Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 14, Issue 2
Neuroprotective Effects of the Aqueous Extract of Leaves of Moringa oleifera (Moringaceae) in Scopolamine-Treated Rats
Sefirin Djiogue1*, Ivana Youmbi Kammogne1, Paul f. Seke Etet2, Antoine Kandeda Kavaye1, Franklin Zemo Gamo1,3,4, Florette Motoum Tedjo1, Rudig N. Djikem Tadah1 and Dieudonne Njamen12Department of Physiological Sciences and Biochemistry, University of Ngaoundere, Garoua, Cameroon
3Department of Psychology, University of Yaounde I, Yaounde, Cameroon
4Department of Health, Private Bilingual Higher Institute les Armandins, University of Ngaoundere, Yaounde, Cameroon
Received: 04-Jan-2022, Manuscript No. BLM-22-15419; Editor assigned: 06-Jan-2022, Pre QC No. BLM-22-15419(PQ); Reviewed: 20-Jan-2022, QC No. BLM-22-15419; Revised: 25-Jan-2022, Manuscript No. BLM-22-15419 (R); Published: 28-Jan-2022, DOI: 10.35248/ 0974-8369.22.14.474
Abstract
Moringa oleifera Lam (Moringaceae) is a medicinal plant used in African traditional medicine against cognitive affections and metabolic diseases. In the present study, the memory-protective and neuroprotective effects of M. oleifera were assessed in scopolamine-treated rats. Wistar rats (n=48) were divided in 6 groups: A healthy control group and five groups treated daily with scopolamine (0.6 mg/kg, i.p.) for 14 days. Concomitantly, they received per os either distilled water (negative control), piracetam at 300 mg/kg (positive control), or aqueous extract of leaves of M. oleifera at doses 100, 200, or 400 mg/kg. Changes in animal cognition were assessed in the Morris Water Maze, in the last five days of treatment. Afterwards, the animals were sacrificed and brains dissected out and processed for biochemical and histopathological studies. Compared to the negative control group, the extract induced significant: (i) decrease in escape latency time (p ˂ 0.001); (ii) increase in the entries (p˂0.001), and time spent (p˂0.001) in the target dial; (iii) increase in GSH (p˂0.001), CAT (p˂0.001), and SOD levels (p˂0.001). The extract also prevented scopolamine-induced hippocampal neuron loss. These results suggest that M. oleifera aqueous extract has potent antioxidant, neuroprotective, and memory-protective effects in scopolamine-treated rats, justifying the traditional medicine use.
Keywords
Moringa oleifera; Scopolamine-treated rat; Memory impairment; Neuronal loss; Alzheimer’s disease; Morris water maze
Introduction
According to World Health Organization, there are nearly 10 million new cases of dementia per year, of which 6 million in low-income countries [1]. Alzheimer’s disease (AD) is the most common cause of dementia (60%-70% of cases). The disease mainly occurs at an advanced age (around 65 years), and at this rate it is estimated that by 2030 about 74.7 million people worldwide will be affected [2]. It is therefore urgent to slow AD progression. AD pathophysiological processes encompass damage to brain tissue resulting from a progressive and irreversible loss of sensitive neuronal populations, notably hippocampal CA1 and CA3 cells [3,4]. This chronic neurodegenerative disease is characterized by memory impairment and other less marked cognitive affections. Disease hallmarks also include hallucinations, loss of appetite, vomiting and diarrhea, and nausea [5,6]. Impairments of learning and memory can be induced chemically in laboratory rodents by administration of scopolamine, a muscarinic cholinergic antagonist and possibly inhibitor of the expression of genes of muscarinic receptor signaling molecules, which leads to memory impairment in humans and learning disorders in animals [7,8]. This substance causes oxidative stress through interference with acetylcholine in brain leading to cognitive impairment [9,10]. Scopolamine-treated rats are often used as an experimental model to test AD drug candidates [8-11].
