Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 12
Mycological Quality of Noni (Morinda citrifolia) Foliar Infection and Susceptibility to a Novel agent
Amadi Lawrence O* and Seth Mercy OReceived: 27-Oct-2021 Published: 30-Dec-2021
Abstract
Foliar infection of Noni poses a great challenge to farmers, microbiologists and phytopathologists despite the broad spectrum of bioactive and therapeutic potentials of the plant. This study investigates the mycological quality of Noni foliar infection and susceptibility to a novel agent (Alum). The mycological quality was assessed by culture-dependent technique using standard mycological procedures and susceptibility of isolates determined by disc and agar well diffusion methods and inhibition zones (IZs) were measured in millimetre. Fungi identified include Aspergillus flavus, A. fumigatus, A. niger and Penicillium species. Relative abundance (%) of fungal species in foliar infection were A. fumigatus (75%) being the most abundant followed by A. niger and A. flavus (50%) respectively and Penicillium (25%) species. Susceptibility assay of these species indicated that Alum compared appreciably with Ketoconazole (control) on dose-dependent fashion by both techniques. Data revealed that A. flavus (36.0 mm), A. fumigatus (32.5 mm) and Penicillium (30.2 mm) were the most susceptible and the least was A. niger (30.0 mm) with the novel agent. This research concludes that Alum could be used as an alternative cost-effective and ecofriendly novel or natural treatment agent to foliar related diseases or infections.
Keywords
Noni; Mycoflora; Alum; Foliar infection; Ketoconazole
Introduction
Morinda citrifolia (Noni) is an important and most popularly cultivated plant of the family (Rubiaceae), genus Morinda, a native of Southeast Asia and now pan-tropically distributed [1,2]. Traditionally, Noni was used as food and for treatment of a variety of diseases [3-6]. Recently, several parts of the plant such as leaves, roots, fruits, stem bark are extensively studied due to wide spectrum bioactivity and therapeutic significance.
A variety of diseases have been associated with the shoot of Noni plant ranging from leaf blight, shot hole, black leaf spot, anthracnose, sooty mould, stem cancer and blight, fruit blight, cracking and rot, black flag to plant death as well as phytoplasma disease [7-10]. The plant leaves with phytoplasma (foliar) disease presents profound reduction in growth, stunted or dwarfed with puckers upwards and inwards as well as lamina shrinkages from petiole to the tip [9]. However, study on the mycoflora of Noni shoot is limited especially those associated with foliar diseases, prevention or management. Many genera of fungi have been implicated as aetiogic agents of Noni shoot diseases such as Phytophthora, Sclerotiumrol, Colletotrichum and Rhizopus [7,11].
Mycoflora from foliar infection was treated with novel or natural compounds such as potash alum (potassium aluminium sulphate) because of its nontoxicity, affordability and eco-friendliness. Alum finds application in a wide variety of anthropogenic activities such as drug, food processing and preservation, domestic and industrial water treatments, antimicrobial agent, cosmetics and pharmaceutical industries as well as for treatment of ‘Ich and white eye‘ diseases in fishes [12-16]. The study attempts to identify the mycoflora associated with foliar infection and their susceptibility to a novel agent, ‘Alum’.
Materials and Methods
Sample Source and Collection
Noni with foliar disease were plucked with sterile hand gloves into sterile Polyethylene bag from Dilomat farm and services, Rivers State University, Nkpolu-Oroworukwo, Port Harcourt.
Fresh Noni with foliar disease (infected foliage with wrinkles or shrinking upward and inward) (Figure 1) were collected/ harvested from Dilomat farms and conveyed to the Department of Microbiology, Rivers State University, Port Harcourt for analyses.

Figure 1: Foliar infection of noni (shrinking upward and inward) as observed at Dilomat farm.
Preparation of alum and discs
Potassium aluminium sulphate (Vickers Laboratories, Ltd, England) was prepared by reconstituting appropriate quantity in sterile distilled water (w/v). Different concentrations of Alum were formulated by reconstituting appropriate quantities such as 2.0, 3.0, 4.0 and 5.0 in 100 ml (w/v) to obtain 2- 5% respectively. Sterile discs (6 mm diameter) were made of Whatman filter paper using an office perforating device.
Preparation of samples for mycological analysis
A 25 gm portion of foliar infected sample was washed, cut in pieces (with sterile knife) and blended with a mechanical grinder in 225 ml of sterile normal saline to obtain the homogenate. Further decimal dilutions were carried out before inoculation of 0.1 ml aliquot onto surface-dried pre-sterilised Sabouraud’s dextrose agar (SDA; Titan Biotech Ltd, India). Followed by incubation at 25 ± 2°C for 2 or more days.
