Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Original Research Article - (2021) Volume 11, Issue 2
O2, N2, CO, CO2 capture technologies in the post-combustion operation of the waste stream (A review)
Malek Hassanpour*Received: 15-Jan-2021 Published: 24-Feb-2021, DOI: 10.35248/2252-5211.21.11.396
Abstract
The presence of post-combustion main gaseous products dissipated into the environment participated in emerging serious damages in the atmosphere. The combustion operation as one of the prominent technologies posed in the disposal of waste stream emits huge quantities of valuable gaseous products as well as its emission of various industrial and non-industrial resources that can be an appropriate feedstock for many industrial units. The objective of the present review conducted towards technologies that came into view in handling and separating gaseous products of O2, N2, CO, CO2 in the post-combustion operation. Therefore, the concepts and implications picked up from scientific references to demystify the overwhelming technologies that emerged in this regard. The findings attract the interests of the researchers by defining the framework of a matrix of criteria and alternatives for the experts' opinion and making the decision for the best alternative possible. Also, the important industrial plants implemented in Iran were explained with the best technologies selected for the gas capturing operation. According to available technologies, it was found the sorbents and molecular sieves as best choices that have been detected by the Iranian assessment team in Environmental Impact Assessment (EIA) plan. The conclusion of the review can be allocated to offering further options in alternative energies based on the new technologies asserted and environmental challenges raised.
Keywords
Screening of Projects; Energy stream; Energy conversion; EIA; Decision theory
Introduction
It is very clear that a huge quantity of our atmosphere accommodated with a variety of gaseous compounds. The nature and origin of gaseous compounds refer to anthropological activities along with the interplay and interaction in the galaxy, nature, environment, and outdoor air. The atmosphere comprised a few enriched gases percentages mostly for N2, O2, CO2, and Ar that can be extracted and exploited for a variety of applications. The abundance of gaseous resources leads to neglect of existing resources. But human duty obligated to preserve and conserve the environment and stop wasting resources and dissipating the air pollutants consequently exploitation of healthy and cost-effectively use of resources. Waste materials appeared as a source of pollution and also income due to the dependence of human life to feed and lots of materials demands in daily life [1-3]. The wastes landfilled are going to be digested by anaerobic systems examined successfully for capturing the CO2 gas via both methods of oxy-fuel and absorption [4]. According to recent reports, there are 4 incinerators equipped with a carbon capture system. The incinerators have been accommodated in Norway (Klemetsrud CHP), Japan (Saga City),and in the Netherlands (Twence and AVR plant). The acquired carbon is employed to grow and breathe the algae in Japan. The quantity of carbon captured; estimated to be around 10 tons/day [5-7].
The main resource of CO2 dissipation is the exploitation of fossil fuels in lots of applications and industries. Using high temperatures for combustion operation in any place generates a high concentration of N2. Despite N2 has been composed approximately 78% of the air atmosphere it produces highly from combustion operation applying high temperatures [8]. The technologies posed to capture and remove CO2 from the gas stream classified into three important tasks of accomplishment such as post-combustion, pre-combustion, and oxy-combustion. The remaining gaseous compounds are able to capture in the following sections [9]. To separate O2 from N2 in oxy-combustion a study requested to employ the ion transport membranes which decline the costs of capturing CO2 in the following step. The post-combustion operation for capturing gaseous products funded by many nations such as China, Canada, Australia, Germany, Jaenschwalde, UK, Hatfield, Netherlands, Maasvlakte, Spain, Compostella, Poland, Belchatow, Italy, Porto Tolle [10].
The cryogenic technique has been recently introduced for gaseous product capturing operation. The principle applied in this technique complies with the temperature difference between gas molecules in a flow that is passing through channels. The energy consumed in this process is relatively high [11,12]. As we know, the environmental rules and regulations have been strictly controlled by governmental offices for the pollutants emitted into the environment. One of the strict rules for the industries with significant pollutant dissipation is offering verdicts to pay fines or penalties. The current scenario has been recognized as a strong and responsible rule against the managers of industries who breach the existing environmental regulations. The study by Hanak et al [13] asserted that this process examined in lots of pilot plants over the world and exposed with the minimum penalty in environmental pollution issues. The research of Wolf and Yan [14] exploited the chemical looping combustion joined to the turbine with input steam which is able to separate H2 from CO2 and the heat produced also utilized to generate electricity.
The high attraction of amine sorbents towards CO2 is the main reason for employing sorbents in CO2 capture operation. Therefore, this kind of sorbent is recommended in a variety of researches. In Iran, the use of amine-based sorbents has been encouraged and many industrial plants have been constructed to capture CO2[15]. Li et al [16] used the CO2 capture operation to promote the energy performance in a plant with both products of natural gas and power based on coal combustion as the fuel in the industry. By the way, the CO2 capture operation also declined the greenhouse gas emissions.Therefore, the main advantages of the post-combustion program include the decline in energy exploited in lines and especially processing parts, observing the environmental regulation as the capture unit is operated in controlling and handling pollutants entered into the environment, minimizing the outlays, and also rising the income by recycling valuable gaseous products consequently escalating the performance and efficiency of industry, minimizing environmental penalties and etc. [17,18].
Scientists are concerned about raising global warming damaging effects especially CO2. The international organizations forecasted to increase the CO2 emissions up to 570 ppmv in the atmosphere by 2100. So, lots of precautions have been taken into consideration that one of them belongs to CO2 capture projects. With regard to this fact that global climate recirculation, interactions in the atmosphere preserve the carbon concentration in fixed and balanced levels but it has been neglected by the industrial revolution and the pollutant dissipation levels are rising as well as waste stream discharged from human activities. It has been estimated that the role of iron, steel, oil refining, and petrochemical manufacturing plants in CO2 emissions raised up to 22%. To cope with global warming challenges the industries obligated to use molecular sieves which are stuff to separate gaseous products based on molecular weight and size. The advantages of the mentioned technology refer to being cheap and efficient and adaptable with different carbon sequestration schemes. Then, the technology extended to introducing the molecular basket adsorbents that are made up of mesoporous molecular sieve in which used polyethylene mine structurally. These sieves highly recommended for employing industrial applications [19-21].