Alarmingly, the Transparency Commission in charge of the evaluation of medical products of the High Authority for Health on the re-evaluation of Alzheimer’s products in 2011, reported that acetylcholinesterase and glutamate inhibitors’ classes of anti-AD drugs have a poor therapeutic interest and are associated with various side effects, including digestive, cardiovascular and neuropsychiatric disorders [12]. Moreover, memantine, the first line drug used against AD, causes headache, insomnia, dizziness, and mental confusion [5,6]. Considering also the high costs of anti-AD drugs and the scarcity of follow-up structures in developing countries, alternative treatment strategies are highly needed in the field.
Medicinal plants are a good source of novel therapeutics. Various studies in experimental models reported the neuroprotective properties of African plant-derived natural products such as naringin, extracted from berries and grapes [13], and black seed oil [14]. Commonly used as a nutritional supplement and against cognitive affections and metabolic diseases in African traditional medicine, the leaves of Moringa oleifera Lam (Moringaceae) were reported strong antioxidant properties and induced neuroprotective-like effects in rats intracerebroventricularly administered with ethylcholine aziridinium ion (AF64A) [15,16]. The leaves of M. oleifera contain secondary metabolites such as phenolic compounds, flavonoids, alkaloids, tannins, and saponins [17,18].
In the present study, the effects of aqueous extract of M. oleifera leaves on memory impairment were assessed in scopolamine-induced dementia, a commonly used rat model of AD. Neuroprotective effects were also assessed on sensible neuronal populations of the hippocampus.
Materials and Methods
Animals
Forty-eight albino male Wistar rats (3-month-old, 166 ± 18 g) were obtained from the Animal Physiology Laboratory of the Faculty of Sciences of the University of Yaoundé I. Animals were housed in a room under a controlled environment (temperature 25°C; humidity 50%-80%; 12 h light-dark cycle). They had ad libitum access to food and water. All experimental procedures were approved by the institutional ethical committee of the Faculty of Sciences, University of Yaoundé I. Animals were handled in accordance with the guidelines of the Cameroon’s Ministry of Scientific Research and Technological Innovation, which has adopted the guidelines of the European Union on Animal Care (CEE Council 86/609; Reg.no.FWA-IRD0001954).
Experimental design
Wistar rats were divided in 6 groups (n=8 per group): A normal control group receiving distilled water, and seven groups treated daily with scopolamine (0.6 mg/kg, i.p, Jiangsu Huayang pharmaceutical Ltd, Zhiongxing, China) for 14 days. Concomitantly, these animals were receiving (orally): distilled water (negative control), piracetam (UCB SA, Braine-l’Alleud, Belgium) at 300 mg/kg (positive control), and for the three test groups, M. oleifera leaves’ aqueous extract at doses 100, 200, or 400 mg/kg. All treatments were administered in a volume of 10 ml/kg. In the five last days of treatment, animals’ memory (spatial and temporal) was assessed in the Morris Water Maze. At the 14th day of treatment, the animals were deeply anesthetized with valium/ ketamine (10 mg/kg and 50 mg/kg, i.p.), and sacrificed. Brains were dissected out. Right hemispheres were processed for histopathological studies, while homogenates of the left hemispheres were processed for biochemical tests assessing oxidative stress indicators.
Plant material and extraction
Fresh leaves of M. oleifera were collected in Yaounde 5th District (Mfoundi Department, Center Region, Cameroun). A sample was identified at the National Herbarium of Cameroon (HNC), and a specimen was deposited (voucher number 49178/HNC). The extraction process was as follows: M. oleifera leaves were washed, shade- dried at room temperature, and grinded. Then, the powder (1100 g) was macerated for 6 hours in 5.5 L of tap water. Afterwards, the solution was filtered with Whatman No.4 filter paper. The filtrate was lyophilized and 31.02 g of dried extract was obtained (extraction yield: 2.82%).
Morris water maze
The Morris Water Maze test is based on rats’ excellent swimming ability and natural tendency to escape from water; here, memory is developed in animals progressively with repetitive trials that resemble human interactions [19]. Standard Morris pool and procedures were used [20-22]. More specifically, the Morris pool was a large circular tank (120 cm diameter × 50 cm height) with a black round platform (8 cm diameter × 29 cm height) placed about 1 cm under the surface of the opaque water, which was the only place where the animals tested could get out of the water and stop swimming. The pool was subdivided into four points labelled North (N), East (E), South (S), and West (W) poles. The platform position was maintained and could be detected by a visual cue.
