Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Hypothesis - (2020) Volume 11, Issue 3
Nano-theragnostic Approach to Mitigate Covid-19
Anita Kamra Verma*Received: 06-May-2020 Published: 17-Jun-2020, DOI: 10.35248/2157-7439.20.11.545
Abstract
Global Coronavirus pandemic has to be strategically addressed. The initial preventive phase has been largely discussed. The gamut of disease is comprehensive as patients hospitalised with COVID-19 manifest respiratory failure, sepsis, pneumonia, and acute respiratory distress syndrome (ARDS). Nano-theragnostics might have the answer to the mightiest challenge of this century in the next phase of diagnostics and therapeutics. Coronavirus causes severe morbidity in the lower respiratory tract, where alveolar epithelial cells are the key targets for dysregulating their function and triggering immune responses. The intensity and continued production of cytokine rush leads to enhanced vascular hyperpermeability that often causes multiorgan failure, and finally death. Nanoparticles (NPs) are being envisaged to target the airway epithelial cells to repair the dysfunctions with therapy, drug repurposing to counter the imbalance caused by the cytokine storm. Because inflammation, according to the literature, induces thrombosis through a complex but well- known pathophysiological mechanism. The way to fight Covid-19 has to experiment not only antibiotics, antivirals, but also anti-inflammatories and anticoagulants.
Keywords
Nano-theragnostics; Covid-19; Alveolar epithelium; Cytokine storm; Hyperpermeability
Introduction
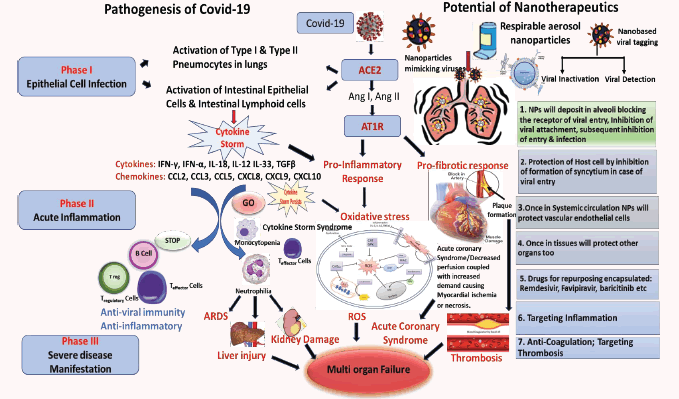
SARS-CoV2 (Severe acute respiratory syndrome coronavirus) binds to ACE2 that are overexpressed in lung epithelial cells and/ or intestine. Binding to ACE2 results in activation of the receptor of the classical renin-angiotensin-aldosterone system. The AT1- R-ACE/angiotensin II/angiotensin type 1/2 receptor system are involved in pro-inflammatory immune responses resulting in tissue injury. The overwhelming cell activation results in production of cytokines & chemokines causing a Cytokine Storm. If the storm persists it causes Neutrophilia, Monocytopenia, Lymphopenia and oxidative stress in the cells. This may lead to intense complications including ARDS-acute respiratory distress syndrome, Liver Injury, viral sepsis shock, kidney damage, Acute Coronary Syndrome, activation of blood coagulation cascade causing Myocardial ischemia or even necrosis, eventually causing multiorgan failure. [Ang I and Ang II: Angiotensin I and Angiotensin II]. Nanoparticles (NPs) are being envisaged to target the airway epithelial cells to repair the dysfunctions with therapy, drug repurposing to counter the imbalance caused by the cytokine storm. NPs can be engineered to mimic viruses and can be made to either deactivate the virus or the receptor inhibiting the entry of the nanoparticles. Once the NPs are internalized and the viral does gain entry, it will inhibit the virus to form syncytium inside the cell, thereby protecting the host cell. They can carry a payload of drugs targeting inflammation, antibiotics, anticoagulants and antivirals Nano- theragnostic approach is the answer to the biggest challenge of this century.
