cells recovered from the inoculated uncoated cotton material immediately after inoculation (at “0” contact time). The antimicrobial results for all tested materials were compared with that of the control samples. The antimicrobial activity for CuO-NPs coated cotton and coated starched cotton against E. coli and S. aureus are given in Figure 8a & b.
The results confirmed that CuO-coated starched cotton samples showed an excellent antimicrobial activity against both E. coli and S.aureus in comparison with that of CuO coated cotton without starch [32]. The starched cotton of (3% content starch) showed almost 100% reduction antimicrobial results against both E. coli and S. aureus (Figure 8). In general, the antimicrobial results for CuO-NPs coated cotton and starched cotton showed a less efficient than that of analogous ZnO-NPs [32]. The antimicrobial activities of CuO-NPs coated cotton or coated starched cotton against E. coli and S. aureus after 10 cycles test were compared with some previous studies (Table 1). The data support the excellent activity of using starched cotton as stabilizing for CuO.
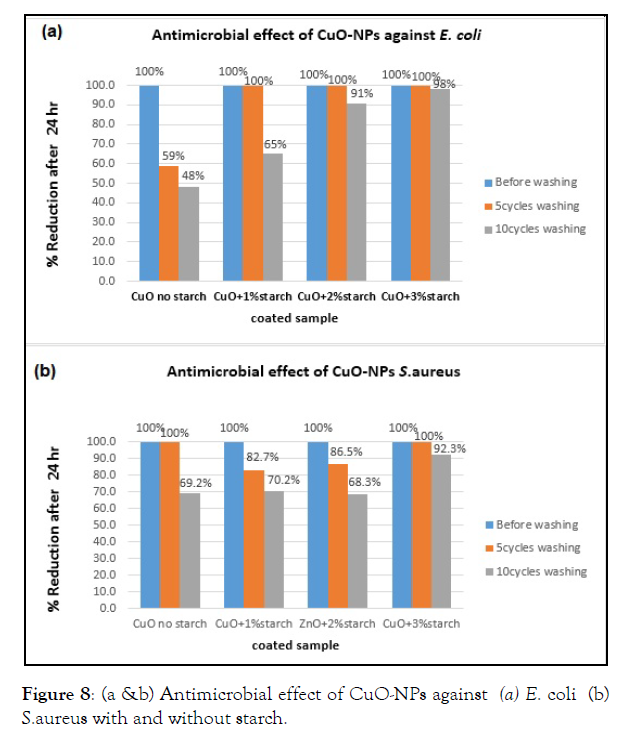
Figure 8: (a &b) Antimicrobial effect of CuO-NPs against (a) E. coli (b) S.aureus with and without starch.
| Material | Reduction of E. coli (%) | Reduction of S. aureus (%) | Ref. |
|---|---|---|---|
| CuO/cotton | 48 | 69 | This work |
| CuO/cotton-starch(3%) | 98 | 92 | This work |
| CuO/cotton-SDS | 95 | 90 | 31 |
| CuO/cotton-HY | 95 | 91 | 31 |
| ZnO/cotton-CTAB | 48 | 82 | 31 |
Table 1: Comparison of antimicrobial activity results of CuO-NPs coated cotton materials against E. coli and S. aureus after 10 cycles.
The antibacterial activity results for curcumin/cotton and its Cu(II) curcumin complex/cotton showed that the Cu(II) curcumin complex coated cotton showed a high reduction of 94-95% for both E. Coli and S. Aureus when compared with that of curcumin/cotton (Figure 9) [32]. The reason for this behavior is that Cu(II) curcumin complex showed a better antimicrobial than that of curcumin/cotton). The antimicrobial activity for CuO-Ag/cotton material is higher for E. coli, than that of CuO/cotton (Figure 8). This is probably that MO-Ag nanocomposite have smaller particle size than that of the corresponding metal oxides [32-35]. It is notable that the antimicrobial activity of CuO-Ag/cotton for E. Coli. is higher than that of S. aureus (Figure 10). The reason for this behavior is probably that the cell wall of S. aureus is thicker than that of E. Coli.
