Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 7
Phytogenic Feed Additive Supplemented Diets as Welfare Promoters under Acute and Chronic Stress Factors in Gilthead Seabream
Eleni Antoniadou, Ioannis T Karapanagiotidis, Panagiota Panagiotaki and Eleni Golomazou*Received: 04-Jul-2023, Manuscript No. JARD-23-21992; Editor assigned: 07-Jul-2023, Pre QC No. JARD-23-21992 (PQ); Reviewed: 21-Jul-2023, QC No. JARD-23-21992; Revised: 28-Jul-2023, Manuscript No. JARD-23-21992 (R); Published: 04-Aug-2023, DOI: 10.35248/2155-9546.23.14.776
Abstract
Stress in aquaculture can be modulated by specific stress-limiting factors such as Phytogenic Feed Additives (PFAs), known for their welfare-promoting effects. Three stress trials with gilthead seabream were, therefore, conducted aiming at evaluating the possible beneficial role of three PFAs as welfare-promoters under stress-induced farming conditions, such as starvation (Trial I: Fish starvation for 14 days), high-density (Trial II: Fish were stocked in aquaria at 1.2 Kg/m3 and 2 Kg/m3) and intense handling procedures (Trial III: Fish were kept out of water in the open air for 5 min). Seven dietary treatments were supplemented with Cannabis sativa seed oil, Origanum vulgare, and Cinnamomum zeylanicum essential oils at 1% and 2%. DNA damage in hepatocytes and erythrocytes and blood cortisol were assessed as stress indices. Diets supplemented with PFAs proved to decrease induced genotoxicity under starvation in most cases and under high-density stocking conditions in the case of OR1% and CAN1% groups. However, their genoprotective role was not clear under intense netting procedures. Their positive impact was more obvious in cortisol values in all trials. Differences presented between the PFAs, the applied doses, and the examined tissues may be related to the toxic effects of PFAs and variations in DNA damage and repair mechanisms.
Keywords
Origanum vulgare; Cinnamomum zeylanicum; Cannabis sativa; Chronic and acute stress factors; Sparus aurata; Aquaculture
Introduction
Aquaculture is one of the fastest-growing sectors of animal production with a significant impact on economic growth [1]. The continuous growth of the sector, which nowadays has reached 122.6 million tons, is based on the intensification of production [2]. However, fish in intensive aquaculture systems come through adverse conditions that impair their health status and well-being [3]. Aquaculture practices tend to cause inevitable acute and chronic stress with fish being vulnerable to diseases associated with significant losses during production [4–6].
Factors such as handling, starvation, and stocking density can be highlighted as crucial. The handling of fish in intensive farms is an unavoidable practice [7,8], and involves chasing, netting, and air exposure [9]. Hypoxia is an extremely stressful condition [10], and the removal of fish from the water should be done only when necessary [11,12]. Handling causes acute stress and can affect the stress response of fish, inducing DNA damage in aquatic organisms [13]. These biochemical and physiological alterations may result in a decrease in fish resistance to pathogenic microorganisms, thus increasing susceptibility to diseases [14,15]. Feeding and feed compensation aquaculture practices are under scientific evaluation for their biological and behavioural consequences, since there is a growing concern for fish welfare [16]. Starvation during feed compensation is a severe stress factor for animals’ survival and development [17]. The organism can adapt to maintain homeostasis and regulate its metabolism, making physiological, biochemical, and behavioral changes [18]. In intensive aquaculture, applying a short period of starvation is a good practice before specific manipulations as it contributes to the cleaning of fish’s digestive tract, and reduces metabolic rate and stress, while it does not degrade water quality [19]. Increasing stocking density in fish farming is an effective method to mitigate operating costs in the aquaculture business. The low stocking density is avoided as the available space should be utilized to the maximum to achieve the desired fish production [20]. Stocking density is an important factor that may affect fish physiology and, thus, fish welfare [21]. Fish density can affect fish growth factors related to water quality and spatial and foraging dominance [22,23]. Many authors report that when increasing stocking density, survival, growth, production, behaviour, and health are affected [5,24]. Indicator parameters or Operational Welfare Indicators (OWI) can be categorized in many ways and can indicate whether aquaculture practices adversely affect fish [12]. Amongst the most reliable direct-biological indicators is cortisol which is considered the major stress hormone [25]. It may affect physiological and brain functions as well as behavioral changes and thus is related to fish welfare. Furthermore, the detection of DNA damage in different cell types under stressful conditions is considered a reliable stress indicator [13]. Stressors linked to intensive aquaculture practices significantly increase Reactive Oxygen Species (ROS) production, leading to DNA damage, which is determined as an after-effect [26].
Stress can be modulated by specific stress-limiting factors related to the aquatic environment and social interactions [27]. It is commonly accepted that medicinal herbal extracts have a positive impact as dietary additives in fish production toward environmental and animal protection [28]. Essential Oils (EOs) are Phytogenic Feed Additives (PFAs) known for their growth, health, and welfare-promoting effects [29,30]. Furthermore, several studies have reported their effects as sedatives, anesthetics, antioxidants, and antimicrobials [31–33]. These medicinal herbal extracts are composed of volatile molecules with useful biological activities, which consider important in fish nutrition, due to their physicochemical characteristics [33].
Oregano (Origanum vulgare) and cinnamon (Cinnamomum zeylanicum) are two of the most commonly used medicinal herbs. O. vulgare is a plant member of the Lamiaceae family, widely distributed in the Mediterranean, which contains bioactive substances (carvacrol, thymol, and their precursors, y-terpinene, and p-cymene) affecting health, physiology, and metabolic pathways in animals [34]. Carvacrol is recognized as a safe component by the U.S. Food and Drug Administration (FDA), the European commission, and the Food and Agriculture Organization (FAO)/World Health Organization (WHO) committee on food additives [35-37]. O. vulgare and its natural compounds are commonly accepted as antioxidant, antidiabetic, anti-inflammatory, antimicrobial, antiviral, antiparasitic, antineoplastic, and immunostimulants [38- 42], while they have proven growth-promoting effects [1,43–45]. Furthermore, it is known as a welfare promoter, reducing stress and mitigating DNA damage induced by stress factors [46,47]. Cinnamon (C. zeylanicum) is used worldwide as a flavouring spice and is known as Ceylon cinnamon [48]. The main components of essential oils (Trans-cinnamaldehyde, Eugenol, and Linalool) are found in the bark of the plant [49,50]. Trans-cinnamaldehyde constitutes 49.0%-62.8% of essential oils components and is related to the medicinal, antimicrobial, antioxidant, and anesthetic properties of cinnamon [51-55]. The concentration of cinnamaldehyde is affected by environmental factors and the form that is applied, as the essential oil has a higher concentration than the powder [56]. The use of cinnamon enhances the innate immune system and promotes welfare under stressful conditions [57,58]. However, very few studies have examined the C. zeylanicum stress-reducing effects.
Cannabis sativa L., commonly known as hemp, is a member of the Cannabaceae family. Cannabinoids, flavones, and terpenes represent the main phytochemical components of the plant [59]. Interest in the seeds of the plant has increased in recent years [60]. Hemp seed oil which is a processing product is known for its health benefits and nutritional value [61]. Cannabinoids are absent from seeds, but their presence in small amounts is possible due to contact with the resin secreted by the glandular structures [62,63], supporting an added health value [61]. The effects of hempseed oil as an additive on fish production under stressful condition have not yet been studied, although C. sativa has recently been the focus of scientific attention worldwide [59].
This study aims to investigate the effects of Phytogenic Feed Additives (PFAs), specifically C. sativa, O. vulgare, and C. zeylanicum, on stress reduction in gilthead seabream (Sparus aurata L.), a key species in Mediterranean aquaculture. The beneficial role of PFAs in aquaculture lacks comprehensive understanding and research, particularly regarding its impact on welfare, with a notable gap in investigating the potential effects on cannabis within this context, making it the first study of its kind in aquaculture.
Materials and Methods
Experimental fish and culture facilities
Ethical statement: For the experimental procedures, the European Union (EU) legal framework on the welfare and protection of animals for scientific purposes (Directive 2010/63/EU) was applied. The experiments were carried out in the licensed experimentation facility of the Aquaculture Laboratory, Department of Ichthyology and Aquatic Environment, University of Thessaly with code EL- 43BIO/exp-01. The Federation of European Laboratory Animal Science Associations (FELASA)-accredited scientists (functions A–D) were responsible to follow the statutory criteria for animal experimentation.
Experimental fish: Experimental fish were transferred to the facilities of the Aquaculture Laboratory from a commercial fish hatchery. In all three trials, the fish were placed in 125-liter glass aquaria with an artificial seawater recirculation system and continuous air supply. The acclimation period lasted 14 days, during which the experimental fish were provided with their control corresponding feed.
Experimental conditions: During the trials, fish were daily fed and water quality parameters were monitored and kept stable at 21.0˚C ± 1.0˚C temperature, 8.0 ± 0.4 pH, 30 g/L salinity, >6.5/L dissolved oxygen, <0.1 mg/L total ammonia-nitrogen and 12 h:12 h (light: dark) photoperiod. The research adhered to the commonlyaccepted principles of the ‘3Rs’ (Replacement, Reduction, Refinement) and followed the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines endorsed for experiments using live animals. Standardized and well-defined protocols were followed for the applied procedures, while the experimental facilities were suitable for conducting the study. The fish were cared for daily and resided in a conducive environment, while unnecessary handling was avoided, to minimize stress.
Experimental diets
Seven isonitrogenous (530 g/kg crude protein) and isoenergetic (21.3 MJ/kg) diets were formulated (Table 1). The Control Diet (CTRL) did not contain any ‘‘treatment’’ oil, while the rest six diets were supplemented at 1% or 2% with Phytogenic Feed Additives (PFAs), either cannabis (C. sativa) seed oil (CAN1%, CAN2%), Oregano (O. vulgare) essential oil (OR1%, OR2%) and Cinnamon (C. zeylanicum) essential oil (CIN1%, CIN2%) at the cost of soybean oil. All diets were formulated to satisfy the amino acid requirements of the species. Fishmeal and corn gluten were the main animal and plant protein sources, respectively, while fish oil was kept constant in all diets to satisfy the fatty acid requirements of fish. Vitamins and minerals were also kept constant in all diets. All dietary ingredients were mixed in a mixer (Maximum MUMXL20G, Bosch) and pellets of 1.5 mm diameter were produced using the California Pellet Mill (CL-2, IRMECO GmbH, The Netherlands) and stored at 4˚C until feeding.
| Experimental diets | |||||||
|---|---|---|---|---|---|---|---|
| CAN1% | CAN2% | OR1% | OR2% | CIN1% | CIN2% | CTRL | |
Formulation (g/kg diet) |
|||||||
| Fishmeal | 535 | 535 | 535 | 535 | 535 | 535 | 535 |
| Corn gluten | 242 | 242 | 242 | 242 | 242 | 242 | 242 |
| Wheat meal | 129 | 129 | 129 | 129 | 129 | 129 | 129 |
| Fish oil | 66 | 66 | 66 | 66 | 66 | 66 | 66 |
| Soybean oil | 10 | 0 | 10 | 0 | 10 | 0 | 20 |
| Essential oil C. zeylanicum | 0 | 0 | 0 | 0 | 10 | 20 | 0 |
| Essential oil O. vulgare | 0 | 0 | 10 | 20 | 0 | 0 | 0 |
| Hempseed oil | 10 | 20 | 0 | 0 | 0 | 0 | 0 |
| Vit. and minerals, premix | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Monocalcium phosphate | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Vitamin E | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Proximate composition (g/kg) | |||||||
| Dry matter | 922 | 923.3 | 918.9 | 920.7 | 920.7 | 920.6 | 920.7 |
| Crude protein | 524.8 | 530.1 | 537.1 | 539.3 | 531.3 | 531 | 532.1 |
| Crude lipid | 154.3 | 155.7 | 147.3 | 146 | 159.8 | 143 | 159.8 |
| NFE* | 149 | 145 | 142.3 | 142.8 | 137.6 | 155 | 136 |
| Ash | 94 | 92.4 | 92.3 | 92.6 | 92 | 91.6 | 92.8 |
| Gross energy (MJ/kg) | 21.3 | 21.2 | 21.2 | 21.4 | 21.1 | 21.2 | 21.3 |
| Note: *Nitrogen-free extract (including fiber)=1000-(moisture+protein+lipid+ash) | |||||||
Table 1: Formulation and proximate composition of the diets containing C. sativa, O. vulgare, and C. zeylanicum oils.