The important CO2 capture technologies allocated to be membrane technologies, chemical absorption using amine sorbents, physical absorption solvent, and cryogenic distillation reported being efficient up to 95%, 95% and upper than 95% [22,23]. Despite this, we can find enough definitions of the above-mentioned technologies that deal with gas capture operation but the technologies mostly developed in theory and pilot plants before 2005 like oxyfuel technology. They are going to be defined in project dimensions and underwent the initial assessments for the implementation steps in non-civilized nations. There is a strong attraction in civilized countries to beckon the stakeholder's interests over the world [24].
To magnify the importance of carbon capture techniques can be mentioned to the studies of the author in the field of acidic sludge management of Used Motor Oil Reprocessing Industries (UMORI) that resulted in the implementation of plasmatron reactor to produce valuable gas compounds so recede the difficulties raised in the field. The difficulties get back to the outcomes of release the acidic sludge into the environment in huge quantities with numerous recycling plants of UMORI. In Iran, there is no other way to solve this kind of environmental problem. The technology replacement in UMORI, refining, re-refining, generation, and regeneration operation of UMO and acidic sludge is completely costly and expensive. So, now we are thinking about the separate handling of gaseous products to supply the demands raised in this regard. It needs to explain that the finishing units of UMORI and similar industries have a heavy demand for valuable gaseous products such as H2, CO2, and N2. Therefore, the present review comes through all relevant articles published to figure out which technologies are dominant in the gaseous product capturing operation [25,26]. It needs to be clear that the current review does not consider sequestration practices like forestation, ocean fertilization, desertification, and mineral carbonization methods.
However, other technologies are also dominant in this regard and they need to taken into consideration for such applications. By assigning relevant processes and technologies the current challenges of the environment can take it back and open the way towards surging interests for business excellence, circular economy, and innovation in products. In the following proclamations for the environment protection against gaseous products dissipated into the environment, recycling and resource conservation are also declared by in-charge organizations along with the EIA plan for further preservation of the environment. The projects outlined at national levels underwent some environmental assessments that help the stakeholders to identify requirements, economic explanation, and select the best technology and process by decision making in a wide range of options and alternatives. This procedure is called screening of projects in the way of decision making for approval or disapproval of projects. It needs to declare the fact here that the objective followed by the present review look at the O2, N2, CO, CO2 capture technologies in the post-combustion operation of the waste stream as projects posed in this way individually. Figure 1 shows the steps mentioned in the flow diagram but the data used and described in this review completely belong to the screening step of project identification only.
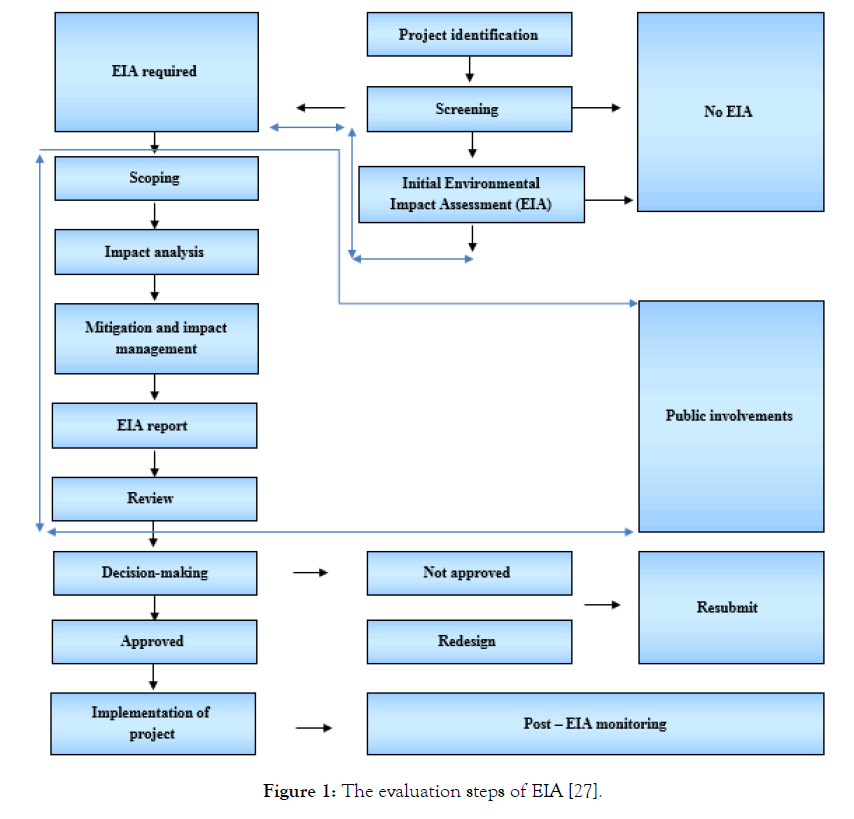
Figure 1: The evaluation steps of EIA [27].