The Morris Water Maze test included a training phase (4 days) and a test phase (1 day). During the training phase, the animal tested was placed in the water close to the border of the pool. To get out of water, the animal had to find the platform and swim to get on it. During each training session, the rat tested was placed at randomly selected locations in the pool, but always close to the border of the pool and facing the tank wall. The animal only had 60 sec to find the platform. Once the platform was found, the rat was allowed to stay there for 30 sec, before being wrung out with a dry cloth and brought back to its cage to rest for 15 min before the next stage. Animals that could not find the platform within 60 sec were slowly guided to the platform by the experimenter. They were also allowed to stay on the platform for 30 sec, and then they received the same treatment as the ones that found the platform by themselves. For each animal, three training sessions were conducted per day for 4 consecutive days.
On the 5th day, for the test phase, the same pool was used but without the platform. To start the test, each animal was placed in the water diagonally from the target quadrant and was allowed to swim freely for 60 sec. The performance of each animal in the pool was video recorded and analyzed offline. The parameters assessed were the time taken by the animal to find the original platform position (escape latency time), the number of entries, and the time spent in the target dial.
Biochemical tests
Left hemispheres were processed to prepare a 10% (w/v) homogenate in 0.1 M phosphate buffer saline (pH 7.5). Homogenates were centrifuged (3000 rpm, 15 min, 5°C). Supernatant concentrations of indicators of oxidative stress, malondialdehyde, glutathione, catalase, and superoxide dismutase (SOD) were determined using spectrometry, as described by [23,24]. Absorbances were read at 480 nm and 412 nm, respectively, using a UV spectrophotometer. Results were expressed as mM per gram of organ.
Histopathological study
Right hemispheres were fixed in 10% formaldehyde, embedded in paraffin, cut (5-μm sections in the coronal plane), and processed for hematoxylin-eosin staining using standard procedures. Affections of brain tissue were assessed in the hippocampal formation using a computerized microscope linked to a camera (Axioskop 40, Zeiss, Hallbermoos, Germany). In addition, CA1 and CA3 cells were counted throughout the hippocampal formation using image J software (version 1.52a).
Statistical analysis
Differences between the normal control group, the negative control group, the positive control group, and the test groups were determined using one-way ANOVA followed by Dennett’s post hoc test (Graph Pad Prism, version 5.03), p values <0.05 were considered statistically significant. Data were expressed as the mean ± SEM.
Results
Assessment of scopolamine-induced memory impairment
Figure 1 shows the effects of Moringa oleifera aqueous extract on the Morris Water Maze parameters. Scopolamine induced a significant increase (p<0.01) in escape latency time in the negative control group, compared to the normal control group in the Morris Water Maze (Figure 1A). At the doses tested, M. oleifera extract mitigated this increase markedly (p<0.001 compared to the negative control), with better effects at 100 mg/kg (Figure 1A). These effects were comparable to piracetam effects (Figure 1A).
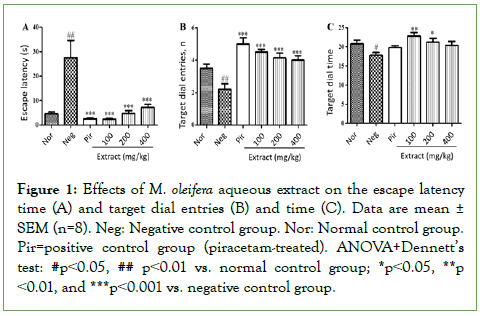
Figure 1: Effects of M. oleifera aqueous extract on the escape latency time (A) and target dial entries (B) and time (C). Data are mean ± SEM (n=8). Neg: Negative control group. Nor: Normal control group. Pir=positive control group (piracetam-treated). ANOVA+Dennett’s test: #p˂0.05, ## p˂0.01 vs. normal control group; *p˂0.05, **p ˂0.01, and ***p˂0.001 vs. negative control group.