Experimental trials
In total, three experimental trials were performed:
Trial I: Impact of starvation on DNA damage and plasma cortisol of gilthead seabream fed previously with PFAs:
Experimental fish: In the ‘‘starvation’’ trial, S. aurata juveniles (n=630, mean weight 5.00 ± 0.12 g) were divided into seven dietary groups (6 aquarium/dietary group, 15 fish/aquarium).
Experimental conditions: Fish were hand-fed ad libitum, twice a day (11:00 and 18:00), 6 days/week for 84 days. At the end of the feeding period, a starvation period of 14 days started in three of the six aquaria for each dietary group.
Sampling procedure: At the end of the starvation period, blood and liver samples were taken for plasma cortisol and DNA damage evaluation by comet assay (see: Sampling procedures).
Prior to tissue sampling, the weight of fish samples from each treatment group was measured as follows:
Feeding groups: CAN1%: 66,56 ± 5,35 g, CAN2%: 70,82 ± 4,28 g, OR1%: 60,88 ± 3,17 g, OR2%: 28,92 ± 8,99 g, CIN1%: 48,58 ± 6,05 g, CIN2%: 29,39 ± 2,26 g, CONTROL: 51,62 ± 5,14 g
Starvation groups: CAN1%: 60,71 ± 6,42 g, CAN2%: 60,12 ± 5,68 g, OR1%: 44,14 ± 5,23 g, OR2%: 22,59 ± 7,04 g, CIN1%: 37,64 ± 3,02 g, CIN2%: 24,04 ± 3,33 g, CONTROL: 41,04 ± 4,68 g.
Trial II: Impact of fish density (crowding) on DNA damage and plasma cortisol of gilthead seabream fed previously with PFAs
Experimental fish: In the ‘‘fish density’’ trial, S. aurata juveniles (n=1680, mean weight 5.00 ± 0.15 g) were stocked in 42 aquaria. Two different densities (in triplicates) were applied for each dietary group: A Low Fish Density (LFD) at 1.2 kg/m3 (30 fish/aquarium) and a High Fish Density (HFD) at 2 kg/m3 (50 fish/aquarium).
Experimental conditions: Fish were fed by hand, according to biomass and temperature, twice a day (11:00 and 18:00), 6 days/ week, for 60 days.
Sampling procedure: At the end of the feeding trial, blood and liver samples were taken for plasma cortisol and DNA damage evaluation by comet assay (see: Sampling procedures). Before the tissue sampling, the fish samples of each treatment were weighted: LFD group: CAN1%: 51,29 ± 2,28 g, CAN2%: 53,14 ± 1,41 g, OR1%: 41,52 ± 3,12 g, OR2%: 24,31 ± 2,19 g, CIN1%: 36,20 ± 5,02 g, CIN2%: 26,18 ± 1,23 g, CONTROL: 47,12 ± 2,96 g, HFD group: CAN1%: 48,28 ± 2,19 g, CAN2%: 50,15 ± 4,62 g, OR1%: 40,24 ± 2,96 g, OR2%: 24,14 ± 3,16 g, CIN1%: 35,41 ± 2,17 g, CIN2%: 24,97 ± 2,39 g, CONTROL: 45,02 ± 2,14 g.
Trial III: Impact of manual handling on DNA damage and plasma cortisol of gilthead seabream fed previously with PFAs
Experimental fish: In the ‘‘handling stress’’ trial, S. aurata juveniles (n=315, mean weight 5.00 ± 0.72 g) were stocked in 21 glass aquaria (3 aquaria/dietary group, 15 fish/aquarium).
Experimental conditions: Fish were fed ad libitum, twice a day (11:00 and 18:00) 6 days/week, for 84 days.
Sampling procedure: At the end of this trial, three fish from each aquarium were randomly captured and kept out of water in the open air for 5 min, using intense netting procedures. The fish underwent a period of 30 minutes of acclimation within the tank prior to blood sampling, following established scientific procedures. Then, they were rapidly anesthetized, and the blood and liver samplings were carried out for plasma cortisol and DNA damage evaluation by comet assay (see: Sampling procedures). Before tissue sampling, fish weight of fish samples of each treatment was measured: CAN1%: 50,47 ± 3,17 g, CAN2%: 51,26 ± 1,98 g, OR1%: 43,12 ± 3,49 g, OR2%: 27,25 ± 2,49 g, CIN1%: 39,41 ± 4,06 g, CIN2%: 28,02 ± 2,31 g, CONTROL: 46,29 ± 4,12 g.
Sampling procedures
At the end of all trials, five fish were randomly selected from each aquarium for blood and liver samples.
Blood sampling: Fish were anaesthetized with a dose (50 mg/L) of tricaine methanesulfonate (MS-222) for blood collection. Blood was obtained from the caudal vein, using heparinized syringes fitted with 21G needles, for plasma cortisol determination and erythrocytes DNA damage evaluation.
Liver sampling: After being euthanized with an overdose of anaesthetic (300 mg/L) of MS-222 according to Directive 2010/63/ EU on the protection of animals used for scientific purposes, fish were individually dissected and liver samples were removed quickly for DNA damage assessment by comet assay.
Analytical techniques
Single cell gel electrophoresis (Comet Assay): At the termination of each trial, after the fish were euthanized, the liver was removed and 2 μl of the blood taken was mixed with 1 ml Phosphate-Buffered Saline (PBS). Liver tissue was placed in a cold HBSS-balanced solution (Mg2+, Ca2+ free) on ice. The tissue was injected with collagenase solution (Biochrom AG, Germany) and minced with razor blades into small pieces on a petri plate. The cell suspension was filtered through sterilized gauze in a centrifuge tube. Cells were centrifuged at 3000 rpm for 5 min and the remaining pellet was resuspended in 10 ml PBS. Centrifugation and resuspension were repeated 2 additional times. DNA damage was assessed by comet assay. The alkaline comet assay protocol was based on applying minor modifications [64]. Frosted slides were precoated with a 300 μl layer of 0.5% Normal Melting Point Agarose (NMA). 20 μl of the cell suspension was mixed with 0.5% Low Melting Point Agarose (LMA) (Sigma, USA) and dropped to the agarose-precoated slide. A 22 mm × 22 mm coverslip was used to cover the agarose and waited until the agarose gelled. The cells were lysed by immersion of the slides, for at least one hour (4˚C), in a freshly prepared lysis buffer (2.5 M NaCl, 100 mM Na EDTA, 10 mM Tris, with 1% Triton X-100 and 10% DMSO).
Subsequently, slides were washed into cold water (3x) and placed on a horizontal gel electrophoresis tray containing an alkaline solution (1 mM Na2EDTA, 300 mM NaOH, pH 13). DNA unwinding was achieved by maintenance of the slides in this buffer for 25 min. The electrophoresis was then carried out for 15 min (30 V, 300 mA), in dim light. Thereafter, the slides were neutralized with 0.4 M Tris buffer (pH 7.5). For DNA damage scoring, images of 50 randomly selected nuclei on each triplicate slide were analyzed for each sample using a fluorescence microscope (Olympus BX43, Tokyo, Japan) in a dark room. DNA migration was analyzed using the Comet Assay IVTM comet scoring system with a camera (Perceptive Instruments- Instem, Suffolk, UK), and the parameter Tail DNA damage (%) was assessed for the DNA damage quantification.
Plasma cortisol: The blood samples were rapidly centrifuged (5000 rpm, 10 min) and the plasma was stored at-20˚C. For the cortisol extraction, 100 μl of plasma was mixed with 1 ml of diethyl ether. After the phase’s separation, the liquid phase was transferred in a clean glass tube and evaporated in a water bath at 45˚C for 45 min. 100 μl of Enzyme Immunoassay buffer (EIA buffer Cayman Chemical, MI, USA) was used for the reconstitution of the residue. Plasma cortisol was determined using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (product number 500360; Cayman Chemical Cortisol ELISA kit, Cayman Chemical, Ann Arbor, MI) by the manufacturer’s instructions. The plates were read at an absorbance of 410 nm in a FLUOstar Omega microplate reader from BMG LABTECH.
Statistical analysis
In order to assess whether the observed differences are likely to occur by chance, the statistical analysis method known as the t-test was employed to compare the averages of two groups. The test utilized the Student’s t-distribution as the statistical criterion under the assumption of the null hypothesis. For the evaluation of hypotheses involving the seven groups, the statistical test employed was the Analysis of Variance (ANOVA). However, as ANOVA alone does not provide detailed insights into specific comparisons between groups, the Tukey’s test was utilized as a post hoc analysis. This additional test allowed a more comprehensive understanding of the data by assessing the significance of differences between pairs of group means. Cortisol values and tail DNA damage (%) data were presented as the mean ± standard error. Mean values were compared among treatments of the same stress factor. In the trial I feeding and starvation data were analyzed using Student’s t-test within the same group and one-way ANOVA and Tukey’s post hoc test were used for the analysis of the same treatment between the groups. In the trial II different fish-density data were compared using Student’s t-test within the same feeding group and one-way ANOVA and Tukey’s post hoc test were used for analysis of the same fish density between the feeding groups. In the trial III, stress data of different feeding groups were analyzed using one-way ANOVA and Tukey’s post hoc test. The SPSS Statistics 26 statistical package was applied in all cases and differences were considered significant at P<0.05.
Results
Experimental results
Trial I: Impact of starvation on DNA damage and plasma cortisol of gilthead seabream previously fed with phytogenic feed additives
StaRvation in the control group significantly induced higher DNA damage in the hepatocytes compared to the control feeding group (feeding groups vs. starvation groups) (Figure 1). Nevertheless, the addition of PFAs to the starved groups resulted in a significant reduction in the induced genotoxicity when compared to the control group (starvation groups). Concerning feeding groups, DNA damage in hepatocytes was similar among the PFAs fed groups and the control fed group, except for OR1% group, in which DNA fragmentation was significantly decreased.
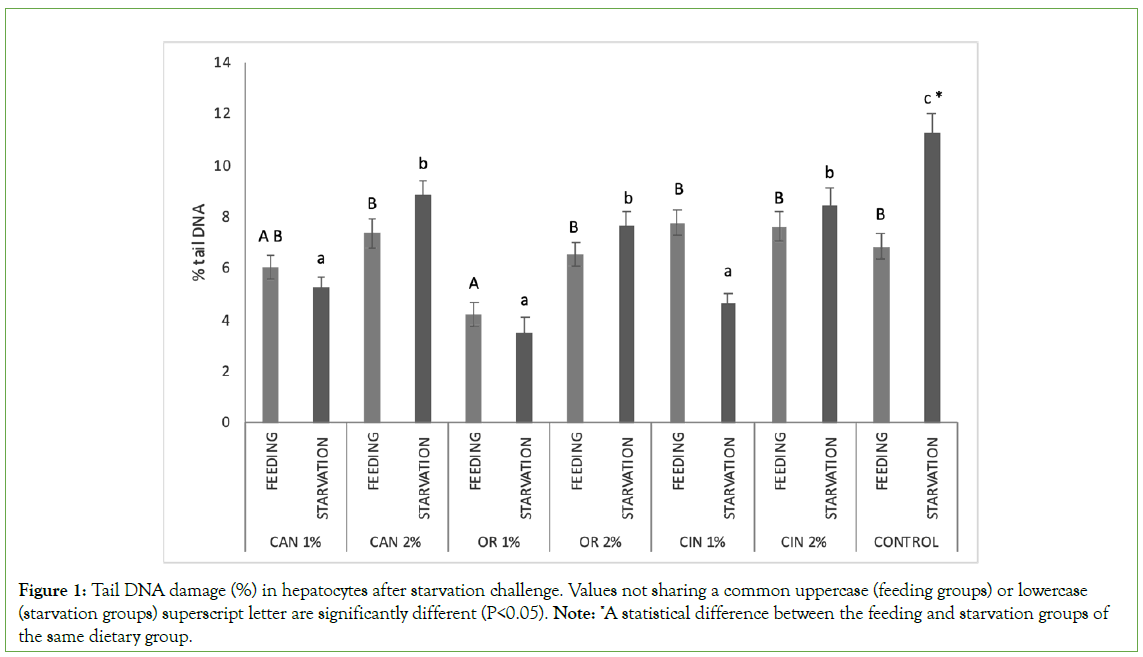
Figure 1: Tail DNA damage (%) in hepatocytes after starvation challenge. Values not sharing a common uppercase (feeding groups) or lowercase (starvation groups) superscript letter are significantly different (P<0.05). Note: *A statistical difference between the feeding and starvation groups of the same dietary group.