Hypothesis
Urgent global need is the development of rapidly positioned, cost-effective, reliable point-of-care diagnostics, antivirals, drug repurposing, vaccines, and additional stratagems to reduce the intense pathologies that go with the infections. Nanomedicine can progress into advance technologies and efficacious therapeutics with a two-pronged approach-diagnostic as well as therapeutics. Precision nano-therapy offers a set of know-hows that will help in diagnosis, therapy through targeted delivery of drugs, antivirals, treatment of co-morbidities, and in exploiting novel pathways that mitigate the pathologies and transmission of infections.
Knowledge of nano-structures and physiological mechanisms can aid in developing therapeutic strategies based upon principles of physiology for design of nanomedicines, along with the principles that reinforce the pathophysiology of SARSCoV-2. The juncture of these two expanses may offer a valuable stage to control the menace of the infective SARS-CoV-2.
Cell Penetrating Peptides have short peptide domains that are also exploited by the viruses to gain entry into the cells, such as ACE- 2 used by coronavirus [1] a herpes virus protein, VP22, [2], that inhibits AIM2 inflammasome, that is initiated DNA, activating caspase-1 and triggering release of pro-inflammatory cytokines interleukin 1β (IL-1β) and IL-18, that is a crucial mediator of host innate immune responses against several pathogens. Nanoparticles mimic the viruses and can be engineered to optimal sizes akin to the size range of virus, to overcome the size limitations of cellular uptake mainly by pinocytosis, mechanisms via caveolae and clathrin-coated pits that are in the size range of 80 to 200 nm, [3] along with the fast elimination of NP by the kidneys, [< 3 nm] are suggestive of the importance of size range to optimize nanotherapeutics, ideally the range should be between 3 nm and 200 nm. Again, the route of administration has a substantial impact on the dosage and time to reach requisite dosing, in maximum tissues the blood vessels have an endothelial layer that is pore- size restrictive, and prevents the leakage of the nanocarriers above a particular size range. For anti-SARS-CoV-2 nanotherapeutics given via inhalation, the vascular epithelial barrier becomes less relevant. Nano-therapeutics administered by this route appears to be effective only in size range between 6 and 600 nm. Viruses generally enter the cells through receptor- mediated endocytosis. The receptor that Covid-19 uses to infect the lung cells is ACE2, a cell surface protein that is expressed on blood vessel cells, in the kidney, in heart, and also in lung AT2 alveolar epithelial cells. These AT2 cells are predominantly disposed to viral infections [4]. A known endocytosis regulator is the AP2-associated protein kinase 1 (AAK1) can be a useful target as disruption of AAK1 can not only further block the cellular internalization of the viruses, but can also disturb formation of the intracellular assembly of virus particles [5]. Six AAK1 high affinity inhibitors include oncology drugs like erlotinib and sunitinib, both have shown inhibition of viral infection. Another regulator of endocytosis is baricitinib, a janus kinase inhibitor, that binds the cyclin G-associated kinase [6]. The plasma concentration of baricitinib (2 mg/4 mg per day) is sufficient as therapeutic dosing to inhibit AAK1, this can be proposed as a promising molecule for trials. This will give a proof of concept for reducing the viral penetration and inflammation.