Today, all civilized and under developing countries are aware that the health of all is the guarantor of sustainable development, and their concept encompasses a wide range of working environments, working manpower, peripheral environments, and society. In addition to combating disease and injury, this approach addresses a wide range of areas of happiness and well-being of life, the work ambient, the environment, and its practical meaning has been exalted in combining economic production with creating a decent standard of living, work, and environment. Therefore, appropriate and popular industrial development leads to health and, conversely, in the unregulated and unsustainable economic development, the health of individuals, safety, and the environment is severely threatened. For this reason, it is necessary to create a balance between nature and society with industrial and economic development. Environmental assessment includes both aspects of the direct and indirect effects of a development plan and examines the chain of changes resulting from the environmental effects that occur as a result of the project while examining the environmental consequences. The environmental assessment predicts and evaluates impacts through a systematic and organized process. The purpose of assessing the environmental consequences of a project is to determine the extent of the hazards anticipated and the measures needed to reduce or avoid them, as well as to determine how to compensate for the inevitable environmental damage. Certainly, the solutions and measures will be done by forecasting financial costs so that the executive body and the planning organization can make the necessary decision-making activities regarding the selection of the best option and the different cracks of the mitigation programs and compare them with each other and the most appropriate selection of them. The first step in environmental assessment is the project description. The project description should provide the assessment team with exactly all the information that may be used during the assessment process. The responsibility for preparing and collecting this information lies with the investor or the executor, and at each stage, if the evaluation team needs more accurate and detailed information, it should be prepared and presented by the executor. Given the fact that the data of the following study are related to the screening of industrial projects, it seems crucial for a comprehensive definition in this field. The purpose of screening is to determine the status of a project to be evaluated and at what level it should be evaluated. Through screening, it is determined what criteria should be observed in different stages of project studies [28-30].
In the screening step of projects must be determined the location, area, geography, properties, materials and energy demands, dependence to water and energy supply networks, facilities required in projects, the dependence on facilities and materials demands to inside and outside the country, number of employees and staff required, prices of materials, devices and facilities, diagram and flow diagram of projects, etc. The necessary and unnecessary information must be cleared and the scope of the project with certain objectives should be notified [31-35].
CO and CO2 Generation, Capture Operation and their Conversion in Industrial Scale
The stages of CO production are as follows: (1) in this stage, natural gas enters the double-walled reactivation tank and burns. Combustion of natural gas produces gaseous products that escape from the end of the device. The length of this device is 4 meters and the diameter is 1.5 meters. Gas combustion takes place in the inner wall of this device. (2) Combustion gases contain amounts of acidic compounds and high temperatures such as CO2. In order to remove CO2 and reduce the temperature of combustion gases, at this stage, the combustion gases enter into the water washing tower. The height of this tower is 5 meters and its diameter is one meter and it is made of steel. This tower is filled with heat resistant materials. (3) The combustion gas collides and then is exposed to the Monoethanolamine (MEA) to rise the absorption process in two steps, counter-current, and co-current. The MEA towers are 7.5 meters high and one meter in diameter. Exhaust gases are discharged into the environment from the top of the second tower. (4) In order to recover the MEA from the enriched solution, and to separate the CO2, first, the solution is preheated in a heat exchanger and then by entering the outer wall of the reactor, the CO2 is separated from the MEA and is stripped perpendicular to the reactivator and is released. (4) MEA, which has lost its CO2, enters the heated reactor to be cooled. (6) The gases from the reactivation tank come into direct contact with water for cooling. (7) Water with gases is separated in the separator tower. (8) In order to chemically purify and remove sulfur and NOx with gas, it is introduced into its soda treatment tank. (9) At this stage, CO is converted to CO2. (10) To convert gas to liquid: First, increases its pressure by a 1000 PSI compressor. The compressor made of torsional type and contains three stages. (11) In order to remove water and dust and also to deodorize the CO2, it must be turned into a liquid. For this reason, the Freon system is used to condense CO2 with a capacity of 25 kg per hour. (12) To store CO2 liquid from two tanks holding a capacity of 25 tons with dimensions of 15 meters and a diameter of 2 meters with a system equipped with a cooler is used. (13) In order to sell the product, a cylinder charging device or transport tankers are used. The cylinder charging system is equipped with a scale to distribute the cylinders according to Figure 2. The CO2 capture operation has been done via employing oxy-combustion and MEA absorbers in a cement plant in NE Scotland, UK [36,37]. Despite, the present study explained the CO2 capture from the air atmosphere with a mixture of other gaseous components, many studies pointed out to direct capture of CO2 from the atmosphere as a novel methodology posed. The required field of CO and CO2 generation industries represented in Table 1.
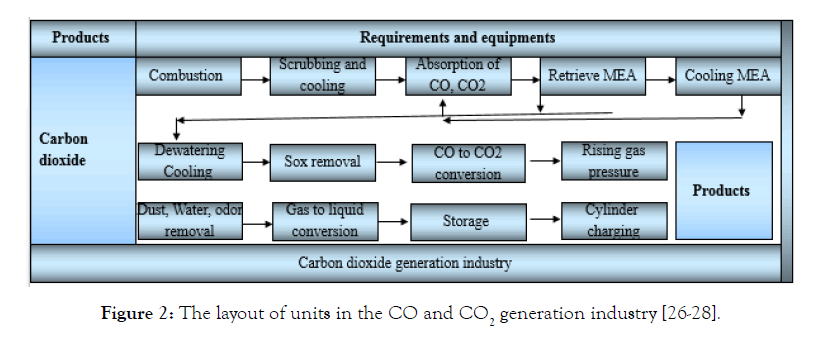
Figure 2: The layout of units in the CO and CO2 generation industry [26-28].
| Main annual materials and equipment | Total annual rates |
|---|---|
| Equipment and devices | |
| Filter 1 m2 | 1 No |
| Air blower, 1800 m3, 3 kW | 1 No |
| Reactivate equipped with stripper | 1 No |
| Scrubber | 1 No |
| MEA Tower | 1 No |
| MEA Exchanger | 2 No |
| Centrifuge pump, equipped with electromotor, 5.5 kW | 3 No |
| Precooler of gas | 1 No |
| De-water tank | 1 No |
| Chemical reaction tank | 1 No |
| Compressor | 1 No |
| The heat exchanger of CO2 | 2 No |
| Silicagel packed | 2 No |
| Active carbon packed | 1 No |
| Gas to liquid conversion system, 250 kg/h | 1 No |
| Solution storage tank, 25 tons, 20 bar | 2 No |
| pH adjusting tank, equipped with 500 liters of soda | 1 No |
| CO2 charging cylinder | 1 No |
| Materials demands | |
| Municipal natural gas | 1296000 m3 |
| MEA | 1200 liters |
| Silica gel | 120 kg |
| CaCO3 | 420 Kg |
| Kmno4 | 300 kg |
| Products | |
| CO2 | 1800 tons |
| Employees | |
| Staff | 18 persons |
| Energy consumption | |
| Required water | 65 m3/day |
| Power consumed | 161 kW/day |
| Required fuel (Stoves) | 134 Giga Joule/day |
| Required land and landscaping | |
| Required land | 2500 m2 |
| Construction of infrastructure (Buildings) | 700 m2 |
Table 1: The annual requirements of the CO2 generation industry (nominal capacity of 1800 tons) [26-28].