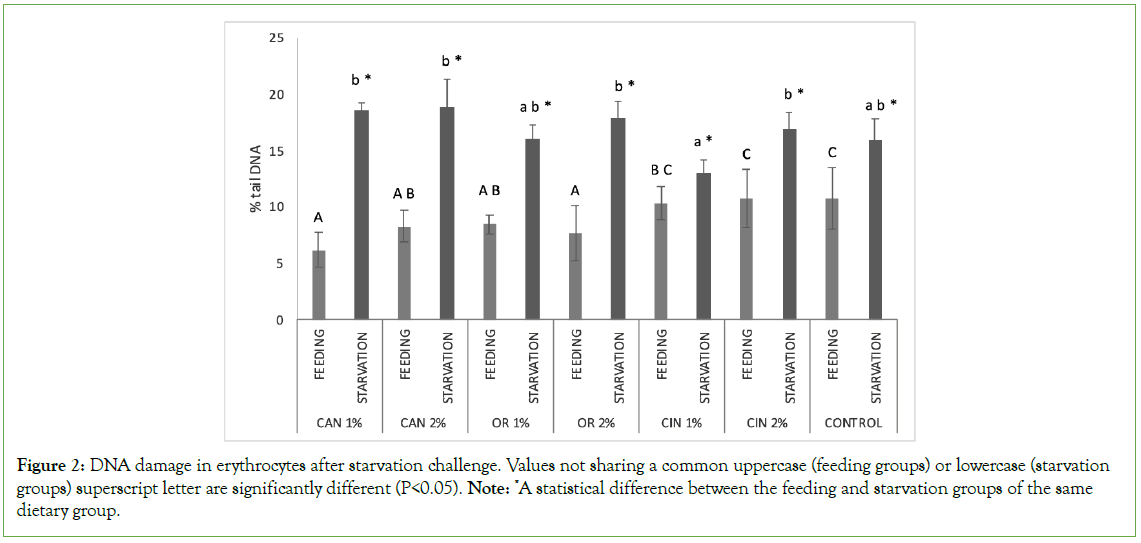
Figure 2: DNA damage in erythrocytes after starvation challenge. Values not sharing a common uppercase (feeding groups) or lowercase (starvation groups) superscript letter are significantly different (P<0.05). Note: *A statistical difference between the feeding and starvation groups of the same dietary group.
Starvation increased significantly the cortisol levels in all dietary groups (feeding groups vs. starvation groups, but starved fish that had previously fed with CAN1%, CAN2%, OR2%, CIN1%, and CIN2% had significantly lower levels compared to the control one (starvation groups, Figure 3). Among the feeding groups, those fed with the oregano oil showed significantly higher cortisol levels compared to the rest groups, including the control one (feeding groups, Figure 3).
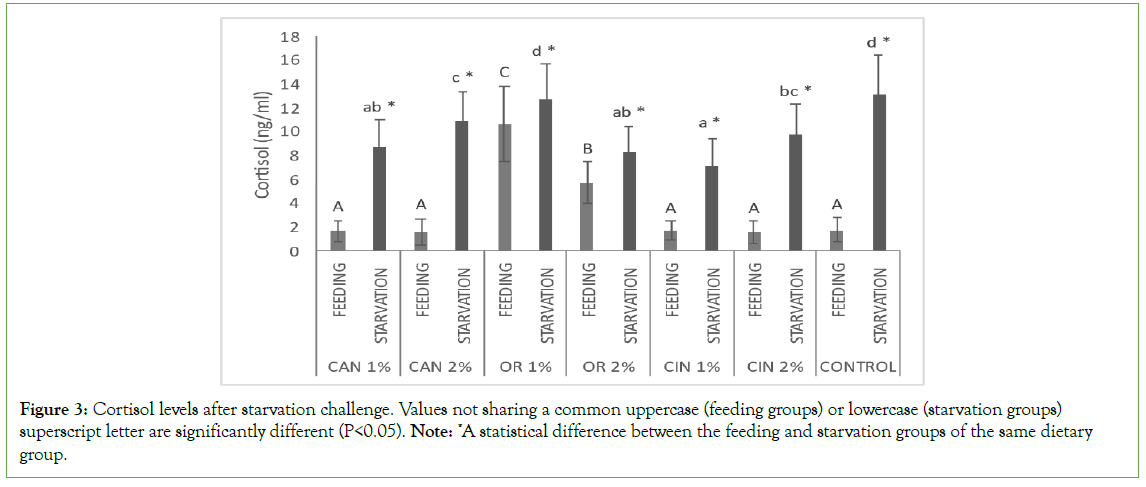
Figure 3: Cortisol levels after starvation challenge. Values not sharing a common uppercase (feeding groups) or lowercase (starvation groups) superscript letter are significantly different (P<0.05). Note: *A statistical difference between the feeding and starvation groups of the same dietary group.
Trial II: Impact of fish density on DNA damage and plasma cortisol of gilthead seabream previously fed with phytogenic feed additives
The impact of PFAs on genotoxicity induced in hepatocytes was more obvious in HFD groups compared to LFD groups, as in most cases, except for the CIN2% group, significantly lower DNA damage values were recorded (LFC vs. HFD groups, Figure 4). Both OR1% and CAN1% HFD groups induced significantly lower genotoxicity compared to the control group (HFD groups, Figure 4). Also, HFD groups fed with 1% PFAs induced lower genotoxicity compared to the 2% PFAs groups (P<0.05), except for cinnamon groups, where no significant differences were mentioned between the two doses applied (HFD groups). Concerning LFD groups, CAN1%, OR1%, CIN2% maintained the genotoxicity at the control group’s level, however CAN2%, OR2%, CIN1% increased significantly DNA damage compared to the control group (LFD groups). In LFD cinnamon groups, the CIN1% group showed higher DNA damage than the CIN2% group (P<0.05), however, in all other LFD groups the opposite pattern was observed.
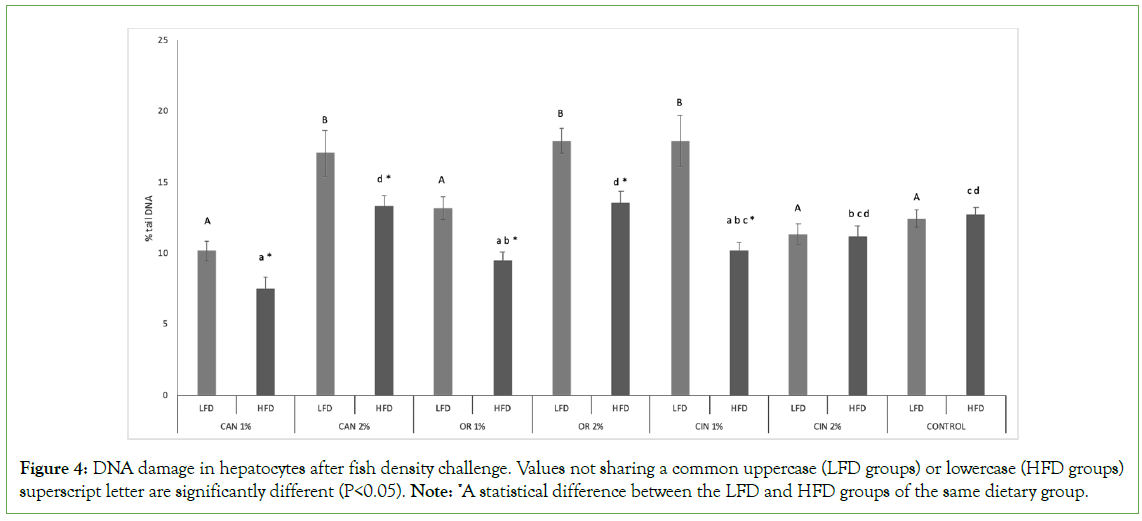
Figure 4: DNA damage in hepatocytes after fish density challenge. Values not sharing a common uppercase (LFD groups) or lowercase (HFD groups) superscript letter are significantly different (P<0.05). Note: *A statistical difference between the LFD and HFD groups of the same dietary group.
PFAs proved to be more effective in HFD groups compared to LFD groups of the same dietary group decreasing genotoxicity in erythrocytes (LFC vs. HFD groups, Figure 5). Concerning both density groups (LFD and HFD), no significant differences were observed among the two doses of the same PFA tested. Both cannabis HFD groups (CAN1% and CAN2%) showed increased DNA damage values, compared to control and other PFAs groups (HFD groups, Figure 5). Concerning LFD groups, PFAs didn’t had any impact on induced genotoxicity (LFD groups).
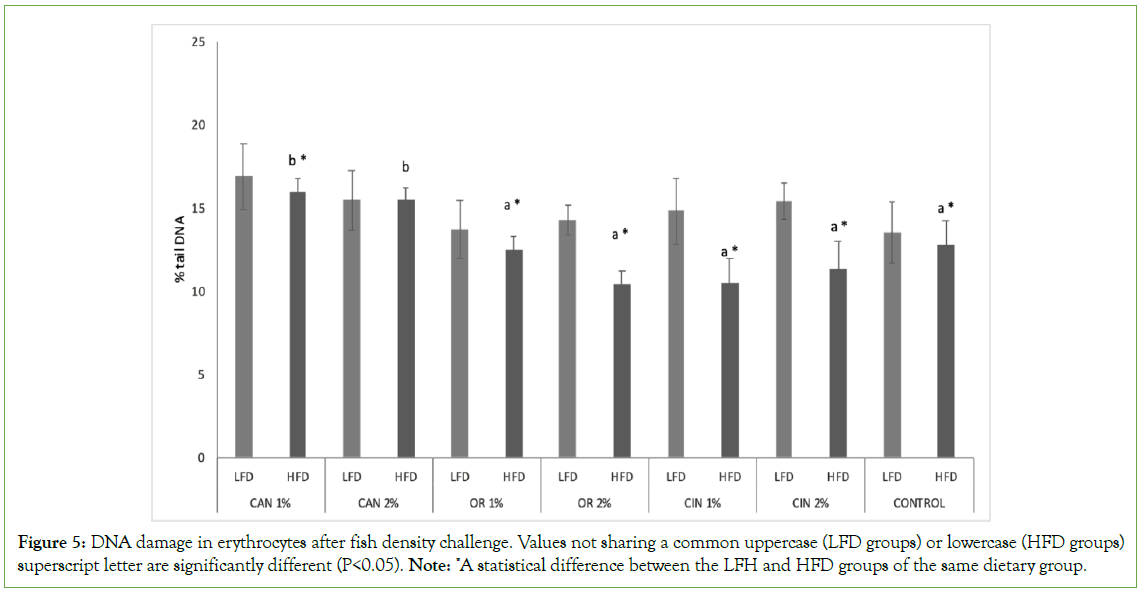
Figure 5: DNA damage in erythrocytes after fish density challenge. Values not sharing a common uppercase (LFD groups) or lowercase (HFD groups) superscript letter are significantly different (P<0.05). Note: *A statistical difference between the LFH and HFD groups of the same dietary group.
Concerning fish fed the same diet, fish density did not affect the cortisol levels in all dietary groups, except for CIN1% in which lower (P<0.05) levels were presented at high density (LFD vs. HFD, Figure 6). CAN1%, OR1%, and CIN1% diets significantly increased the cortisol levels in fish under low fish density conditions, compared to those found in the control and other groups (LFD groups, Figure 6). CAN2%, OR2%, and CIN2% supplement diets proved to be more effective in HFD groups, reducing cortisol levels, compared to those found in the control and other PFAs groups (HFD groups). Differences between the two doses of the same PFA were observed in all cases in both LFD and HFD groups, while more effective were diets supplemented with the higher doses.