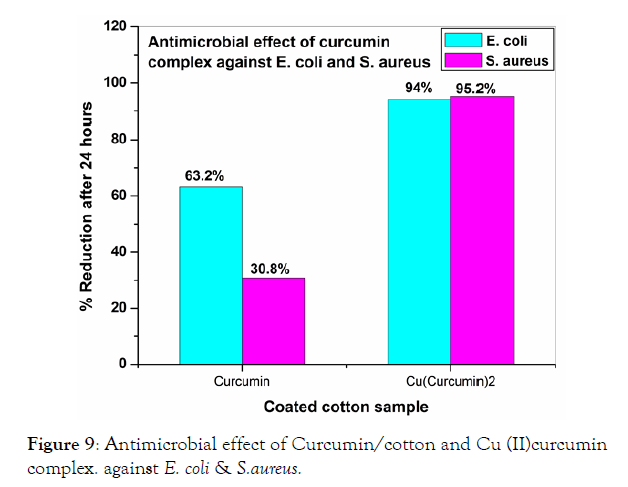
Figure 9: Antimicrobial effect of Curcumin/cotton and Cu (II)curcumin complex. against E. coli & S.aureus.
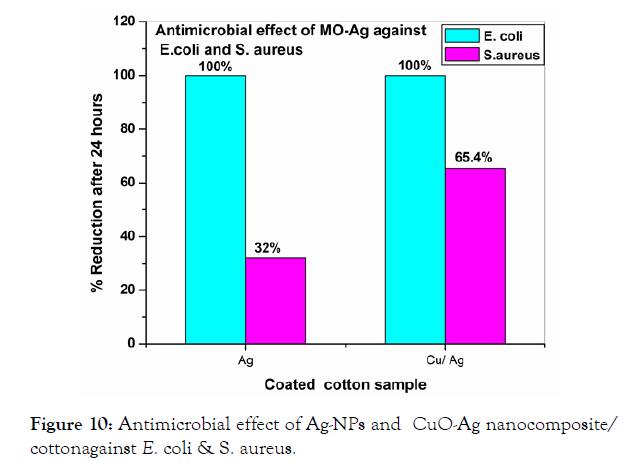
Figure 10: Antimicrobial effect of Ag-NPs and CuO-Ag nanocomposite/ cottonagainst E. coli & S. aureus.
The antibacterial activity of CuO particles mechanism of action is not fully understood. The generation of hydrogen peroxide could be the main factor of antibacterial activity, or binding of CuO particles on bacterial surface via electrostatic forces could be a mechanism. The results of this study showed notable higher antibacterial activity compared with other tested preparations. This might be due synergy produced by the different mechanisms of killing exhibited by Silver and copper.
Conclusion
In this work, CuO-NPs are immobilized in/onto the surface of cotton and starched cotton substrates via ultrasonic irradiation method. The used of starch as none toxic material for coating cotton fibers is to improve the adhesion properties of the cotton fibers and to increase the stability of CuO-NPs coated cotton fabrics. The durability of the coated CuO-NPs/starched cotton is significantly enhanced resulting of less leaching of the coated species from the surface of coated starched cotton. This is apparently increased remarkably its antimicrobial activity. Functionalized of CuO-Ag nanocomposite coated cotton and Cu(II) curcumin complex/ cotton materials exhibit that the antimicrobial activity results for curcumin/cotton is significantly enhanced when the curcumin solution is added to the CuO/cotton upon formation Cu(II) curcumin complex. The antimicrobial activity of CuO-NPs coated cotton fabrics was enhanced when CuO-Ag/cotton is formed.
Conflicts of Interest
The authors have no conflict of interest with respect with current manuscript
Acknowledgments
The authors would like to thank the chemistry department of Al- Azhar University of Gaza for their generous financial support of this research.