O2, Ar, and N2 Generation Industries
The product of this unit is Ar, N2, and O2 gases with very high purity. To produce a product with high purity, liquid air distillation, water electrolysis, absorption separation, and hydrocarbon combustion with air can be used. The selected method in this unit is liquid air distillation. The steps are as follows: (1) The air is compressed after passing through the filter in the compressor and by cooling in the pre-cooler, part of its water will be lost (2) then compressed air enters the molecular sieve beds to absorb water and CO2 in the sieves. Then, it enters the powder filter to absorb the suspended particles of the molecular sieve. (3) The cleaned and compressed air enters into the heat exchanger to reduce the temperature to about 100 to 120°C (4) Cold and compressed air then enters into the expansion machine (turbine) and the temperature decreases to -180°C with decreasing pressure. (5) The air that has become liquid in the previous stage enters into the two-stage air distillation column. It is divided into several parts, where N2 and O2 are released directly, but the argon bulk in the raw argon column is distilled again. (6) The N2 released transfers to the evaporator after equalization that will be charged by diaphragm compressors and are stored in 40-liters capsules and transport tanks. (7) The output O2 is charged exactly like N2. (8) In the next step, the output Ar released from the raw Ar column is combined and reacted with H2 within the reactor containing catalyst, and the produced water is wiped via desiccators. Finally, Ar is purified in the secondary distillation system (9) the output Ar, after being balanced in the storage tank, is transferred to the evaporator and is charged by diaphragm compressors in 40-liter capsules. In lots of studies, this kind of technology for separating gaseous products is called cryogenic air separation according to Figure 3 [40]. Table 2 shows the annual requirements of Ar, O2 and N2 generation industries.
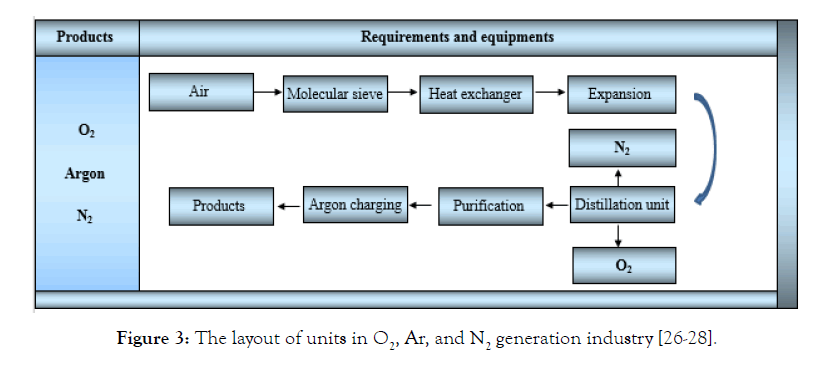
Figure 3: The layout of units in O2, Ar, and N2 generation industry [26-28].
| Main annual materials and equipment | Total annual rates |
|---|---|
| Equipment and devices | |
| Air filter | 1 No |
| Air compressor | 1 No |
| Ar distillation system | 2 No |
| Heat exchanger | 1 No |
| Turbine | 1 No |
| Capsules, 40 liters | 5000 No |
| Lab facilities | I unit |
| Materials demands | |
| Atmospheric air (contains 78% N2, 21% O2, 1% Ar | - |
| Molecular sieve (the type of 13x, to remove water and CO2 | 1400 kg |
| H2, as Capsules of 40 liters | 480 No |
| Products | |
| Ar (purity of 99/9999%), O2 (purity of 99/5%), and N2 (purity of 99/999%, capsules of 40 liters, 150 bar) | 1800 tons |
| Employees | |
| Staff | 32 persons |
| Energy consumption | |
| Required water | 310 m3/day |
| Power consumed | 542 kW/day |
| Required fuel (Stoves) | 13 Giga Joule/day |
| Required land and landscaping | |
| Required land | 8800 m2 |
| Construction of infrastructure (Buildings) | 2500 m2 |
Table 2: The annual requirements of Ar, O2, and N2 generation industries (nominal capacity of 43200, 2160000, and 1440000 m3 respectively) [26-28].
New Developments
Generally, the CO2 capture technologies divided into four main sections such as (1) adsorption (2) absorption (physical and chemical absorption) that the chemical absorption also includes (amine and inorganic solvents), (3) membrane separation, and (4) cryogenics. Different techniques of accelerated carbonation for CO2 capture can be allocated as (1) direct carbonation (1.1) dry routes composed from the gas-solid form and (1.2) aqueous carbonation such as brine/wastewater or gas-liquid-solid exposures. (2) Acid extraction based on aqueous carbonation and pH swing with laying out acid and base additive units along with aqueous carbonation. The second route in precipitation of calcium carbonate is out of objective followed by present review [38,39].
Oxygen Transport Membranes (OTM)
The emergence of mixed metal oxide ceramic materials has been introduced as a dominant technology that is able to generate high stream rates of O2 under high temperature and pressure conducted. The ceramic materials used have diffusing characters for the gas content passed through from that. Also, they comprised to capture CO2 with an efficiency of around 99% that is upper than conventional oxyfuel processes with insensible NOx dissipation. This technology is cheaper than cryogenic systems up to 70% low power consumption and a 50% thrift in capital costs. This system can be optimized with plasma reactors with high temperature ambient in future developments [40]. Also, the newly made-up microporous metal-organic frameworks reported offering high efficiency in capturing gaseous products from gas flows.