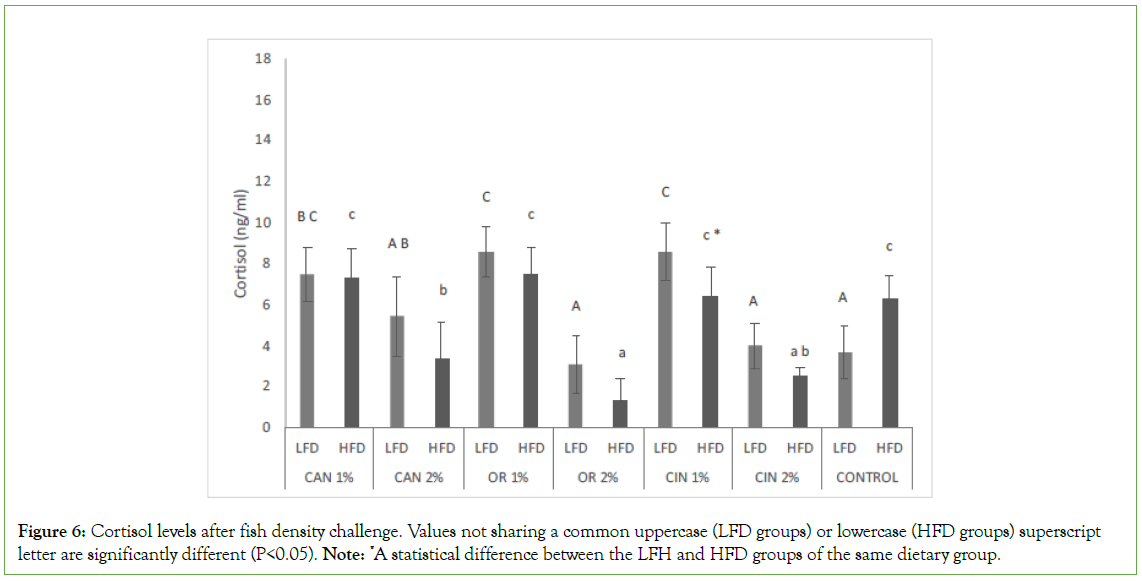
Figure 6: Cortisol levels after fish density challenge. Values not sharing a common uppercase (LFD groups) or lowercase (HFD groups) superscript letter are significantly different (P<0.05). Note: *A statistical difference between the LFH and HFD groups of the same dietary group.
Trial III: Impact of manual handling on DNA damage and plasma cortisol of gilthead seabream previously fed with phytogenic feed additives
The fish feed supplementation with PFAs didn’t mitigate the genotoxicity in hepatocytes, induced by the manual handling of experimental fish. After the handling stress challenge, DNA damage levels in hepatocytes of the CAN1% group were similar to those of the control group (Figure 7). On the other hand, significantly higher genotoxicity was recorded in all the other groups supplemented with PFAs, compared to the control and CAN1% groups.
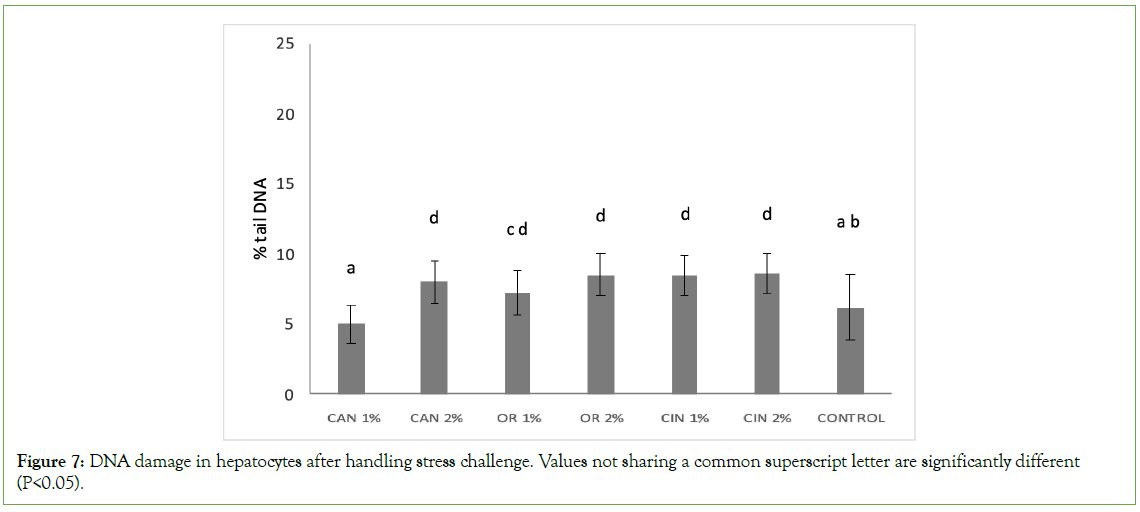
Figure 7: DNA damage in hepatocytes after handling stress challenge. Values not sharing a common superscript letter are significantly different (P<0.05).
In groups supplemented with PFAs, DNA fragmentation in erythrocytes was kept at the control group’s level, in most cases, while the CIN2% group was the only one which showed significantly higher genotoxicity (Figure 8). Furthermore, this was the only group that revealed higher DNA fragmentation compared to the CIN1% group (P<0.05), while DNA damage among the two applied doses did not vary significantly in the C. sativa and O. vulgare groups.
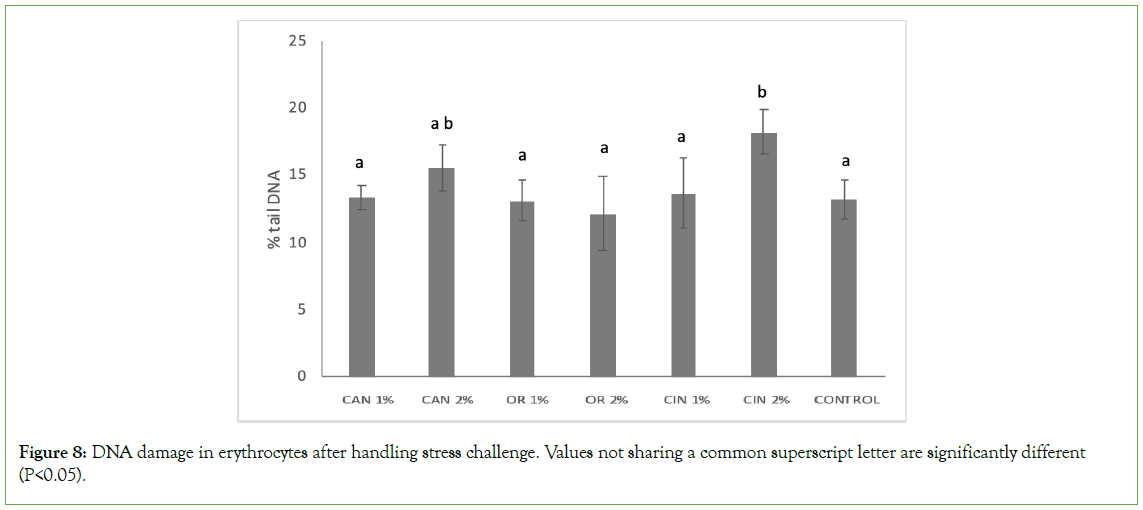
Figure 8: DNA damage in erythrocytes after handling stress challenge. Values not sharing a common superscript letter are significantly different (P<0.05).
Concerning cortisol levels, there were significant variations between the control group and groups fed with diets supplemented with PFAs (Figure 9). Cortisol levels in groups fed with CAN1%, CIN1%, and CIN2% were significantly lower compared to the control group, while fish fed with O. vulgare diets (OR1% and OR2%) had significantly higher cortisol levels compared to the other groups supplemented with PFAs and the control group.
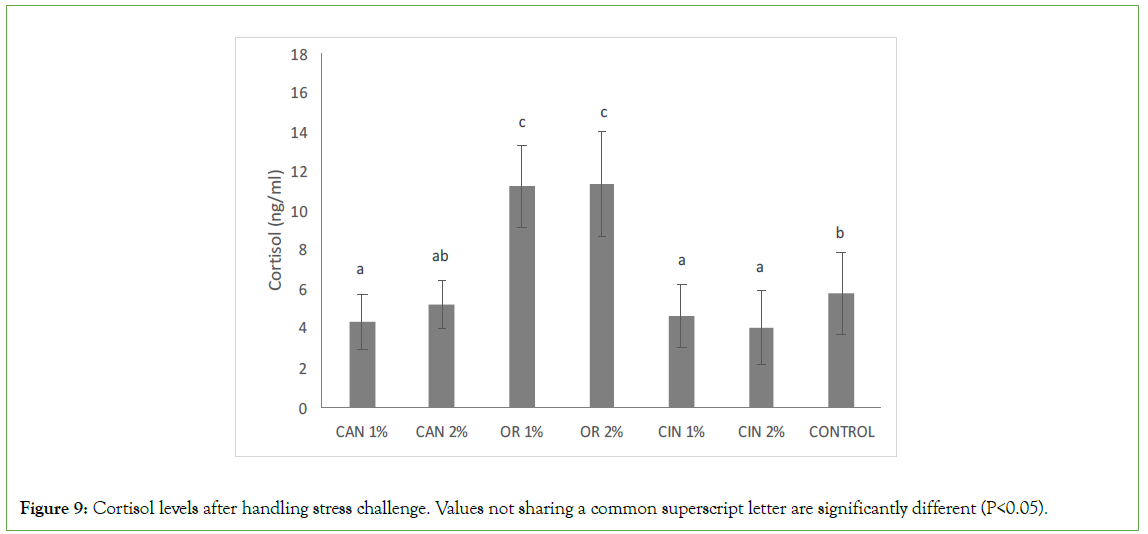
Figure 9: Cortisol levels after handling stress challenge. Values not sharing a common superscript letter are significantly different (P<0.05).
Discussion
Starvation
Intensive fish culture practices create the necessity to search for medicinal-plant extracts that may act as stress-reducing agents under fish farming conditions. In recent years, given the growing concern about fish welfare, the inclusion of beneficial feed additives to mitigate stress induced by aquaculture practices has been an important research issue. Extracts from medicinal plants and fruits possess several medicinal properties related to substantial antioxidant effects [38]. However, there has been little research on natural products with antioxidant properties used in the feeding of fish [65].
In aquaculture, although starvation before slaughter is a common practice, is considered a severe stress factor that may affect flesh quality, reproduction, development, growth, and welfare of fish [19,66]. Also, alternating periods of food deprivation and refeeding is a cost-effective strategy resulting in enhanced growth and feed utilization [67]. In the present study, the two-week starvation proved to be a significant stressor, as shown mainly by the increased cortisol levels and DNA damage in the control groups. This is a reasonable finding considering that starvation is known to lead to oxidative stress and the production of ROS has been linked with molecular damage in living cells leading to cell death [17,68- 71]. Starvation is also known to be an important stress factor as it has been shown that the induced DNA damage produces ROS during the first weeks of food deprivation, but repair mechanisms are activated during the following weeks [72]. Furthermore, DNA damage in liver cells in gilthead seabream has been reported after a period of 7, 14, and 21 days of starvation [13]. Presently, all the PFAs-based diets proved to have a genoprotective role in the hepatocytes of seabream given that their induced DNA damage after starvation was significantly lower compared to that found in the control group. However, the fact that the genotoxicity level in the hepatocytes of the feeding control group was similar to that of the feeding PFAs groups denotes that the genoprotective role of PFAs is stronger after a starvation challenge than during feeding. Nevertheless, fish fed with oregano oil at 1% of the diet showed a higher genoprotective role compared to the rest dietary groups. Previous studies have indicated the genoprotective role of Oregano EO, including fish [47,73,74]. No significant differences were presented between the feeding and the starvation group of the same PFA, indicating the protective effect of medicinal plants under starvation. In the starvation groups, low doses of each PFA proved to be adequate for their genoprotective effect, as they presented lower DNA fragmentation values compared to higher ones. The same pattern concerning the dose’s impact has been also presented in the case of oregano EO-supplemented diets in common carp, in which the DNA fragmentation induced was proportional to the applied dose [47]. Natural products and essential oils may have adverse effects on fish, as it is possible for the high concentration of bioactive compounds to act as an extra stress factor inducing extra genotoxicity.