Scopolamine also induced significant decreases in the target dial entries (p<0.01 compared to normal control) (Figure 1B) and time (p<0.05 compared to normal control) (Figure 1C). Similar to piracetam, M. oleifera extract significantly prevented the negative effects of scopolamine on the target dial entries at the doses of 100 (p<0.05), 200 (p<0.01) and 400 (p<0.001) mg/kg (Figure 1B). Better than piracetam, the doses 100 and 200 mg/kg prevented significantly the negative effects of scopolamine on target dial time (p<0.01 and p<0.05, respectively) (Figure 1C).
Biochemical indicators of oxidative stress
Figure 2 shows the effects of M. oleifera aqueous extract on malondialdehyde (Figure 2A), glutathione (Figure 2B), catalase (Figure 2C) and SOD (Figure 2D) levels in brain homogenates. Scopolamine induced a significant increase (p<0.01) in malondialdehyde in the negative control group, compared to the normal control group, which was mitigated by concomitant treatment with M. oleifera (Figure 2A).
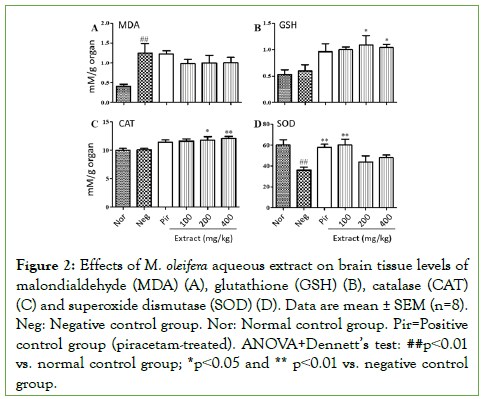
Figure 2: Effects of M. oleifera aqueous extract on brain tissue levels of malondialdehyde (MDA) (A), glutathione (GSH) (B), catalase (CAT) (C) and superoxide dismutase (SOD) (D). Data are mean ± SEM (n=8). Neg: Negative control group. Nor: Normal control group. Pir=Positive control group (piracetam-treated). ANOVA+Dennett’s test: ##p˂0.01 vs. normal control group; *p˂0.05 and ** p˂0.01 vs. negative control group.
Scopolamine did not induce marked changes in the levels of glutathione (Figure 2B) and catalase (Figure 2C) compared to the normal control group. Instead, interestingly, treatment with M. oleifera extract at doses 200 and 400 mg/kg induced significant increases in the levels of glutathione (p˂0.05) and catalase (p˂0.01) compared to both negative and normal control animals (Figures 2B and 2C). Piracetam and extract dose 100 mg/kg induced slight increases in glutathione (p˂0.05) and catalase (p˂0.01) also compared to both negative and normal control animals (Figures 2B and 2C). In addition, scopolamine induced a significant decrease in brain SOD levels in the negative control group compared to the normal control group (Figure 2D). Piracetam and M. oleifera extract dose 100 mg/kg prevented this decrease significantly (p˂0.01) and maintained SOD level at normal control values (no significant change compared to the normal control group) (Figure 2D).
Histopathological studies and hippocampal cell counts
Figure 3 presents micrographs of representative cases of animals from the normal control group (Figure 3A), negative control group (Figure 3B), positive control group (Figure 3C), and animals treated with M. oleifera extract doses 100 mg/kg (Figure 3D), 200 mg/kg (Figure 3E), and 400 mg/kg (Figure 3F). Histopathological analysis of hippocampal formation revealed that scopolamine induced a marked loss of neurons in CA1 and CA3 in the negative control group (Figure 3B), compared to the normal control group (Figure 3A). Instead, the hippocampi of animals treated with either piracetam (Figure 3C) or M. oleifera extract at all doses tested (Figure 3D-3F) were comparable to normal control group animals (Figure 3A).