Polymeric Membranes (PMs)
The PMs are applied to capture gaseous products. PMs configured from solid zeolite structure and work at a low flow diagram of O2 and temperatures. The high degree of separation by membranes need to set up multiples stages of the membrane to pass through the gas flows [40]. The PMs Technology contains a low footprint, simple facility with a steady-state operation. Its configuration and flexibility are impressive. The prominent drawbacks for PMs come from the cut-off & clogging in the system in long time service and negative points posed about inefficiency and costs of large scale reactors. Also, the demand for facilities of pressure supply is the main disadvantage of running the PMs. PMs are emerged as both symmetrical and asymmetrical in their structures and frameworks. That is why they have a homogeneous or heterogeneous distribution in terms of appearance. An asymmetric membrane is made up of two layers of the same materials with different porosity (a membrane that can change phase) or two layers of different materials (a composite membrane). The active layer of the membranes is dense and thin, about one micrometer, which regulates the efficiency and permeability of the membrane in the feeding area. The secondary porous layer plays the supporting and holding mode. Asymmetric membranes are used in high flow fluxes, especially in solution diffusion membrane processes. Both composite membranes and polymer-modified membranes; can be made porous or non-porous as products of plasma technology based on conversions in polymers frameworks [41,42]. Plasma technology plays a major role in the fabrication and production of membranes. Membranes made of 5% cellulose acetate, 95% polyamide, cellulose acetate, sulfonate polyether, zirconium dioxide, polyamide, polyvinylidene fluoride, titanium oxide, sulfonate polyether, polyacrylic nitrile, cellulose, polysulfone membrane component have changed the phase with plasma technology and ceramic and composite materials have been used in their structure recently. The structure of these membranes is porous and asymmetric. The active material of many membranes is polymer and ceramic with porous and asymmetric structures such as polypropylene, fluorinated polyvinylidene, polysulfone, aluminum oxide, refined metals, titanium oxide, and zirconium oxide. Today, there are very common types of membranes in the global market. Determining the type of membranes depends on the composition and characteristics of the gas being treated. Cellulose membranes are less used today or not used at all. Unlike cellulose membranes, PMs such as polysulfone, polyacrylonitrile, and polyethersulfone are resistant to high temperatures and chemical stresses; the use of minerals such as ceramics, aluminum, refined metals, and glass is very important [43].
Chemical solvents
The chemical solvents (like MEA, Dipropylamine, Butanediamine and, etc) are used is scrubbers to clean the CO2 from input gas and convert it into the pure product. It is a possible practice for low gas flow and small plants [44]. The commercial scrubbing solvents which are employed in industrial application for CO2 are as physical solvents of rectisol, puisol, selexol, flour and the chemical solvents are allocated as organic amines, MEA, amin guard, econamine, flexsorb, Benfield, wet potassium carbonate,and other versions. The physic-chemical solvents are amisol and sulfinol-D and Sulfinol-M [45,46]. The limestone as a sorbent is utilized to capture CO2 in post-combustion operation that thrift the energy exploited and is an efficient process. The process is called carbonating looping [47,48].
Oxy-fuel combustion
This process demand pure O2 for burning fuel and the air superseded by pure O2 in this technology. Therefore, the outcome of this reaction is water and CO2 in which the gas capture process is easy. The main advantage of this process is generating an enriched CO2 content of around 80%. Therefore, it demands a simple purification for CO2 captured to be stored [45]. The recent developments and studies are surging interests towards minor and simple purification of gaseous products released from oxyfuel technology using pure O2 in combustion operation.
Plasma reactors
The plasma technology included a variety of hot and cold reactors for the processing waste stream and generating highly enhanced and enriched gaseous products in post-combustion operation. Most compounds are converted to CO, CO2, N2, CH4, and H2 with a huge capacity for capturing purposes in an easy way. The gas stream called working fluid has been composed of a mixture of CO2 captured in presence of O2. Using MATIANT cycles resulted to extract excess CO2 via simple valves. The resource for lots of adsorbents also refers to plasma technology that creates nanomaterials, porous structures, functional composites, and high strength and flexible adsorbents in a variety of applications and technologies [49-51].
The new technologies of hydrogen generation like plasma technology (gassifiers like plasmatron) need a swig adsorption step to capture H2 from other compounds with a high purity of H2 generated up to 99.9999% [52-54]. However, we know that the output gases of plasma reactors especially plasmatron restricted to H2-35%; N2-47%; CO-3%; CO2-13% in post-combustion operation [55] but it needs to notice the typical impurities of gaseous compounds stream as: (1) in post-combustion (SO2, NOx, N2/Ar/O2 ≤0.01 vol%; Pre-combustion H2S, H2, CO, CH4 around 0.01-0.6, 0.8-2, 0.03-0.4, 0.01 vol%; Oxyfuel, SO2, NOx, N2/Ar/ O2; 0.5, 0.01, 3.7 vol% respectively [56-59].
The study of Sadat and Archer [60] introduced the O2-assisted Al/ CO2 electrochemical cell to capture CO2 in an electrochemical ambient that the product has been used to recover the energy consumed. This type of pilot-plants also can be called a type of plasma cold reactor.
Integrated methods
The presence of a mixture of a few different types of gas flows makes the gas capture process a little cumbersome. For these situations, we recommend using integrated technologies and methods like the layout of selective chemicals, physical absorption, and even oxyfuel combustion. The green algae like Chlorella Vulgaris have been used to capture CO2 from the photobioreactor. Coupling the reactor with membrane enhanced the CO2 capturing process and raised the efficiency estimated. In other studies, the developments moved towards using the same algae and microorganisms in immobilization technology inside a porous biocomposite paper, and high efficiency achieved by spinning disc bioreactor [61-65]. The use of ionic liquids has been emphasized in many studies but employing them in post-combustion gas capturing operation demands high costs and special consideration in the process because of the complexity of technology. The advanced sorption reformers coupled to plasma reactors like plasmatron are able to convert hydrocarbons and produce enrich gas content of H2 along with capturing of gases. It is an integrated method of sorbents with a water-gas conversion reaction. Also, it has been recognized as zero-emission systems that lithium silicates and hydrotalcitelike materials are used in its structures. The study of Friday et al [66] takes into consideration the CO2 hydrate and electrochemical pump as new methods of CO2 capture without any further description in this regard. The use of hybrid technologies has been encouraged by Al-Mamoori et al [67].