On the other hand, the genoprotective role of PFAs during starvation was not obvious in the fish erythrocytes, where DNA damage was similar among all starved groups irrespectively of the diet previously fed. DNA damage in erythrocytes is often evaluated by comet assay as it is an easily collected and processed tissue [75–78]. However, in fish, ROS are produced under food deprivation conditions and are bio-accumulated in the liver, a fact that probably is related to the differences observed between the two examined tissues in our study [79]. Furthermore, during starvation, the nutrients are stored in the liver to maintain homeostasis, while structural changes have been reported, including a reduction in the size of hepatocytes and lipid vacuoles affecting metabolic activity [80,81]. On the other hand, an increase in RBCs attributed to the changes in whole-body water content has been reported during starvation [82]. Therefore, different genotoxicity responses may be related to the different effects of starvation in target tissues. Also, these differences between hepatocytes and erythrocytes may be related to differences in DNA single-strand breaks between the two types of cells, as the repair mechanism and metabolic activity are involved [83].
Cortisol is recognized as a key player in the stress response across vertebrates and stress response and is strongly related to the nutritional state in gilthead sea bream [84]. However, there is an inconsistency in cortisol response under starvation as cortisol levels may be increased, remain stable, or decrease [80,85,86]. Furthermore, cortisol response may be suppressed in starved fish as food deprivation inhibits the cortisol secretion mechanism [87]. In the present study cortisol response was not restrained in the starved fish fed with PFAs. The protective role of PFAs was indicated by the cortisol values as in most cases they mitigated efficiently the cortisol secretion after the starvation challenge, compared to the control group. Previous studies revealed the use of phytogenic feed additives as natural anti-stress agents in Nile tilapia, reducing cortisol levels after a starvation period and dietary supplementation with PFAs [88]. This may be related to the antioxidant properties of O. vulgare, C. zeylanicum, and C. sativa [45,54,60]. Cortisol is the primary corticosteroid hormone and has a major role in the mobilization of energy during stress under farming conditions, affecting the activity of important antioxidant enzymes [25,89]. The reduction of cortisol may be explained by the blockage of sensory information transmission to the hypothalamus [90,91]. Furthermore, it may be related to a decrease in cholesterol levels, a primary precursor in cortisol biosynthesis in starved fish [92]. The inclusion of O. vulgare EO in the diets of sea bass (Dicentrarchus labrax) has been proven to decrease cholesterol in serum while oregano in a mixture with other PFAs may reduce cholesterol levels in common carp (Cyprinus carpio) [43,93,94]. The reduction of cholesterol levels after dietary supplementation with Oregano may be attributed to the suppression of 3-hydroxy-3- methylglutaryl coenzyme A reductase (HMG-CoA), which is a regulatory enzyme for cholesterol de novo biosynthesis [95]. Similar results have also been described after dietary supplementation of C. zeylanicum and C. sativa in Nile tilapia and common carp, respectively proving their beneficial role in reducing cholesterol [96,97]. Decreased cholesterol levels after administration of dietary cinnamon may be explained by the enhancement of activity of antioxidant enzymes to block the HMG-CoA reductase enzyme and prevent lipid peroxidation [98]. Beneficial effect of hemp seed oil in reducing hypercholesterolemia has been also highlighted and could be due to the phytosterol β-sitosterol which prevents cholesterol absorption through crystallization and coprecipitation [99].
Fish density
High stocking density is a common husbandry practice in aquaculture [100,101]. It may affect fish physiology, and disturb the balance of homeostasis in farmed aquatic animals and, thus, fish welfare [102–104]. In the present study, diet supplementation with PFAs affected stress indices assessed, under different fish density conditions. Although the density applied in this study was not as high as typically seen in commercial aquaculture, the observed differences between the two applied densities provide valuable insights, highlighting that conditions encountered in large-scale farming operations could not be fully replicated in laboratory settings. The genoprotective role of PFAs diets proved to be more obvious under high fish density conditions compared to lower ones, in both hepatocytes and erythrocytes, as DNA damage values were significantly reduced in HFD groups compared to LFD groups. It is already confirmed that high stocking density induces chronic stress in fish increasing the production of Reactive Oxygen Species (ROS), which may damage DNA, proteins, and lipids [10,105]. High stocking density under farming conditions changes the antioxidative status in aquatic animals causing oxidative stress conditions, affecting the scavenging capacity of the antioxidant defense system and its ability to remove ROS and protect against oxidative damage [106-108].
PFAs are commonly accepted as antioxidants that may activate the antioxidant defense system affecting the activity of antioxidant enzymes under high stocking density. The genoprotective role of PFAs under high-density conditions was confirmed in the case of OR1% and CAN1% supplemented diets in hepatocytes, while the same pattern was not obvious in erythrocytes. Lower doses proved to be sufficient in both hepatocytes and erythrocytes. In some cases, CAN2%, OR2%, and CIN1% diets increased genotoxicity induced in hepatocytes, while both cannabis diets increased genotoxicity induced in erythrocytes. Concerning the genotoxicity induced, there is not a clear pattern as the antioxidant parameters related to high stocking density may be increased or inhibited indicating either that the antioxidant defense system may be activated to cope with the adverse stimulation or that it may depress the activity of antioxidant enzymes as its consumption could be greater than its synthesis [106,109,110]. The differences in genotoxicity induced between the examined tissues were previously discussed and may be explained by variations in DNA damage and repair mechanisms [83]. Furthermore, concerning hepatocytes, high stocking density in fish increases malonaldehyde as a final product of lipid peroxidation [111,112]. The genoprotective role of some PFAs exclusively in hepatocytes inhibits the liver’s functional abnormalities and lipid peroxidation caused by the imbalance between oxidant and antioxidant agents [113]. Lipid peroxidation and its products activate cell necrosis through the apoptotic Fasligand pathway, which is important for the homeostasis of cells in the immune system, while this way of cell killing is related to DNAdamaging agents causing lesions that up-regulate Fas receptor [114]. Moreover, high stocking density as a stressor has been confirmed to induce changes in amino acid carbohydrate and triglyceride metabolism and abnormal lipid metabolism in the fish liver [115,116]. The high lipid utilization of the organism to cope with stress may reduce the lipid content in the liver of gilthead seabream under high stocking density, while multiple omics analysis has revealed abnormal lipid metabolism in fish [117-119].
PFAs diets in high doses supported the trend to reduce or mitigate the cortisol levels in both examined stocking densities, as significantly lower values were presented, compared to low doses. It is already known that high stocking density increases the circulating cortisol [120]. In the present study, high doses of all PFAs applied proved to reduce cortisol secretion compared to the control group under high-density conditions. Many medicinal plants have proven their effectiveness to mitigate plasma cortisol levels after exposure to high stocking densities [121,122]. The protective role of cinnamon under chronic environmental stress factors was also investigated in Nile tilapia showing its impact to mitigate cortisol levels [96]. The ability of EOs to affect cortisol levels in fish reared under high stocking density has also been described in the cases of supplementation of the daily meal of silver catfish and gilthead seabream with Lippia alba and Myrcia sylvatica, respectively [123,124]. Additionally, the beneficial effect of O. vulgare EO as a dietary additive has been reported under different stocking densities in Nile tilapia [44,125], and under different chronic environmental stressors [41,93,126]. In LFD significantly higher cortisol level was presented in the CAN1%, OR1%, and CIN1% groups compared to the control group. Cortisol secretion is influenced by dietary oils as it may be increased after the crowding challenge of gilthead sea bream [84,127]. However, the effect of EOs on cortisol secretion and the precise mechanism remains unknown [128]. Fish species, other factors related to the animals living, the type, intensity, and duration of the stressor may affect stress response which is handled through different stress pathways in S. aurata [21,129].
Handling
Handling procedures are considered acute stressors and refer to the activities that take place during the production cycle, including capture (chasing, netting, and air exposure) and transportation within the rearing system, between farms and before the products are marketed for human consumption and slaughter [12]. During the last years, PFAs have proved their stress-reducing efficacy against stressful aquaculture practices, including handling [130–132].
In the present study, the CAN1% group proved to reduce DNA damage induced after handling stress in hepatocytes. However, all other PFAs supplemented diets applied increased the genotoxicity induced in hepatocytes. On the other hand, increased genotoxicity in erythrocytes was induced only in the case of the CIN2% group, while in most cases no differences were recorded between the groups supplemented with PFAs and the control group. Genotoxicity of natural products and essential oils has been already reported in mammals, as despite their health benefits they may cause toxic effects and oxidative stress [133]. Acute stressors affect DNA damage and apoptosis in aquatic organisms, while vigorous movements have been shown to cause oxidative stress and DNA degradation in fish [13,134,135]. Limited effectiveness of PFAs to mitigate induced genotoxicity may be related to handling procedures being strongly related to hypoxia caused by a shortage of adequate oxygen due to air exposure, which is a very stressful condition and should be kept at a minimum level [10]. Exposure to hypoxia has adverse effects on health, disturbing physiological homeostasis and inducing stress responses [136]. Adaptive responses using physiological and biochemical adjustments are activated by fish, towards homeostasis achievement and survival [137]. It is known that liver physiology has been found to respond to hypoxia, showing a robust correlation of hepatic response with gill damage and stress response [138,139]. Adaptation to hypoxia has been described in blood erythrocytes, which are responsible for oxygen transportation. An increase in the number of erythrocytes and haematocrit is usually recorded under low oxygen availability, due to the action of catecholamines and contraction of the spleen, however, this is not an exclusive pattern [140-143]. Differences in adaptation mechanisms to hypoxia may be related to the different responses of examined tissues concerning the genotoxicity induced. These differences have been previously discussed and may be also explained by differential gene expression and regulation of different cells in a multicellular organism [83].
It is well known that rapid exposure to hypoxia results in elevated cortisol levels in fish [136,144,145]. In the present study, significantly lower cortisol levels in fish fed with hempseed oil (1%) and Cinnamon EO were recorded, indicating that these phytogenic additives were capable of restraining the elevated cortisol levels triggered by intense handling. The negative impact of acute handling stress on cortisol and other blood parameters in gilthead seabream has been reported [13,146,147]. Phytobiotics restrain cortisol, as reduction of cortisol levels has been observed in the case of C. zeylanicum incorporated diets, under stress conditions induced by hypoxia in fish [148]. Moreover, a similar pattern has been observed in the case of Aloysia triphylla after handling [91]. Conversely, fish presently fed with oregano EO incorporated diets showed significantly higher cortisol values compared to the control group, which may be related to the blockage of the cortisol response, as previously discussed in European sea bass [46].
Conclusion
In summary, the utilization of PFAs as natural stress-reducing agents with welfare-enhancing properties was demonstrated, highlighting their potential in optimizing intensive practices O. vulgare and C. zeylanicum Essential Oils (EOs) are widely recognized as commonly used phytobiotics; however, their effects have primarily been evaluated in non-Mediterranean fish species exposed to different stressors. Furthermore, while the scientific community has shown considerable interest in C. sativa oil worldwide due to its recognized health benefits and nutritional value, this study contributes by addressing a critical knowledge gap and investigating the effects of C. sativa oil on fish production under stressful conditions, thus expanding the understanding in this field. Oregano and cinnamon essential oils, along with hemp seed oil, exhibited their advantageous effects as PFAs in alleviating stress induced by common acute and chronic stressors. Their capacity to promote welfare was particularly evident when faced with challenging circumstances, resulting in decreased levels of cortisol and genotoxicity. Notably, even at lower doses, these PFAs demonstrated efficacy in mitigating the stress response. However, further scientific inquiry is warranted to ascertain the most suitable and efficacious dosage for specific stress factors and fish species, while additional stress indices should be investigated to provide a comprehensive understanding of the physiological and behavioral responses of fish under various stress conditions.
Acknowledgment
This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research–2nd cycle” (MIS-5000432), implemented by the State Scholarships Foundation (ΙΚΥ). The authors thank the postgraduate student Ms. Liliana Kolaiti, and Dr. Pier Psofakis for their help during the experiment and the fish sampling, and the anonymous reviewers of this manuscript for their constructive comments.
Conflicts of Interest
There are no conflicts of interest.
References
- Abdel-Latif HM, Abdel-Tawwab M, Khafaga AF, Dawood MA. Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio L.) fingerlings. Aquaculture. 2020;526:735432.
- Bianchi MC, Chopin F, Farme T, Franz N, Fuentevilla C, Garibaldi L, et al. FAO: The state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations: Rome, Italy. 2014:1-230.