REFERENCES
- ZorrillaVaca A, EscandónVargas K. La Importancia del control y prevención de enfermedades infecciosasen anestesiología. Rev Colomb Anestesiol. 2017;45:69–77.
- Van Baarle D, Bollaerts K, Del Giudice G, Lockhart S, Luxemburger C, Postma MJ et al. Preventing infectious diseases for healthy ageing: The VITAL public-private partnership project." Vaccine. 2020; 38, 5896–5904.
- B. Bakan CT, Kayhan ÇK, Karayıldırım M, Dağdeviren S, Gülcemal Y, Yıldırım S, et al. “Synthesis, characterization, toxicity and in vivo imaging of lysine graft polymeric nanoparticles. J Polym Res.2019;26:239.
- Perelshtein I, Lipovsky I, Perkas N, Tzanov T, Gedanken A. Sonochemical co-deposition of antibacterial nanoparticles and dyes on textiles. Beilstein J Nanotechnol. 2016;7:1–8.
- Salat M, Petkova P, Hoyo J, Perelshtein I, Gedanken A, Tzanov T et al. Durable antimi-crobial cotton textiles coated sonochemically with ZnO nanoparticles embedded in an in-situ enzymatically generated bioadhesive. Carbohydr Polym. 2018;189:198–203.
- El-Nahhal MI, Elmanama AA, ElAshgar MN, Amara N, Selmane M, Chehim MM et al. Stabilization of nano-structured ZnO particles onto the surface of cotton fibers using different surfactants and their antimicrobial activity Ultrason. Sonochem. 2017;38:478-487.
- Borah JP, Barman J, Sarma KC. Structural and optical properties of ZnS nanoparticles. Chalcogenide Lett. 2008;5:201–208.
- Wang H, Zakirov A, Yuldashev S U, Lee J, Fu D, Kang T et al. ZnO films grown on cotton fibers surface at low temperature by simple two-step process. Mater Lett. 2011;65:1316-1318.
- Borkow G, Gabbay J, Copper an ancient remedy returning to fight microbial and viral infections. Curr. Chem Biol. 2009;3:272–278.
- Abramov OV, Gedanken A, Koltypin Y, Perkas N, Perelshtein I, Joyce E et al. Pilot scale sonochemical coating of nanoparticles onto textile to produce biocidal fabrics. Surf Coat Technol. 2009;204:718–722.
- El-Nahhal IM, Zourab SM, Kodeh FS, Elmanama AA, Selmane M, Genois I et al. Nano-structured zinc oxide–cotton fibers: synthesis, characterization and applications. J Mater Sci Mater. 2013;24:3970-3975.
- El-Nahhal IM, Zourab SM, Kodeh FS, Selmane M, Genois I, Babonneau F et al. Nanostructured copper oxide-cotton fibers: synthesis, characterization, and applications. Int Nano Lett. 2012;2:1-5.
- Perelshtein I, Applerot G, Perkas N, Wehrschetz-Sigl E, Hasmann A, Guebit G et al. CuO-cotton nanoparticles: formation, morphology and antibacterial activity. Surf Coat Technol. 2009;204:54-57.
- Dorfman D, Raz M. Mask Exemptions During the COVID 19 Pandemic — A New Frontier for Clinicians. JAMA Heal Forum. 2020;1(7): e200810.
- Skaria SD, Smaldone GC. Respiratory source Control using surgical masks with nanofiber media. Ann Occup Hyg. 2014; 58(6):771-781.
- Huang JT, Huang VJ. Evaluation of the efficiency of medical masks and the creation of new medical masks. J Int Med Res. 2007;35(2):213-223.
- Centers for Disease Control and Prevention. Infection Control: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | CDC. Published. 2020;8:10, 2020.
- Chughtai AA, Seale H, Macintyre CR. Effectiveness of Cloth Masks for Protection Against Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020;26:(10).
- HospiMedica International. Reusable Antimicrobial Mask Neutralizes Close to 99% Coronavirus Even After 100 Washings -COVID-19.2020;8:10.