Economic aspect
According to a review of texts conducted in recent years, it shows that the gas capture technologies have found their economic justification in solving environmental problems and impede paying penalties, forfeits, and fines for environmental pollution issues. In addition to the fact that job creation, production promotion, obtaining international certificates, and implementing a circular economy and many other benefits make this technology more economical and the need for implementation as much as possible [68-71]. Studies developed in the field of techno-economic evaluation and assessments with the help of data envelopment analysis are confirmation records in this field. The outcome or output of these technologies is alternative energy for various demands and applications [71-75].
Conclusion
By present review pointed out the role of gaseous products is rising added value to the country assets as well as procuring business and job opportunities. Also, the gas capturing technologies are a way of handling greenhouse gases dissipated into the environment and making a profit for stakeholders. Employing combustion operation as a dominant technology in handling waste stream needs such technologies in reaching the zero-emission pollutants.On the other hand, the combustors equipped with plasma technologies provide highly enriched gaseous products that are feedstock for lots of other resources as mixed gases or separated. Therefore, the gas capturing techniques pave the way towards both integrated applications of gaseous products and individual. The main objective of the present review was to identify the best and common procedure of gas capturing techniques. Despite the review introduced the relevant technologies in this regard but to make a decision about the best capturing process the decision-making theory with lots of multi-criteria decision-making models will help researchers to find the right one in future studies. There are many units of gas capturing for the mentioned cases in which the air atmosphere plays the main role in the initial feed of manufacturing. We recommend the attention towards waste conversion technologies for the gas capturing operation. However, the outputs of waste conversion techniques are inputs of other industries so it supplies the frameworks and dimensions of the circular economy by this process as well as protection and conservation of the environment. The present review with a glance view of the implementation of gas capturing projects in many nations encouraged other nations to take into consideration the privileges and economic expansion created in this pathway.
Acknowledgment
This research was conducted as part of the corresponding author's Ph.D. research work (Entitled; Evaluation of 405 Iranian Industries). The tabulated data were picked up from the screening step of project identification in environmental impact assessment. The author thanked colleagues and evaluators of both the Iranian environment protection agency and Iranian industries organization for the data assessed.
Competing Interests
The author declares that there is no competing interest.
Conflicts of Interest
There is no conflict of interest.
Ethical Considerations
Ethical issues have been completely observed by the author.
REFERENCES
- Romano MC, Anantharaman R, Arasto A, Ozcan DC, Ahn H, Dijkstra JW, et al. Application of advanced technologies for CO2 capture from industrial sources. Energy Procedia.2013; 37: 7176-7185.
- 2.Koornneef J, Ramirez A, Harmelen TV, Horssen AV, Turkenburg W, Faaij A. The impact of CO2 capture in the power and heat sector on the emission of SO2, NOx, particulate matter, volatile organic compounds and NH3 in the European Union. Atmospheric Environment. 2010; 44: 1369-1385.
- Sitaras IE, Siskos PA. The role of primary and secondary air pollutants in atmospheric pollution: Athens urban area as a case study. Environ Chem Lett. 2008; 6: 59–69.
- Li H, Tan Y, Ditaranto M, Yan J, Yu Z. Capturing CO2 from biogas plants. Energy Procedia. 2017; 114: 6030-6035.
- Toshiba: News Release (10 Aug. Toshiba complete installation of world’s first commercial-use CCU system in incineration plant, 2016.
- Wienchol P, Szlek A, Ditaranto M. Waste-to-energy technology integrated with carbon capture Challenges and opportunities.Energy. 2020; 198: 117352, 1-11.
- Fernandez ES, Goetheer ELV, Manzolini G, Macchi E, Rezvani S, Vlugt TJH. Thermodynamic assessment of amine based CO2 capture technologies in power plants based on European Benchmarking Task Force methodology. Fuel. 2014; 129: 318-329.
- Chen X, Huang G, An C, Yao Y, Zhao S. Emerging N-nitrosamines and N-nitramines from amine-based postcombustion CO2 capture – A review. Chemical Engineering J. 2018; 335: 921–935.
- Florin N, Fennell P. Review of Advanced Carbon Capture Technologies. Work stream 2, Report 5A of the AVOID program (AV/WS2/D1/R05A). 2010; 1-28.
- Figueroa JD, Fout T, Plasynski S, McIIvried H, Srivastava RD. Advances in CO2 capture technology–The U.S. Department of Energy's Carbon Sequestration Program. Int J Greenhouse Gas Control. 2008; 2(1): 9–20.
- Awe OW, Zhao Y, Nzihou A, Minh DP, Lyczko N. A Review of Biogas Utilisation, Purification and Upgrading Technologies: Review. Waste and Biomass Valorization. 2017; 8 (2): 267-283.
- Zhao M, Minett AI, Harris AT. A review of techno-economic models for the retrofitting of conventional pulverised-coal power plants for post-combustion capture (PCC) of CO2. Energy Environ Sci. 2013; 6: 25-40.
- Hanak DP, Anthony EJ, Manovic V. A review of developments in pilot-plant testing and modelling of calcium looping process for CO2 capture from power generation systems. Energy Environ Sci. 2015; 8: 2199-2249.
- Wolf J, Yan J. Parametric study of chemical looping combustion for tri-generation of hydrogen, heat, and electrical power with CO2 capture. Int J Energy Res. 2004; 28: 1-15.
- Iranian industries organization database. (2019).
- Li S, Jin H, Gao L, Zhang X, Ji X. Techno-economic performance and cost reduction potential for the substitute/synthetic natural gas and power cogeneration plant with CO2 capture. Energy Conversion and Management. 2014; 85: 875–887.