- Dawood MA, El Basuini MF, Yilmaz S, Abdel-Latif HM, Alagawany M, Kari ZA, et al. Exploring the roles of dietary herbal essential oils in aquaculture: A review. Animals.2022;12(7):823.
- Beltrán JM, Espinosa C, Guardiola FA, Esteban MÁ. In vitro effects of Origanum vulgare leaf extracts on gilthead seabream (Sparus aurata L.) leucocytes, cytotoxic, bactericidal and antioxidant activities. Fish Shellfish Immunol. 2018;79:1-10.
[Crossref] [Google Scholar] [Pubmed]
- El‐Sayed AF. Effects of stocking density and feeding levels on growth and feed efficiency of Nile tilapia (Oreochromis niloticusL.) fry. Aquac Res. 2002;33(8):621-626.
- Ellis T, Berrill I, Lines J, Turnbull JF, Knowles TG. Mortality and fish welfare. Fish Physiol Biochem. 2012;38:189-199.
- Barcellos LJ, Woehl VM, Wassermann GF, Quevedo RM, Ittzés I, Krieger MH. Plasma levels of cortisol and glucose in response to capture and tank transference in Rhamdia quelen (Quoy and Gaimard), a South American catfish. Aquac Res. 2001;32(2):121-123.
- Grutter AS, Pankhurst NW. The effects of capture, handling, confinement and ectoparasite load on plasma levels of cortisol, glucose and lactate in the coral reef fish Hemigymnus melapterus. J Fish Biol. 2000;57(2):391–401.
[Crossref]
- Fagundes M, Urbinati EC. Stress in pintado (Pseudoplatystoma corruscans) during farming procedures. Aquaculture. 2008;276(1–4):112–119.
- Ni M, Wen H, Li J, Chi M, Bu Y, Ren Y, et al. The physiological performance and immune responses of juvenile Amur sturgeon (Acipenser schrenckii) to stocking density and hypoxia stress. Fish Shellfish Immunol. 2014;36(2):325–335.
- Ashley PJ. Fish welfare: Current issues in aquaculture. Appl Anim Behav Sci. 2007;104(3–4):199–235.
- Segner H, Reiser S, Ruane N, Rösch R, Steinhagen D, Vehanen T. Welfare of fishes in aquaculture. FAO Fisheries and Aquaculture Circular no. 1189. Budapest: FAO. 2019.
- Malandrakis EE, Dadali O, Golomazou E, Kavouras M, Dailianis S, Chadio S, et al. DNA damage and differential gene expression associated with physical stress in gilthead seabream (Sparus aurata). Gen Comp Endocrinol. 2016;236:98–104.
- Kvamme BO, Gadan K, Finne-Fridell F, Niklasson L, Sundh H, Sundell K, et al. Modulation of innate immune responses in Atlantic salmon by chronic hypoxia-induced stress. Fish Shellfish Immunol. 2013;34(1):55–65.
- Douxfils J, Deprez M, Mandiki SNM, Milla S, Henrotte E, Mathieu C, et al. Physiological and proteomic responses to single and repeated hypoxia in juvenile Eurasian perch under domestication-Clues to physiological acclimation and humoral immune modulations. Fish Shellfish Immunol. 2012;33(5):1112–1122.
- Lines JA, Spence J. Safeguarding the welfare of farmed fish at harvest. Fish Physiol Biochem. 2012;38(1):153–162.
- Morales AE, Pérez-Jiménez A, Carmen Hidalgo M, Abellán E, Cardenete G. Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentexliver. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139(1–3):153–161.
- Fan X, Hou T, Sun T, Zhu L, Zhang S, Tang K, et al. Starvation stress affects the maternal development and larval fitness in zebrafish (Danio rerio). Sci Total Environ. 2019;695.
- Waagbø R, Jørgensen SM, Timmerhaus G, Breck O, Olsvik PA. Short-term starvation at low temperature prior to harvest does not impact the health and acute stress response of adult Atlantic salmon. PeerJ. 2017;(4):1–22.
- Liu B, Liu Y, Liu Z, Qiu D, Sun G, Li X. Influence of stocking density on growth, body composition and energy budget of Atlantic salmon Salmo salarL. in recirculating aquaculture systems. Chinese J Oceanol Limnol. 2014;32(5):982–990.
- Barton BA. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol.2002;42(3):517–525.
- Ellis T, North B, Scott AP, Bromage NR, Porter M, Gadd D. The relationships between stocking density and welfare in farmed rainbow trout. J Fish Biol. 2002;61(3):493–531.
- Håstein Τ, Scarfe AD, Lund VL. Science-based assessment of welfare: Aquatic animals. 2005.
- De Oliveira EG, Pinheiro AB, De Oliveira VQ, Da Silva ARM, De Moraes MG, Rocha ÍRCB, et al. Effects of stocking density on the performance of juvenile pirarucu (Arapaima gigas) in cages. Aquaculture. 2012;370–371:96–101.
- Sadoul B, Geffroy B. Measuring cortisol, the major stress hormone in fishes. J Fish Biol. 2019;94(4):540–555.
- Chowdhury S, Saikia SK. Oxidative stress in fish: A review. J Sci Res. 2020;12(1):145–160.
- Macintyre CM. Water quality and welfare assessment on United Kingdom trout farms. Inst Aquac Univ Stirling, UK. 2008;1–203.
- Bandeira G, Pês TS, Saccol EMH, Sutili FJ, Rossi WR, Murari AL, et al. Potential uses of Ocimum gratissimum and Hesperozygis ringens essential oils in aquaculture. Ind Crops Prod. 2017;97:484–491.
- Azambuja CR, Mattiazzi J, Riffel APK, Finamor IA, Garcia L de O, Heldwein CG, et al. Effect of the essential oil of Lippia alba on oxidative stress parameters in silver catfish (Rhamdia quelen) subjected to transport. Aquaculture. 2011;319(1–2):156–161.
- Zeppenfeld CC, Toni C, Becker AG, Miron D dos S, Parodi TV, Heinzmann BM, et al. Physiological and biochemical responses of silver catfish, Rhamdia quelen, after transport in water with essential oil of Aloysia triphylla (L’Herit) Britton. Aquaculture. 2014;418:101–107.
- Da Cunha JA, Heinzmann BM, Baldisserotto B. The effects of essential oils and their major compounds on fish bacterial pathogens: A review. J Appl Microbiol. 2018;125(2):328–344.
- Hoseini SM, Mirghaed AT, Yousefi M. Application of herbal anaesthetics in aquaculture. Rev Aquac. 2018.
- Sutili FJ, Gatlin DM, Heinzmann BM, Baldisserotto B. Plant essential oils as fish diet additives: Benefits on fish health and stability in feed. Rev Aquac. 2018;10(3):716–726.
- Kokkini S, Karousou R, Hanlidou E, Lanaras T. Essential oil composition of greek (Origanum vulgare ssp. Hirtum) and turkish (O.onites) Oregano: A tool for their distinction. J Essent Oil Res. 2004;16(4):334–338.
- Food and Drug Administration, HHS. Food additives permitted for direct addition to food for human consumption; folic acid. Final rule. Federal register. 2016;81(73):22176-22183.
- Commission implementing regulation (EU) No 672/2013. In annual meeting on 2012;17:21).
- Joint FA, WHO expert committee on food additives, World Health Organization. Evaluation of certain food additives and contaminants: Fifty-fifth report of the Joint FAO/WHO Expert Committee on Food Additives. 2001.
- Alagawany M, Abd El-Hack ME, Farag MR, Shaheen HM, Abdel-Latif MA, Noreldin AE, et al. The applications of origanum vulgare and its derivatives in human, ruminant and fish nutrition: A Review. Ann Anim Sci. 2020;20(2):389–407.
- Ferreira PMF, Caldas DW, Salaro AL, Sartori SSR, Oliveira JM, Cardoso AJS, et al. Intestinal and liver morphometry of the Yellow Tail Tetra (Astyanax altiparanae) fed with oregano oil. An Acad Bras Cienc. 2016;88(2):911–922.
- García Beltrán JM, Silvera DG, Ruiz CE, Campo V, Chupani L, Faggio C, et al. Effects of dietary Origanum vulgare on gilthead seabream (Sparus aurata L.) immune and antioxidant status. Fish Shellfish Immunol. 2020;99:452–461.
- Rafieepour A, Hajirezaee S, Rahimi R. Moderating effects of dietary oregano extract (Origanum vulgare) on the toxicity induced by organophosphate pesticide, diazinon in rainbow trout, Oncorhynchus mykiss: Metabolic hormones, histology and growth parameters. Turkish J Fish Aquat Sci. 2020;20(3):207–219.
- Thulluru A, Varma MM, Setty CM, Chintamaneni PK, Samayamanthula S. Effect on histology and nutrient digestibility of supplemented Origanum onites essential oil to rainbow trout diets (Oncorhynchus mykiss). Indian J Pharm Educ Res. 2015;49(4):293-303.
- Dinardo FR, Deflorio M, Casalino E, Crescenzo G, Centoducati G. Effect of feed supplementation withOriganum vulgare L. essential oil on sea bass (Dicentrarchus labrax): A preliminary framework on metabolic status and growth performances. Aquac Reports. 2020;18:100511.
- El-Hawarry WN, Mohamed RA, Ibrahim SA. Collaborating effects of rearing density and oregano oil supplementation on growth, behavioral and stress response of Nile tilapia (Oreochromis niloticus). Egypt J Aquat Res. 2018;44(2):173–178.
- Mohammadi G, Rafiee G, El Basuini MF, Van Doan H, Ahmed HA, Dawood MAO, et al. Oregano (Origanum vulgare), St John’s-wort (Hypericum perforatum), and lemon balm (Melissa officinalis) extracts improved the growth rate, antioxidative, and immunological responses in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquac Reports. 2020;18:100445.
- Bodur T, León-Bernabeu S, Navarro A, Tort L, Afonso JM, Montero D. Effects of new plant based anesthetics Origanum sp. and Eucalyptus sp. oils on stress and welfare parameters in Dicentrarchus labrax and their comparison with clove oil. Aquaculture. 2018;495:402–408.
- Khafaga AF, Naiel MAE, Dawood MAO, Abdel-Latif HMR. Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in Common carp (Cyprinus carpio). Aquat Toxicol. 2020;228:105624.
- Suriyagoda L, Mohotti AJ, Vidanarachchi JK, Kodithuwakku SP, Chathurika M, Bandaranayake PCG, et al. “Ceylon cinnamon”: Much more than just a spice. Plants People Planet. 2021;3(4):319–336.
- Chericoni S, Prieto JM, Iacopini P, Cioni P, Morelli I. In vitro activity of the essential oil of Cinnamomum zeylanicum and eugenol in peroxynitrite-induced oxidative processes. J Agric Food Chem. 2005;53(12):4762–4765.
- Wong YC, Ahmad-Mudzaqqir MY, Wan-Nurdiyana WA. Extraction of essential oil from cinnamon (Cinnamomum zeylanicum). Orient J Chem. 2014;30(1):37–47.
- Simić A, Soković MD, Ristić M, Grujić‐Jovanović S, Vukojević J, Marin PD. The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother Res. 2004;18(9):713-717.
[Crossref] [Google Scholar] [PubMed]
- Perina FJ, de Andrade CCL, Moreira SI, Nery EM, Ogoshi C, Alves E. Cinnamomun zeylanicum oil and trans-cinnamaldehyde against Alternaria brown spot in tangerine: Direct effects and induced resistance. Phytoparasitica. 2019;47(4):575–589
- Singh G, Maurya S, deLampasona MP, Catalan CAN. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol. 2007;45(9):1650–1661.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Tawwab M, Samir F, Abd El-Naby AS, Monier MN. Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nila tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018;74:19–25.
[Crossref] [Google Scholar] [PubMed]
- Golomazou E, Malandrakis EE, Kavouras M, Karatzinos T, Miliou H, Exadactylos A, et al. Anaesthetic and genotoxic effect of medicinal plant extracts in gilthead seabream (Sparus aurata L.). Aquaculture. 2016;464:673–682.
- Ghafoor F. Importance of herbs in aquaculture; Cinnamon a potent enhancer of growth and immunity in fish, Ctenopharyngodon idella. Iran J Aquat Anim Heal. 2020;6(1):78–92.