Coronavirus causes severe morbidity in the lower respiratory tract, where alveolar epithelial cells are an important target for dysregulating their function and triggering immune responses. The aggressive production of early response pro-inflammatory cytokines include IL1-β, IL-6 and tumour necrosis factor [TNF], causing the so-called cytokine storm. The unrestrained release of chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) and other pro-inflammatory cytokines (IFN-γ, IFN-α, IL-18, IL-12 IL-33, TGFβ, etc.) by immune effector cells in COVID-19 infection [7,8]. The level of CXCL10 may be predictive of the subsequent clinical course [9,10]. CXCL10 is an excellent interferon responsive gene that has presence in the response of alveolar type II cells to SARS-CoV and influenza [11,12], and has further been reported to be valuable disease biomarker in SARS [7-12]. Therefore, neo- therapeutic strategies have to be undertaken to investigate immunomodulators or anti-cytokine therapies targeting the overproduction of cytokines. Understanding the hosts’ innate immune response may expand our gamut of predictions on how aggressively to monitor the subsequent course of the disease. The intensity and continued production of cytokine rush leads to enhanced vascular hyperpermeability that often causes multiorgan failure, and finally death [13]. During the heightened immune response to balance the infection, the blood coagulation cascade gets initiated. Even though primarily thrombin promotes formation of clot by stimulating platelets, thereby converting the inactive fibrinogen to active fibrin [14], thrombin can exert several cellular effects causing more inflammation through PARs-the proteinase activated receptors, mainly PAR-1 [15] Release of thrombin is firmly regulated by negative feedback loops and other physiological anticoagulants, such as inhibitor of tissue factor pathway, antithrombin III and protein C system [16]. All these regulatory mechanisms can be dysfunctional during inflammation, i.e decreased concentrations of anticoagulants due to over consumption and reduced production. This defective procoagulant–anticoagulant balance inclines to the progress of micro-thrombosis, dispersed intra-vascular coagulation, and eventually multi-organ failure, as evidenced in severe COVID-19. Because inflammation, according to the literature, induces thrombosis through a complex but wellknown pathophysiological mechanism. The way to fight Covid-19 has to experiment not only antibiotics, antivirals, but also antiinflammatories and anticoagulants.
Conclusion
There are significant information gaps regarding pathogenesis of COVID-19, that are expected to be filled in near future. In the background of design of nano-therapeutics along with the very basic premise of understanding of pathogenesis of SARS-CoV-2, it is anticipated that this viewpoint is useful.
REFERENCES
- Dhama, K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Human Vaccines & Immunotherapeutics 2020 1-7.
- Maruzuru Y, Ichinohe T, Sato R, Miyake K, Okano T, Suzuki T, et al. Herpes Simplex Virus 1 VP22 Inhibits AIM2- Dependent Inflammasome Activation to Enable Efficient Viral Replication; Cell Host Microbe 2018 23:254-265.
- Papasani MR, Wang G, Hill RA. Gold nanoparticles: the importance of physiological principles to devise strategies for targeted drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2012;8(6):804-14.
- Zhao Y, Zhao Z, Wang Y, et al. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov.
- Lu R, Zhao X, Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020.
- Pu SY, Xiao F, Schor S. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antiviral Res 2018, 155:67-75.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 2019 395(10223):497-506.
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. In Seminars in immunopathology Springer Berlin Heidelberg 2017;39:529-539.
- Reyfman PA, Walter JM, Joshi N. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 2019;199:1517-1536.
- Tang NL, Chan PK, Wong CK. Early enhanced expression of interferon-inducible protein- 10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem 2005;51:2333-2340.
- Hancock AS, Stairiker CJ, Boesteanu AC. Transcriptome analysis of infected and bystander type 2 alveolar epithelial cells during influenza A virus infection reveals in vivo Wnt pathway downregulation. J Virol 2018;92:e01325-18.
- Qian Z, Travanty EA, Oko L. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol 2013;48:742- 748.
- Wang J, Nikrad MP, Phang T. Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am J Respir Cell Mol Biol 2011;45:582-591.
- Rockx B, Baas T, Zornetzer GA. Early upregulation of acute respiratory distress syndrome- associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol 2009;83:7062-7074.
- Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A, et al. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 1995;108:1303-14.
- José RJ, Williams AE, Chambers RC. Proteinase activated receptors in fibroproliferative lung disease. Thorax 2014;69:190-92.
Citation: Verma AK (2020) Nano-theragnostic Approach to Mitigate Covid-19. J Nanomed Nanotech. 10:544. doi: 10.35248/2157-7439.19.10.s545
Copyright: © 2020 Verma AK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