- Plaza JM, Chen E, Rochelle GT. Absorber intercooling in CO2 absorption by Piperazine-promoted Potassium Carbonate. AICheE Journal. 2010; 56(4): 905:914.
- Wang M, Lawal A, Stephenson P, Sidders J, Ramshaw C, Yeung H. Post-combustion CO2 Capture with Chemical Absorption: A State-of-the-art Review. Chemical Engineering Research and Design. 2011; 89(9): 1609-1624.
- Yang H, Xu Z, Fan M, Gupta R, Slimane RB, Bland AE, et al. Progress in carbon dioxide separation and capture: A review. J Environ Sci. 2008; 20: 14–27.
- Stewart C, Hessami MA. A study of methods of carbon dioxide capture and sequestration–the Sustainability of a photosynthetic bioreactor approach. Energy Conversion and Management. 2005; 46: 403–420.
- Güleç F, Meredith W, Snape CE. Progress in the CO2 Capture Technologies for Fluid Catalytic Cracking (FCC) Units—A Review. Frontiers in Energy Research. 2020; 8(62): 1-14.
- Sukor NR, Shamsuddin AH, Mahlia TMI, Isa MFM. Techno-Economic Analysis of CO2 Capture Technologies in Offshore Natural Gas Field: Implications to Carbon Capture and Storage in Malaysia. Processes. 2020; 8: 1-22.
- 23.Adánez-Rubio I, Abad A, Gayán P, De Diego LF, García-Labiano F, Adánez J. Performance of CLOU process in the combustion of different types of coal with CO2 capture. Int J Greenhouse Gas Control. 2013: 12: 430-440.
- Buhre BJP, Elliott LK, Sheng CD, Gupta RP, Wall TF. Oxy-fuel combustion technology for coal-fired power generation Progress in Energy and Combustion Science. 2005; 31: 283–307.
- Jafari JA, Hassanpour M. Survey of economic indices of the used motor oil industry equipped to acidic sludge recycling unit (a case study). Merit Research Journal of Engineering, Pure and Applied Sciences. 2014; 2(2): 22-9.
- Hassanpour M. Techno-economic assessment of recycling acidic sludge project of reprocessing industries to value-added gaseous products using Plasmatron. Environmental Health Engineering and Management Journal. 2020; 7(3): 1-10.
- Hassanpour M. Evaluation of Iranian Small and Medium-Sized Industries. Ph.D. thesis.Osmania University, College of Science. 2020; 1-350.
- Hassanpour M. Evaluation of Iranian Chemical Industries. International Journal of chemistry materials research. 2020; 8(1): 26-48.
- Brian D, Clark PADC. Environmental Impact Assessment, Principles and Procedures.Scope 5. John Wiley and Sons, New York. 1979; 1-439.
- Vallero DA. Environmental Contaminants: Assessment and Control. Elsevier Academic Press, Burlington. 2004; 1-820.
- Ward M, Wilson J. Application of Strategic Environmental Assessment to Regional Land Transport Strategies, Land Transport New Zealand Research Report. Ward- Wilson Research. No: 275: 2005. Available: www.landtransport.govt.nz
- Assessment SE. Integrated Ecosystem Services in Strategic Environmental Assessment: A guide for Practitioners, United Nations Environment program (UNEP). 2014, 1-68.
- Audouin MA, K Govender. Strategic Environmental assessment: Integrated Environmental Management. 2004.
- Beanlands G. Scoping Methods and Baseline Studies in EIA Taylor and Francis. London and New York, 1988.
- HeuserIL. Milestones of Soil I Protection in EU Environmental Law. J European Environmental & Planning Law. 2006; 3(3): 190-203.
- Barker DJ, Turner SA, Napier-Moore PA, Clark M, Davison JE. CO2 Capture in the Cement Industry.Energy Procedia. 2009; 1(1): 87-94.
- Bosoaga A, Masek O, Oakey JE. CO2 Capture technologies for Cement Industry. Energy Procedia. 2009; 1(1): 133-140.
- Pan S-Y, Chang EE, Chiang P-C. CO2 Capture by Accelerated Carbonation of Alkaline Wastes: A Review on Its Principles and Applications. Aerosol and Air Quality Research. 2012; 12: 770–791.
- Krevor SCM, Lackner KS. Enhancing Serpentine Dissolution Kinetics for Mineral Carbon Dioxide Sequestration. Int J Greenhouse Gas Control. 2011; 5(4): 1073–1080.
- Blomen E, Hendriks C, Neele F. Capture technologies: Improvements and Promising Developments. Energy Procedia. 2009; 1(1): 1505–1512.
- Abanades JC, Arias B, Lyngfelt A, Mattisson T, Wiley DE, Li H, et al. Emerging CO2capture systems. Int J Greenhouse Gas Control. 2015; 40: 1750-5836.
- Wang M, Joel AS, Ramshaw C, Eimer D, Nuhu M. Musa Process Intensification for Post combustion CO2 capture with Chemical Absorption: A critical review. Applied Energy. 2015; 158: 275-291.
- Shavalieva G, Kazepidis P, Papadopoulos AI, Seferlis P, Papadokonstantakis S. Environmental, health and safety assessment of post-combustion CO2 capture processes with phase-change solvents. Sustainable Production and Consumption. 2021; 25: 60–76.
- Gupta M, Coyle I, Thambimuthu K. CO2 capture technologies and opportunities in Canada. 1st Canadian CC&S Technology roadmap workshop, 18-19 September 2003, Calgary, Alberta, Canada. 2003.
- Chen S, Lior N, Xiang W. Coal gasification integration with solid oxide fuel cell and chemical looping combustion for high-efficiency power generation with inherent CO2 capture. Applied Energy. 2015; 146: 298–312.
- Yang X, Qiao Y, Yu H, Han X, Liu B, Shah KJ, et al. Sensitivity Analysis of Carbonate Looping Process Using Twin Fluidized Bed Model. Aerosol Air Qual Res. 2020; 1-12.