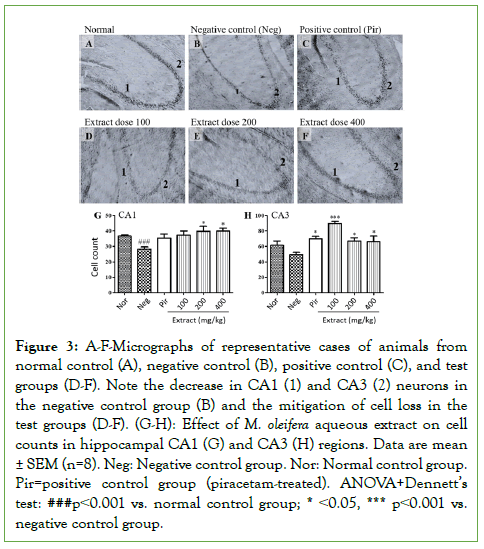
Figure 3: A-F-Micrographs of representative cases of animals from normal control (A), negative control (B), positive control (C), and test groups (D-F). Note the decrease in CA1 (1) and CA3 (2) neurons in the negative control group (B) and the mitigation of cell loss in the test groups (D-F). (G-H): Effect of M. oleifera aqueous extract on cell counts in hippocampal CA1 (G) and CA3 (H) regions. Data are mean ± SEM (n=8). Neg: Negative control group. Nor: Normal control group. Pir=positive control group (piracetam-treated). ANOVA+Dennett’s test: ###p˂0.001 vs. normal control group; * ˂0.05, *** p˂0.001 vs. negative control group.
Cell counts in hippocampal CA1 (Figure 3G) and CA3 (Figure 3H) confirmed marked decreases in the negative control group compared to the normal control group (p<0.001). Treatment with piracetam and M. oleifera extracts at all doses tested maintained cell counts to control group values, with significant differences compared to the negative control group (p˂0.05 and p<0.001 for CA1 and CA3, respectively, for extract dose 100 mg/kg and p<0.05 for other treatments with significant changes) (Figure 3G and 3H).
Discussion
Findings of this study suggest that as piracetam, treatment with M. oleifera aqueous leaves extract prevented or mitigated scopolamine- induced: (i) increase in escape latency time and decreases in both target dial entry and time in the Morris Water Maze; (ii) increase in malondialdehyde level and decrease in SOD level in the brain tissue; and (iii) neuron loss in the hippocampus.
Scopolamine induces cognitive affection, especially spatial learning and acquisition [25] partly by reducing brain cholinergic signaling output pivotal for memory processes [8,26,27], justifying its common use to induce AD-like memory impairment in laboratory rodents. In this study, scopolamine-induced memory impairment was revealed by a significant increase in escape latency time and marked decreases in target dial entry and time. Treatment with piracetam or M. oleifera extract prevented or mitigated the development of these alterations in scopolamine-treated rats, suggesting that the extract is endowed with memory-protective properties, possibly by countering the detrimental effects of scopolamine on cholinergic neurotransmission.
Oxidative damage to cellular components results in alterations of cell membrane properties, hence affection of neuronal signaling. It is well established that scopolamine mediates marked oxidative stress in brain tissue by increasing brain levels of lipid peroxidation and free radical generation agent malondialdehyde, and by decreasing the activities of antioxidant enzymes like SOD and glutathione in the brain [10,28,29]. In the present study, these alterations were observed in brains of scopolamine-treated animals receiving distilled water (negative control) but not in those treated with M. oleifera, suggesting that the extract prevented scopolamine-induced oxidative stress. These results confirm the findings of a previous report suggesting that M. oleifera is endowed with antioxidant properties [15]. These effects could be mediated by its second metabolites, which include many classes of compounds with antioxidant potential such as phenolic compounds, flavonoids, alkaloids, tannins, and saponins [17,18,30]. The antioxidant properties of M. oleifera partly explain its ability to prevent scopolamine-induced memory impairment.
Moreover, scopolamine-mediated oxidative damage results in the death of sensible neurons [29,31]. In this study, M. oleifera extract maintained the number of CA1 and CA3 cells at normal values in scopolamine-treated animals, suggesting neuroprotective properties, in accordance with the work by [32]. This finding is in agreement with the results of an early study in a model of age-related dementia induced by bilateral intraventricular administration of the neurotoxin ethylcholine mustard azirinium (AF64A), where M. oleifera improved hippocampal cell density [16]. The findings further supports that the extract has antioxidant properties and indicates that scopolamine-mediated memory impairment was prevented at least partly by mitigating the development of detrimental oxidative stress and related tissue damage in the hippocampus.