- Ahmad MH, Mesallamy AMDEL, Samir F, Zahran F. Effect of Cinnamon (Cinnamomum zeylanicum) on Growth Performance , Feed Utilization , Whole-Body Composition , and Resistance to Aeromonas hydrophila in Nile Tilapia. J Appl Aquac. 2011;289–298.
- Rattanachaikunsopon P, Phumkhachorn P. Potential of cinnamon (Cinnamomum verum) oil to control Streptococcus iniae infection in tilapia (Oreochromis niloticus). Fish Sci. 2010;76(2):287–293.
- Pellati F, Brighenti V, Sperlea J, Marchetti L, Bertelli D, Benvenuti S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L.(hemp). Molecules. 2018;23(10):2639.
[Crossref] [Google Scholar] [PubMed]
- Farinon B, Molinari R, Costantini L, Merendino N. The seed of industrial hemp (Cannabis sativa L.): Nutritional quality and potential functionality for human health and nutrition. Nutrients. 2020;12(7):1935.
[Crossref] [Google Scholar] [PubMed]
- Leizer C, Ribnicky DM, Poulev A, Dushenkov D, Raskin I. The composition of hemp seed oil and its potential as an important source of nutrition. J Nutraceuticals Funct Med Foods. 2000;2(4):35–53
- Citti C, Pacchetti B, Vandelli MA, Forni F, Cannazza G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of Cannabidiolic Acid (CBDA). J Pharm Biomed Anal. 2018;149:532–540
[Crossref] [Google Scholar] [PubMed]
- Escrivá Ú, Andrés-Costa MJ, Andreu V, Picó Y. Analysis of cannabinoids by liquid chromatography–mass spectrometry in milk, liver and hemp seed to ensure food safety. Food Chem. 2017;228:177–185.
[Crossref] [Google Scholar] [PubMed]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191
[Crossref] [Google Scholar] [PubMed]
- Kokou F, Sarropoulou E, Cotou E, Kentouri M, Alexis M, Rigos G. Effects of graded dietary levels of soy protein concentrate supplemented with methionine and phosphate on the immune and antioxidant responses of gilthead sea bream (Sparus aurata L.). Fish Shellfish Immunol. 2017;64:111–121.
[Crossref] [Google Scholar] [PubMed]
- Secci G, Parisi G. From farm to fork: Lipid oxidation in fish products. A review. Ital J Anim Sci. 2016;15(1):124–136.
- Gaylord TG, Gatlin III DM. Dietary protein and energy modifications to maximize compensatory growth of channel catfish (Ictalurus punctatus). Aquaculture. 2001;194(3-4):337-348.
- Bowden TJ. Modulation of the immune system of fish by their environment. Fish Shellfish Immunol. 2008;25(4):373–383.
[Crossref] [Google Scholar] [PubMed]
- Faheem M, Abbas RZ, Liaqat I, Hoseinifar SH, Maneepitaksanti W, Van Doan H. Bio-active components in medicinal plants: A mechanistic review of their effects on fish growth and physiological parameters-A Review. Ann Anim Sci. 2022;22(4):1127–1149.
- Martínez-Álvarez RM, Morales AE, Sanz A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev Fish Biol Fish. 2005;15(1–2):75–88.
- Regoli F, Giuliani ME. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res. 2014;93:106–117.
[Crossref] [Google Scholar] [PubMed]
- Robinson MK, Rustum KR, Chambers EA, Rounds JD, Wilmore DW, Jacobs DO. Starvation enhances hepatic free radical release following endotoxemia. J Surg Res. 1997;69(2):325–330.
[Crossref] [Google Scholar] [PubMed]
- Arami S, Ahmadi A, Haeri SA. The radioprotective effects of Origanum vulgare extract against genotoxicity induced by 131I in human blood lymphocyte. Cancer Biother Radiopharm. 2013;28(3):201–206
[Crossref] [Google Scholar] [PubMed]
- Habibi E, Shokrzadeh M, Chabra A, Naghshvar F, Keshavarz-Maleki R, Ahmadi A. Protective effects of Origanum vulgare ethanol extract against cyclophosphamide-induced liver toxicity in mice. Pharm Biol. 2015;53(1):10–15.
[Crossref] [Google Scholar] [PubMed]
- Belpaeme K, Delbeke K, Zhu L, Kirsch-Volders M. Cytogenetic studies of PCB77 on brown trout (Salmo trutta Fario) using the micronucleus test and the alkaline comet assay. Mutagenesis. 1996;11(5):485–492.
[Crossref] [Google Scholar] [PubMed]
- Belpaeme K, Cooreman K, Kirsch-Volders M. Development and validation of the in vivo alkaline comet assay for detecting genomic damage in marine flatfish. Mutat Res. 1998;415(3):167–184.
[Crossref] [Google Scholar] [PubMed]
- Mitchelmore CL, Chipman JK. DNA strand breakage in aquatic organisms and the potential value of the comet assay in environmental monitoring. Mutat Res. 1998;399(2):135–147.
[Crossref] [Google Scholar] [PubMed]
- Sumathi M, Kalaiselvi K, Palanivel M, Rajaguru P. Genotoxicity of textile dye effluent on fish (Cyprinus carpio) Measured Using the Comet Assay. Bull Environ Contam Toxicol. 2001;66(3):407–414.
[Crossref] [Google Scholar] [PubMed]
- Pascual P, Pedrajas JR, Toribio F, López-Barea J, Peinado J. Effect of food deprivation on oxidative stress biomarkers in fish (Sparus aurata). Chem Biol Interact. 2003;145(2):191–199.
[Crossref] [Google Scholar] [PubMed]
- Davis KB, Gaylord TG. Effect of fasting on body composition and responses to stress in sunshine bass. Comp Biochem Physiol A Mol Integr Physiol. 2011;158(1):30–36
[Crossref] [Google Scholar] [PubMed]
- Li H, Xu W, Jin J, Yang Y, Zhu X, Han D, et al. Effects of starvation on glucose and lipid metabolism in gibel carp (Carassius auratus Gibeliovar. CAS III). Aquaculture. 2018;496:166–175.
- McCue MD. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol A Mol Integr Physiol. 2010;156(1):1–18.
[Crossref] [Google Scholar] [PubMed]
- Lee RF, Steinert S. Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat Res. 2003;544(1):43–64.
[Crossref] [Google Scholar] [PubMed]
- Pérez-Sánchez J, Borrel M, Bermejo-Nogales A, Benedito-Palos L, Saera-Vila A, Calduch- Giner JA, et al. Dietary oils mediate cortisol kinetics and the hepatic mRNA expression profile of stress-responsive genes in gilthead sea bream (Sparus aurata) exposed to crowding stress. Implications on energy homeostasis and stress susceptibility. Comp Biochem Physiol Part D Genomics Proteomics. 2013;8(2):123–130.
[Crossref] [Google Scholar] [PubMed]
- Jia Y, Gao Y, Chen X, Huang B. Determination of optimal fasting time before blood sampling to get baseline data on serum biochemical characteristics in juvenile turbot (Scophthalmus maximus). Aquaculture. 2018;487(106):83–88.
- Small BC. Effect of fasting on nychthemeral concentrations of plasma Growth Hormone (GH), Insulin-Like Growth Factor I (IGF-I), and cortisol in channel catfish (Ictalurus punctatus). Comp Biochem Physiol B Biochem Mol Biol. 2005;142(2):217-223.
[Crossref] [Google Scholar] [PubMed]
- Poursaeid S, Falahatkar B. Starvation alters growth, stress metabolites and physiological responses in juvenile great sturgeon (Huso huso). Anim Feed Sci Technol. 2022;294:115429.
- Elabd H, Soror E, El-Asely A, El-Gawad EA, Abbass A. Dietary supplementation of Moringa leaf meal for Nile tilapia Oreochromis niloticus: Effect on growth and stress indices. Egypt J Aquat Res. 2019;45(3):265–271.
- Goncharova ND, Shmaliy AV, Bogatyrenko TN, Koltover VK. Correlation between activity of antioxidant enzymes and circadian rhythms of corticosteroids in Macaca mulatta monkeys of different age. Exp Gerontol. 2006;41(8):778–783.
[Crossref] [Google Scholar] [PubMed]
- Iversen M, Finstad B, McKinley RS, Eliassen RA. The efficacy of metomidate, clove oil, Aqui- STM and Benzoak® as anaesthetics in Atlantic salmon (Salmo salar L.) smolts, and their potential stress-reducing capacity. Aquaculture.2003;221(1–4):549–566.
- Teixeira RR, de Souza RC, Sena AC, Baldisserotto B, Heinzmann BM, Couto RD, et al. Essential oil of Aloysia triphylla in Nile tilapia: Anaesthesia, stress parameters and sensory evaluation of fillets. Aquac Res. 2017;48(7):3383–3392.
- Miller LL, Hontela A. Species-specific sensitivity to selenium-induced impairment of cortisol secretion in adrenocortical cells of rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis). Toxicol Appl Pharmacol. 2011;253(2):137–44.
[Crossref] [Google Scholar] [PubMed]
- Dinardo FR, Maggiolino A, Centoducati G, Casalino E, Deflorio M. A multi-biomarker approach in european sea bass exposed to dynamic temperature changes under dietary supplementation with Origanum vulgare essential oil. Animals. 2021;11(4).
[Crossref] [Google Scholar] [PubMed]
- Ghafarifarsani H, Hoseinifar SH, Adorian TJ, Goulart Ferrigolo FR, Raissy M, Van Doan H. The effects of combined inclusion of Malvae sylvestris, Origanum vulgare, andAllium hirtifolium boiss for common carp (Cyprinus carpio) diet: Growth performance, antioxidant defense, and immunological parameters. Fish Shellfish Immunol. 2021;119:670–677.
[Crossref] [Google Scholar] [PubMed]
- Lee KW, Everts H, Beynen AC. Essential oils in broiler nutrition. Int J Poult Sci. 2004;3(12):738–752.
- Hamed HS, Ismal SM, Abdel-Tawwab M. Modulatory effects of dietary cinnamon (Cinnamomum zeylanicum) against waterborne lead toxicity in Nile tilapia fingerlings: Growth performance, haemato-biochemical, innate immunity, and hepatic antioxidant indices. Aquac Reports. 2022;25:101190.
- Audu BS, Ajima MNO, Ofojekwu PC. Enzymatic and biochemical changes in common carp, Cyprinus carpio (L.) fingerlings exposed to crude leaf extract of Cannabis sativa (L.). Asian Pacific J Trop Dis. 2015;5(2):107–115.
- Lee JS, Jeon SM, Park EM, Huh TL, Kwon OS, Lee MK, et al. Cinnamate supplementation enhances hepatic lipid metabolism and antioxidant defense systems in high cholesterol-fed rats. J Med Food. 2003;6(3):183–191.
[Crossref] [Google Scholar] [PubMed]
- Mattson H, Crouse R. Optimizing cholesterol the effect absorption of plant sterols in man. Am J Clin Nutr.1982;35(4):697–700.
[Crossref] [Google Scholar] [PubMed]
- Ani JS, Manyala JO, Masese FO, Fitzsimmons K. Effect of stocking density on growth performance of monosex Nile Tilapia (Oreochromis niloticus) in the aquaponic system integrated with lettuce (Lactuca sativa). Aquac Fish. 2022;7(3):328–335.
- Hossain MA, Hossain MA, Haque MA, Mondol MMR, Harun-Ur-Rashid M, Das SK. Determination of suitable stocking density for good aquaculture practice-based carp fattening in ponds under drought-prone areas of Bangladesh. Aquaculture. 2022;547:737485.
- Costas B, Aragão C, Dias J, Afonso A, Conceição LEC. Interactive effects of a high-quality protein diet and high stocking density on the stress response and some innate immune parameters of Senegalese sole Solea senegalensis. Fish Physiol Biochem. 2013;39(5):1141–1151.
[Crossref] [Google Scholar] [PubMed]
- Gonçalves AT, Núñez-Acuña G, Détrée C, Gallardo-Escárate C. Coding/non-coding cross-talk in intestinal epithelium transcriptome gives insights on how fish respond to stocking density. Comp Biochem Physiol - Part D Genomics Proteomics. 2019;29:14–23.