- Yaqub ZT, Oboirien BO.Process Modelling of Chemical Looping Combustion of Paper, Plastics, Paper/Plastic Blend Waste, and Coal. ACS Omega. 2020; 5: 22420−22429.
- Denes FS, Manolache S. Macromolecular plasma-chemistry: an emerging field of polymer science. Prog Polym Sci. 2004; 29 (8): 815–895.
- Yazicioğlu O, Katircioğlu YT. Applications of plasma technology in energy sector. Yazıcıoğlu & Katırcıoğlu / Kirklareli University J Engineering and Science. 2017; 3: 18-44.
- Li L, Zhao N, Wei W, Sun Y. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences. Fuel. 2013; 108: 112–130.
- Luis P, Van Gerven T, Van Der Bruggen Bruggen B. Recent developments in membrane-based technologies for CO2 capture. Progress in Energy and Combustion Science. 2012; 38: 419-448.
- Kaarstad O,Audus H. Hydrogen and electricity from decarbonized fossil fuels. Energy Conversion and Management. 1997; 38: S431-S436.
- Klett MG, White JS, Schof RL, Buchanan TL. Hydrogen production facilities: plant performance and cost comparisons, final report, prepared for the national energy technology laboratory, US DOE. Reading: Parsons Infrastructure and Technology Group, Inc; 2002.
- Damen K, Troost MV, Faaij A, Turkenburg W. A comparison of electricity and hydrogen production systems with CO2 capture and storage. Part A: Review and selection of promising conversion and capture technologies. Progress in Energy and Combustion Science. 2006; 32: 215–246.
- Hassanpour M. Projects Management Addressed to Iranian Government (A case study). Proceedings of Business and Economic Studies. 2018; 1(2): 1-15.
- B Metz, O Davidson, H De Coninck. Carbon Dioxide Capture and Storage-Special Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, England, 2005.
- IEA, Impurities on CO2 capture, transport and storage, London, 2004.
- Bongartz R., Linssen J., Markewitz P. CO2 Transportation. In: Kuckshinrichs W., Hake JF. (eds) Carbon Capture, Storage and Use. Springer, Cham. 2015; 47-65.
- Markewitz P, Kuckshinrichs W, Leitner W, Linssen J, Zapp P, Bongartz R, et al. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ Sci. 2012; 5: 7281, 1-25.
- Sadat WIA, Archer LA. The O2-assisted Al/CO2 electrochemical cell: A system for CO2 capture/conversion and electric power generation. Archer Sci Adv. 2016; 2(7): e1600968, 1-10.
- Moreira D, Pires JCM. Atmospheric CO2 capture by algae: negative carbon dioxide emission path. Bioresour Technol. 2016; 215: 371–379.
- 62.Tongprawhan W, Srinuanpan S, Cheirsilp B. Biocapture of CO2 from biogas by oleaginous microalgae for improving methane content and simultaneously producing lipid. Bioresour Technol. 2014; 170: 90–99.
- Cheng L, Zhang L, Chen H, GAO C. Carbon Dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep Purif Technol. 2006; 50(3): 324–329.
- Ekins-Coward T, Boodhoo KVK, Velasquez-Orta S, Caldwell G, Wallace A, Barton R, et al. A microalgae biocomposite-integrated spinning disk bioreactor (SDBR): toward a scalable engineering approach for bioprocess intensification in light-driven CO2 absorption applications. Ind Eng Chem Res. 2019; 58(15): 5936–5949.
- Adamu A, Russo-Abegão F, Boodhoo K. Process intensification technologies for CO2 capture and conversion – a review. Adamu et al. BMC Chemical Engineering.2020; 2(2): 1-18.
- Ochedi FO, Liu Y, Adewuyi YG. State-of-the-art review on capture of CO2 using adsorbents preparedfrom waste materials. Process Safety and Environmental Protection.2020; 139: 1–25.
- Al-Mamoori A, Krishnamurthy A, Rownaghi AA, Rezaei F. Carbon Capture and Utilization Update. Energy Technol. 2017; 5: 834 – 849.
- Norouzi N, Talebi S, Shahbazi A. An overview on the carbon capture technologies with an approach of green coal production study. Chem Rev Lett.2020; 3: 65-78.
- Masudi A, Jusoh NWC, Muraza O. Recent progress on low rank coal conversion to dimethyl ether as clean fuel: A critical review. J Cleaner Production. 2020; 277: 124024, 1-12.
- Chaffee AL, Knowles GP, Liang Z, Zhang J, Xiao P, Webley PA. CO2 capture by adsorption: Materials and process development. International journal of greenhouse gas control. 2007; 1(1): 11-18.
- Hossain MM, De Lasa HI. Chemical-looping combustion (CLC) for inherent CO2 separations- a review. Chemical Engineering Science. 2008; 63: 4433-4451.
- Olajire AA. CO2 capture and separation technologies for end-of-pipe applications - A review. Energy. 2010; 35: 2610-2628.
- Pikon K. Bogacka M. Contemporary problems of power engineering and environmental protection. Published by Department of Technologies and Installations for Waste Management, Silesian University of Technology Copyright © Department of Technologies and Installations for Waste Management, Silesian University of Technology 2020. Available online: waste.polsl.pl
- Adánez J, Abad A, García-Labiano F, Gayán P, De Diego LF. Progress in Chemical-Looping Combustion and Reforming Technologies.A review. Progress in Energy and Combustion Science. 2012; 38: 215-282.
- Khan MN, Shamim T. Techno-economic assessment of a plant based on a three reactor chemical looping reforming system. Int J Hydrogen Energy. 2016; 41(48): 22677-88.
Citation: Hassanpour M (2021) O2, N2, CO, CO2 Capture Technologies in the Post-Combustion Operation of the Waste Stream (A Review). Int J Waste Resour 11: 396.
Copyright: © 2021 Hassanpour M. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.