Conclusion
In this study, rat treatment with scopolamine-induced memory impairment, oxidative stress, and neuron loss in the hippocampus. Concomitant treatment with M. oleifera aqueous extract prevented or mitigated these alterations and prevented neuron loss. Altogether, the results of this study suggest that M. oleifera extract prevented scopolamine-induced memory impairment, at least partly, through the extract’s potent antioxidant activity and neuroprotective effects. These results justify the extract’s use in traditional medicine.
Conflicts of Interest
Authors declare no competing financial interests.
Acknowledgments
The authors are thankful to the members of the Laboratory of Animal Physiology, Department of Animal Biology and Physiology, Faculty of Science, University of Yaounde I, for technical assistance.
Funding
This work was not supported by any specific grant from any funding agency.
REFERENCES
- OMS, Organisation Mondiale de la Santé. The number of people with dementia is expected to triple over the next 30 years. Press release.2017.
- Prince M, Wimo A, Guerchet M, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015: The Global Impact Of Dementia. Int J Alzheimers. 2015.
- Badrul A, Ekramul H. Anti-Alzheimer and antioxidant activity of Celastrus paniculatus seed. Iranian J. Pharm. Sci. 2011;7:49-56. [Cross Ref] [Google Scholar]
[Pub Med]
- Fan L, Mao C, Hu X, Zhang S, Yang Z, Hu Z et al. New insights into the pathogenesis of Alzheimer’s Disease. Front. Neurol. 2020;10:1312.
- Chandra V, Pandav R, Dodge HH. Incidence of Alzheimer’s Disease in a Rural Community in India: The Indo-US Study. Neurol. 2001;57:985-989.
[Cross Ref] [Google Scholar] [Pub Med]
- MacShane R. Drug treatment for Alzheimer’s disease. Factsheet 407. 2012;12:1-5.
- Hsieh MT, Hsieh CL, Lin LW, Wu CR, Huang G. Differential gene expression of scopolamine-treated rat oh hippocampus-application of cDNA microarray technology. Life Sci. 2003;73:1007-1016.
[Cross Ref] [Google Scholar] [Pub Med]
- Cheon SY, Koo BN, Kim SY, Kam EH, Nam J, Kim EJ. Scopolamine promotes neuroinflammation and delirium-like neuropsychiatric disorder in mice. Nat Sci Rep. 2021;11:8376.
[Cross Ref] [Google Scholar] [Pub Med]
- Hashimoto M, Hashimoto T, Kuriyama K. Protective effect of WEB 1881 FU on AF64A (ethylcholine aziridinium ion)-induced impairment of hippocampal cholinergic neurons and learning acquisition. Eur J Pharmacol 1991;209: 9-14.
[Cross Ref] [Google Scholar] [Pub Med]
- Goverdhan P, Sravanthi A, Mamatha T. Neuroprotective effects of meloxicam and selegeline in scopolamine induced cognitive impairment and oxidative stress. Int J Alzheim Dis. 2012.
[Cross Ref] [Google Scholar] [Pub Med]
- Kwon SH, Kim HC, Lee SY, Jang CG. Loganing improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol. 2009;619:44-49.
[Cross Ref] [Google Scholar] [Pub Med]
- HAS, Haute Autorité de Santé. Reassessment of drugs indicated in the treatment of Alzheimer's disease. Eval Rep. 2011;1-72.
- Hala F, Zaki, May A, Abd-El-Fattah, Amina S, Attia. Naringin protects against scopolamine induced-dementia in rats. Bulletin of faculty of pharmacy, Cairo university. 2014; 52:15-25.
- Imam A, Ajao M, Ajibola M, Amin A, Abdulmajeed WI, Lawal AZ et al. Black seed oil ameliorate scopolamine induced memory dysfunction and cortico-hippocampal neural alternation in male wistar rats. Bull Fac Pharm. 2016; 54: 49-57.
- Vongsak B, Sithisarn P, Gritsanapan W. Bioactive contents and free radical scavering activity of Moringa oleifera leaf extract under different storage conditions. Ind Crops Prod. 2013; 49: 419-421.