Macroscopic and microscopic fungal cultures
Discrete fungal colonies were isolated, characterised and identified according to their shape, colour, texture and contour, and microscopically under X10 and X40 magnification by infiltration with lactophenol cotton blue [17,18]. Cultures of each fungus was further grown for viability, confirmed and maintained on SDA and slants at 5°C for further assay.
Antibiogram/susceptibility testing procedure
Antimycotic susceptibility test was carried out by agar well diffusion (AWD) and disc diffusion (DD) methods [19]. After adjustment of fungal cultures of 2-5days on SDA to a 0.5 McFarland turbidity standard, they were asceptically plated with the various Alum concentrations and equidistantly spaced from one another and incubated. An antimycotic drug, Ketoconazole (2.0 mg/ml; Janseen, Pharmaceuticals, New Jersey, USA) was used as control both for AWD and DD methods respectively. Colony diameters were measured and the mean and standard
Statistical analysis
Means of duplicate measurements were determined for each sample using Microsoft Excel® 2016.
Results
Table 1 depicts the inhibitory effects of Alum to fungi isolated from foliar disease/infection of Noni. Inhibition zones or susceptibility of fungal isolates to Alum were observed with increasing concentrations of Alum treatment (dose dependent). The inhibitory pattern of different concentrations of Alum by AWD and DD techniques revealed more profound effects on the isolates by AWD than with DD. The data revealed that two (2) genera of fungi were isolated; Aspergillus and Penicillium. The most sensitive to Alum at various concentrations was A. flavus, followed by A. fumigatus and Penicillium sp with A. niger as the least. Results showed relatively larger IZs as Alum concentrations increased which almost equals those of control.
| Concentration of Alum (%) and Mean IZ (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fungi | AWDT | DDT | Control | |||||||
| 2.0% | 3.0% | 4.0% | 5.0% | 2.0% | 3.0% | 4.0% | 5.0% | 2.0mg/mL | ||
| Aspergillus flavus | 28.0 | 30.0 | 34.0 | 36.0 | 22.0 | 25.0 | 30.0 | 34.0 | 39.0 | 24.8 |
| A. fumigatus | 22.0 | 29.0 | 30.3 | 32.5 | 15.0 | 25.0 | 29.5 | 30.0 | 35.2 | 25.0 |
| A. niger | 15.0 | 19.4 | 21.0 | 30.0 | 10.0 | 18.2 | 20.4 | 22.0 | 25.3 | 20.2 |
| Penicillium sp. | 20.0 | 22.5 | 25.0 | 30.2 | 20.0 | 21.4 | 23.0 | 29.6 | 35.0 | 22.53 |
Table 1: Susceptibility of fungi isolated from foliar infected noni to alum by AWD and DD techniques.
The relative abundance (%) of fungal isolates associated with Noni foliar disease/infection are shown in Figure 2. The data shows that there are two (2) genera of fungi; Aspergillus and Penicillium. The dominant genus, Aspergillus, has A. fumigatus (75%) as most abundant species, followed by A. niger and A. flavus (50%) respectively, the least being Penicillium species (25%).
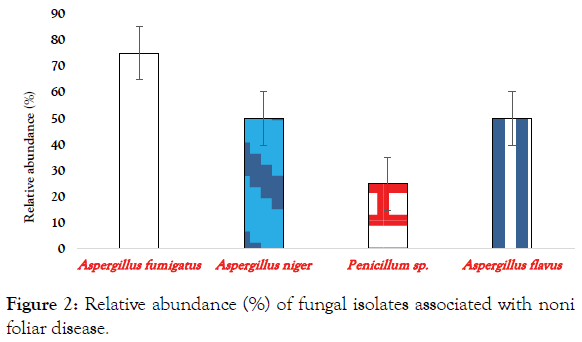
Figure 2: Relative abundance (%) of fungal isolates associated with noni foliar disease.
Discussion
Foliar infection or disease has over the years been à major challenge to farmers, mycobiologists and phytopathologists considering the huge economic potentials of Noni. The major genera of fungi identified were Aspergillus and Penicillium and may not be unconnected with foliar phyoplasma disease first reported by [9]. However, the report did not identify the microbes associated with the disease.