- Bort-Swiss Orthopaedic Supply. Copper, Silver infused Cotton Face Mask, Antimicrobial, Reusable, Washable 30 times, Essential-BSOS-Bort-Swiss Orthopedic Supply. 2020;08:10.
- Horvath H. Best antimicrobial face masks, according to medical experts. NBC News.2020;08:10.
- Bashiri Rezaie A, Montazer M, Mahmoudi Rad M. A cleaner route for nanocolouration of wool fabric via green assembling of cupric oxide nanoparticles along with antibacterial and UV protection properties. J Clean Prod. 2017;08:046
- Radetic M, Darka RR, Markovic M, Radetic´MR, Markovic D. Nano-finishing of cellulose textile materials with copper and copper oxide nanoparticles. Mater Sci Forum. 2019;26 :(17)8-28.
- El-Nahhal IM, Elmanama AA, Amara N, Qodih FS, Selmane M, Chehimi MM at al. The efficacy of surfactants in stabilizing coating of nano-structured CuO particles onto the surface of cotton fibers and their antimicrobial activity. Mater Chem Phys. 2018;215:221-228.
- Mitrano DM, Motellier S, Clavaguera S, Nowack B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int. 2015;77:132-147.
- Hanczvikkel A, Víg A, Tóth Á. Survival capability of healthcare-associated, multidrug-resistant bacteria on untreated and on antimicrobial textiles. J Ind Text. 2018;48:1113–1135.
- Muller MP, Mac Dougall C, Lim M. Antimicrobial surfaces to prevent healthcare-associated infections: A systematic review. J Hosp Infect. 2016;92: 7–13.
- Luz E Román, Enrique D Gomez, José L, Solís Mónica M. Antibacterial Cotton Fabric Functionalized with Copper Oxide Nanoparticles. Molecules. 2020;25: 5801.
- Varaprasad K, Raghavendra G M, Jayaramudu T, Seo J. Nano zinc oxide– sodium alginate antibacterial cellulose fibres. Carbohydr Polym. 2016;135:349–355.
- Ghayempour S, Montazer M. Ultrasound irradiation based in-situ synthesis of star-like Tragacanthgum/zinc oxide nanoparticles on cotton fabric. Ultrason Sonochem. 2017;34:458–465.
- El-Nahhal M I, Elmanama AA, Amara N, Qudih F, Selmane M, Chehim M et al. The efficacy of surfactants in stabilizing coating of nano-structured CuO particles onto the surface of cotton fibers and their antimicrobial activity. Mater Chem Phys. 2018;215:221-228.
- El-Nahhal IM, Salem JK, Anbar R, Kodeh FS, Elmanama A. Preparation and antimicrobial activity of ZnO-NPs coated cotton starch and their functionalized ZnO-Ag/ cotton and Zn(II) curcumin/cotton materials. Scientific reports. 2020;10: 5410.
- Joydeb M, Srishti G, Nagaraju S, Nivedita S, Rohit KR. Biomimetic Method To Assemble Nanostructured Ag@ZnO on Cotton Fabrics: Application as Self-Cleaning Flexible Materials with Visible-Light Photocatalysis and Antibacterial Activities. ACS ApplMaterInterfaces.2015;7:8076−8082.
- Sofia M, Costa DP, Ferreira AF, Filipe VRF. Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures. Nanomaterials. 2018;8:1069.
- Nasrin G, Farid J, Roya Z. CuO and Ag/CuO nanoparticles: Biosynthesis and antibacterial properties. Materials Letters. 2017;196:78–82.
Citation: El-Nahhal IM, Salem J, Kodeh FS, Anbar R, Elmanama A (2021) Preparation of CuO-NPs coated cotton, starched cotton and its CuO-Ag nanocomposite, Cu(II) curcumin complex coated cotton and their Antimicrobial Activities. J Nanomed Nanotech. 12: 566.
Copyright: © 2021 El-Nahhal IM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