- Chatchada S, Jintanaporn W, Supapoen M, Wipawee T. Moringa oleifera Mitigates Memory impairment and Neurodegeneration in Animal model of age-related dementia. Oxid Med Cell Longev. 2013.
[Cross Ref] [Google Scholar] [Pub Med]
- Ugwu O, Nwodo, Joshua P, Abubakar B, Ossai. Phytochemical and acute toxicity studies of Moringa ethanol leaf extract. Int J Life Sci Biotechnol Pharma Res. 2013;2:66-71.
- Layele O, Ahissou H, Olounlade A, Azando E, Layele. A Bibliographic study of antidiabetic plants: Khaya Senegalais (Der) A. Juss (Meliaceae), Momordica charantia Lim (Cucurbitacae) and Moringa oleifera Lam (Moringacae). Int J Biol Chem 2015; 9:2682-2700.
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1982;11:47-60.
[Cross Ref] [Google Scholar] [Pub Med]
- Morris R, Garrud P. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681-683.
[Cross Ref] [Google Scholar] [Pub Med]
- Saxena G, Singh SP, Agrawal R, Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur J Pharmacol. 2008;581:283-289.
[Cross Ref] [Google Scholar] [Pub Med]
- Lee B, Sur B, Shim I, Lee H, Hahm DH. Phellodendron amurense and its major alkaloid compound, Berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J Physiol Pharmacol 2012;16:79-89.
[Cross Ref] [Google Scholar] [Pub Med]
- Misra H, Fridovish I. Determination of the level of superoxide dismutase in whole blood. Yale University Press New Haven. 1972;101-109. [Cross Ref] [Google Scholar] [Pub Med]
- Ellman G. Tissue sulfhydryl group. Arch Biochem Biophys. 1959;82:70-77.
[Cross Ref] [Google Scholar] [Pub Med]
- Saraf MK, Prabhakar S, Khanduja KL, Anand A. Bacopa monniera attenuates scopolamine induced impairment of spatial memory in mice. Evid. Based Complementary Altern. Med. 2011
- Attrey DP, Singh AK, Naved T, Roy B. Effect of seabuckthorn extract on scopolamine induced cognitive impairment. Ind J Exper Biol. 2012;50:690-695.
- Hirokawa S, Nose M, Ishge A, Amagaya S, Oyama T, Ogihara Y. Effect of Hachimi-jio-gan on scopolamine-induced memory impairment and on acetylcholine content in rat brain. J ethnopharmacol. 1996;50:77-84.
[Cross Ref] [Google Scholar] [Pub Med]
- Abhinav K, Jogender M, Madhusudana K, Vegi GMN, Yogendra KG, Ramakrishna S. Anti-Amnesic Activity of Vitex negundo in Scopolamine Induced Amnesia in Rats. Pharmacol Pharm. 2010;1:1-8.
- Zhang Z-J. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci. 2004;75:1659-1699.
[Cross Ref] [Google Scholar] [Pub Med]
- Rajamurugan R, Deepa V, Sivashanmugam M, Raghavan CM. Phytochemistry, antioxidant and antibacterial activities of medicinal plants: A comparative Study. Int J Contemp Res Rev. 2013;5:8-19.
- Roy C. Role of Moringa oleifera on hippocampal cell morphology and senile plaque formation in colchicine induced experimental rat model of Alzheimer’s disease. Int J Curr Pharm Res. 2014;6:51-54.
- Zhou J, Yang W, Suo D, Li Y, Peng L, Xu L et al. Moringa oleifera seed extract alleviates scopolamine-induced learning and memory impairment in mice. Front. Pharmacol. 2018;9:389.
[Cross Ref] [Google Scholar] [Pub Med]
Citation: Djiogue S, Kammogne IY, Etet PFS, Kavaye AK, Gamo FZ, Tedjo FM, et al. (2022) Neuroprotective Effects of the Aqueous Extract of Leaves of Moringa oleifera (Moringaceae) in Scopolamine-Treated Rats. Bio Med. 14:474.
Copyright: © 2022 Djiogue S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