[Crossref] [Google Scholar] [PubMed]
- Ellison AR, Uren Webster TM, Rodriguez-Barreto D, de Leaniz CG, Consuegra S, Orozco- terWengel P, et al. Comparative transcriptomics reveal conserved impacts of rearing density on immune response of two important aquaculture species. Fish Shellfish Immunol. 2020;104:192–201.
[Crossref] [Google Scholar] [PubMed]
- Morel Y, Barouki R. Repression of gene expression by oxidative stress. Biochem J. 1999;342(3):481–496.
[Crossref] [Google Scholar] [PubMed]
- Dorothy MS, Vungarala H, Sudhagar A, Reddy AK, Rani Asanaru Majeedkutty B. Growth, body composition and antioxidant status of Litopenaeus vannamei juveniles reared at different stocking densities in the biofloc system using inland saline groundwater. Aquac Res. 2021;52(12):6299–6307.
- Campa-Córdova ÁI, Angulo C, Zarain-Herzberg M, Pacheco-Marges R, Ascencio F, Guzmán- Murillo MA, et al. Stressing stocking density and rearing time effect on whiteleg shrimp (Penaeus vannamei) reared intensively in floating cages. Lat Am J Aquat Res. 2022;50(2):158–167.
- Zhang W, Dan Z, Zhuang Y, Zheng J, Gong Y, Liu Y, et al. Effects of dietary lipid levels on growth, digestive enzyme activities, antioxidant capacity, and lipid metabolism in turbot (Scophthalmus maximus L.) at three different stages. Aquac Nutr. 2022.
- Choi CY, Choi JY, Choi YJ, Kim BS, Kim JW. Effects of green wavelength light on antioxidant and non-specific immune responses of the olive flounder Paralichthys olivaceus maintained at different stocking densities. Aquac Eng. 2019;84:23–28.
- Onxayvieng K, Piria M, Fuka MM, Gavrilović A, Liang X, Liu L, et al. High stocking density alters growth performance, blood biochemical profiles, and hepatic antioxidative capacity in gibel carp (Carassius gibelio). Fish Physiol Biochem. 2021;47(2):203–212.
[Crossref] [Google Scholar] [PubMed]
- Jia R, Liu BL, Han C, Huang B, Lei JL. Influence of stocking density on growth performance, antioxidant status, and physiological response of juvenile turbot, Scophthalmus Maximu, reared in land-based recirculating aquaculture system. J World Aquac Soc. 2016;47(4):587–599.
- Wang Y, Xu P, Nie Z, Li Q, Shao N, Xu G. Growth, digestive enzymes activities, serum biochemical parameters and antioxidant status of juvenile genetically improved farmed tilapia (Oreochromis niloticus) reared at different stocking densities in in-pond raceway recirculating culture system. Aquac Res. 2019;50(4):1338–1347.
- Yin H, Xu L, Porter NA. Free radical lipid peroxidation: Mechanisms and analysis. Chem Rev. 2011;111(10):5944–5972.
[Crossref] [Google Scholar] [PubMed]
- Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412(15–16):1297–1305.
[Crossref] [Google Scholar] [PubMed]
- Oyarzún R, Paredes R, Saravia J, Morera FJ, Muñoz JLP, Ruiz-Jarabo I, et al. Stocking density affects the growth performance, intermediary metabolism, osmoregulation, and response to stress in Patagonian blennie Eleginops maclovinus. Aquaculture. 2020;515:734565.
- Dong Y, Jia R, Hou Y, Diao W, Li B, Zhu J. Effects of stocking density on the growth performance, mitophagy, endocytosis and metabolism of Cherax quadricarinatus in integrated rice–crayfish farming systems. Front Physiol.2022;13.
[Crossref] [Google Scholar] [PubMed]
- Montero D, Robaina LE, Socorro J, Vergara JM, Tort L, Izquierdo MS. Alteration of liver and muscle fatty acid composition in gilthead seabream “Sparus aurata” juveniles held at high stocking density and fed an essential fatty acid deficient diet. Fish Physiol Biochem. 2001;24(1):63–72.
- Liu B, Fei F, Li X, Wang X, Huang B. Effects of stocking density on stress response, innate immune parameters, and welfare of turbot (Scophthalmus maximus). Aquac Int. 2019;27(6):1599–1612.
- He Y, Yu H, Zhao H, Zhu H, Zhang Q, Wang A, et al. Transcriptomic analysis to elucidate the effects of high stocking density on grass carp (Ctenopharyngodon idella). BMC Genomics. 2021;22(1):1–11.
[Crossref] [Google Scholar] [PubMed]
- Abreu JS, Takahashi LS, Hoshiba MA, Urbinati EC. Biological indicators of stress in pacu (Piaractus mesopotamicus) after capture. Brazilian J Biol. 2009;69(2):415–421.
[Crossref] [Google Scholar] [PubMed]
- Fazelan Z, Vatnikov YA, Kulikov EV, Plushikov VG, Yousefi M. Effects of dietary ginger (Zingiber officinale) administration on growth performance and stress, immunological, and antioxidant responses of common carp (Cyprinus carpio) reared under high stocking density. Aquaculture. 2020;518:734833.
- Yousefi M, Hoseini SM, Vatnikov YA, Kulikov EV, Drukovsky SG. Rosemary leaf powder improved growth performance, immune and antioxidant parameters, and crowding stress responses in common carp (Cyprinus carpio) fingerlings. Aquaculture. 2019;505:473–480.
- Saccol EM, Parrado‐Sanabria YA, Gagliardi L, Jerez‐Cepa I, Mourão RH, Heinzmann BM, et al. Myrcia sylvatica essential oil in the diet of gilthead sea bream (Sparus aurata L.) attenuates the stress response induced by high stocking density. Aquac Nutr. 2018;24(5):1381-1392.
- Souza C de F, Salbego J, Gressler LT, Golombieski JI, Ferst JG, Cunha MA, et al. Rhamdia quelen (Quoy and Gaimard, 1824), submitted to a stressful condition: Effect of dietary addition of the essential oil of Lippia alba on metabolism, osmoregulation and endocrinology. Neotrop Ichthyol. 2015;13(4):707–714.
- Shourbela RM, El-Hawarry WN, Elfadadny MR, Dawood MAO. Oregano essential oil enhanced the growth performance, immunity, and antioxidative status of Nile tilapia (Oreochromis niloticus) reared under intensive systems. Aquaculture. 2021;542:736868.
- Rafieepour A, Hajirezaee S, Rahimi R. Dietary oregano extract (Origanum vulgare L.) enhances the antioxidant defence in rainbow trout, Oncorhynchus mykissagainst toxicity induced by organophosphorus pesticide, diazinon. Toxin Rev. 2019;39(4):397–407.
- Ganga R, Montero D, Bell JG, Atalah E, Ganuza E, Vega-Orellana O, et, al. Stress response in sea bream (Sparus aurata) held under crowded conditions and fed diets containing linseed and/or soybean oil. Aquaculture. 2011; 311(1-4): 215-223.
- Saccol EMH, Jerez-Cepa I, Ourique GM, Pês TS, Gressler LT, Mourão RHV, et al. Myrcia sylvatica essential oil mitigates molecular, biochemical and physiological alterations in Rhamdia quelen under different stress events associated to transport. Res Vet Sci. 2018;117:150–160.
- Martos-Sitcha JA, Wunderink YS, Straatjes J, Skrzynska AK, Mancera JM, Martínez- Rodríguez G. Different stressors induce differential responses of the CRH-stress system in the gilthead sea bream (Sparus aurata). Comp Biochem Physiol -Part A Mol Integr Physiol. 2014;177:49–61.
- De Oliveira Hashimoto GS, Neto FM, Ruiz ML, Acchile M, Chagas EC, Chaves FCM, et al. Essential oils of Lippia sidoides and Mentha piperita against monogenean parasites and their influence on the hematology of Nile tilapia. Aquaculture. 2016;450:182–186.
- Fogliarini CO, Garlet QI, Parodi T V, Becker AG, Garcia LO, Heinzmann BM, et al. Anesthesia of Epinephelus marginatus with essential oil of Aloysia polystachya: An approach on blood parameters. An Acad Bras Cienc. 2017;89(1):445–456.
- Hoseini SM, Hoseinifar SH, Doan H Van. Effect of dietary eucalyptol on stress markers, enzyme activities and immune indicators in serum and haematological characteristics of common carp (Cyprinus carpio) exposed to toxic concentration of ambient copper. Aquac Res. 2018;49(9):3045–3054.
- Mossa ATH, Mohafrash SM, Chandrasekaran N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. Biomed Res. Int. 2018.
- Cheng CH, Guo ZX, Luo SW, Wang AL. Effects of high temperature on biochemical parameters, oxidative stress, DNA damage and apoptosis of pufferfish (Takifugu obscurus). Ecotoxicol Environ Saf. 2018;150:190–198.
- Aniagu SO, Day N, Chipman JK, Taylor EW, Butler PJ, Winter MJ. Does exhaustive exercise result in oxidative stress and associated DNA damage in the chub (Leuciscus cephalus)? Environ. Mol. Mutagen. 2006;47(8):616-623.
- Chronic impact of exposure to low dissolved oxygen on the physiology of Dicentrarchus labrax and Sparus aurata and its effects on the acute stress response. Aquaculture. 2023;15(562):738830.
- Farrell AP, Richards JG. Defining hypoxia: An integrative synthesis of the responses of fish to hypoxia. Fish Physiol. 2009;27(C):487–503.
- Beemelmanns A, Zanuzzo FS, Xue X, Sandrelli RM, Rise ML, Gamperl AK. The transcriptomic responses of Atlantic salmon (Salmo salar) to high temperature stress alone, and in combination with moderate hypoxia. BMC Genomics. 2021;22:1–33.
- Emam M, Caballero-Solares A, Xue X, Umasuthan N, Milligan B, Taylor RG, et al. Gill and liver transcript expression changes associated with gill damage in Atlantic salmon (Salmo salar). Front Immunol. 2022;13.
- Silkin YA, Silkina EN. Effect of hypoxia on physiological-biochemical blood parameters in some marine fish. J Evol Biochem Physiol. 2005;41(5):527–532.
- Aboagye DL, Allen PJ. Effects of acute and chronic hypoxia on acid–base regulation, hematology, ion, and osmoregulation of juvenile American paddlefish. J Comp Physiol B Biochem Syst Environ Physiol. 2018;188(1):77–88.
- Wendelaar Bonga SE. The Stress Response in Fish. Physiol. Rev. 1997;77(3):591-625.
- Araújo-Luna R, Ribeiro L, Bergheim A, Pousão-Ferreira P. The impact of different rearing condition on gilthead seabream welfare: Dissolved oxygen levels and stocking densities. Aquac Res. 2018;49(12):3845–3855.
- De Mercado E, Larrán AM, Pinedo J, Tomás-Almenar C. Skin mucous: A new approach to assess stress in rainbow trout. Aquaculture. 2018;484:90–97.
- Morales-Lange B, Djordjevic B, Gaudhaman A, Press CML, Olson J, Mydland LT, et al. Dietary inclusion of hydrolyzed Debaryomyces hansenii yeasts modulates physiological responses in plasma and immune organs of atlantic salmon (Salmo salar) parr exposed to acute hypoxia stress. Front Physiol. 2022;13:1-13.
- Fazio F, Ferrantelli V, Fortino G, Arfuso F, Giangrosso G, Faggio C. The influence of acute handling stress on some blood parameters in cultured sea bream (Sparus aurata Linnaeus, 1758). Ital J Food Saf. 2015;4(1):4–6.
- Sunyer JO, Gómez E, Tort L, Navarro V, Quesada J. Physiological responses and depression of humoral components of the immune system in gilthead sea bream (Sparus aurata) following daily acute stress. Can J Fish Aquat Sci. 1995;52(11):2339–2346.
- M. dos Santos W, S. de Brito T, de A. Prado S, G. De Oliveira C, C. De Paula A, C. De Melo D, et al. Cinnamon (Cinnamomum sp.) inclusion in diets for Nile tilapia submitted to acute hypoxic stress. Fish Shellfish Immunol. 2016;54:551–555.
Citation: Antoniadou E, Karapanagiotidis IT, Panagiotaki P, Golomazou E (2023) Phytogenic Feed Additive Supplemented Diets as Welfare Promoters under Acute and Chronic Stress Factors in Gilthead Seabream. J Aquac Res Dev.14:776.
Copyright: © 2023 Antoniadou E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