Aspergillus plays significant role phytopathogenetically as well as ecologically by production of enzymes, e.g., polygalacturonase, capable of degrading starches, hemicelluloses, celluloses, etc [20- 22]. However, It could be said that, these enzymes can negatively affect the tender structural architecture of Noni foliage to present the typical condition shown in Figure 1. The relative abundance (%) or diversity of foliar mycoflora may be attributed to soil, climate/weather and other environmental conditions which in turn impacts the microbial community structure where the plant is grown [7,20, 23-25].
The antimycotic potentials of Alum has been well documented in our previous studies (in vitro and in fields) against fungi such as Aspergillus niger, A. flavus, A. terreus, Penicillium crystallium, Saccharomyces cerevisiae, Candida albicans, Geotrichum candidum and Fusarium species [15,26]. Its activities against fungi in the present study was in consonance with previous reports. As an eco-friendly natural agent it could be exploited and adopted to ameliorate or combat phytoplasma disease as well as other mycotic infections of plants. Its close comparison with Ketoconazole underscores antifungal efficacy.
Conclusion
The study revealed that two genera (Aspergillus and Penicilluim) of fungi were identified to be associated with phytoplasma disease of Noni. Alum treatment against these fungi exhibited appreciable mycotic activity comparable to the control which if explored could serve as a novel agent for the prevention, control and/or management of fungal infections.
Acknowledgement
We appreciate the magnanimity of Chief MM. Chinda, CEO/ MD and his Technical team lead by Mr. Francis U. Abel all of Dilomat Farms and Services Ltd, Rivers State University, Nkpolu- Oroworukwo, Port Harcourt for supplying the Noni plant samples used for this study.
REFERENCES
- Nelson SC, Elevitch CR. Noni: The Complete guide for consumers and growers. Permanent Agriculture Resources. 2006:67-80.
- Almeida ES, Oliveira D, Hotza D. Properties and applications of Morinda citrifolia (Noni): A review. Compr Rev Food Sci Food Saf. 2019;18:883-909.
- Wang MY, West BJ, Jensen CJ, Nowicki D, Su C. Morinda citrifolia (noni): A literature review and recent advances in noni research. Acta Pharmacol Sin. 2002;23(12):1127-1141.
- Sibi G, Parul C, Sayak A, Ravikumar KR. Pyhtoconstituents and their influence on antimicroial properties of Morinda cirifolia L. Res J Med Plant. 2012;6(6):441-448.
- Assi RA, Darwis Y, Abdulbaqi IM, khan AA, Vuanghao L. Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab J Chem. 2017;10:691-707.
- Amadi LO, Nwala H. Preliminary investigation on microflora associated with fermentation of noni (Morinda citrifolia) fruit and its nutritional components. Int J Biotechnol Microbiol. 2021;3(2):1-4.
- Nelson SC, Abad ZG. Phytophthora morindaea new species causing black flag disease on noni (Morindacitrifolia) in Hawaii. Mycologia. 2010;102(1):122-134.
- Jackson G, McKenzie E. Guignardia morindae and noni shot-hole disease (313). Pacific pests, Pathogens and Weeds – Online (edn). Produced with Support from the Australian Centre International Agriculture Research. 2013.
- Sarwade PP, Sarwade KP, Chavan SS. New first report of foliar phytoplasma disease on Bartondi plant in India. J Plant Pathol Microb. 2015;6(3):260.
- Jumar, Yusriadi, Surtinah. Control of Anthracnose disease in chili with several doses of noni leaf extracts.IOSR J Agric Vet. Sci.2020;13(4):33-38.
- Kavitha PG, Umadevi M. Medicinal Properties and pests and diseases of noni – A review. Res J Pharmacogn Phytochem. 2016;8(1):41-48.
- Alzomor AK, Moharram AS, Al Absi NM. Formulation and evaluation of potash alum as deodorant lotion and after shaving astringent as cream and gel. Int Curr Pharm J. 2014;2:228-233.
- Efiuvwevwere BJO, Amadi LO. Effects of Preservatives on the bacteriological, chemical and sensory qualities of mangrove oyster (Crassostrea gasar) harvested from the Niger Delta Region, Nigeria. Br J App Sci Technol. 2015;5(1):76-84.
- Al-Talib H, Nasir MSIN, Yaziz H, Zulkafli FN, Adani AN. Potassium aluminium sulphate (Alum) inhibits growth of human axillary malodour-producing skin flora in vitro. J Clin Health Sci.2016;1(1):59-63.
- Amadi LO, Udoh EJ, Thompson RU, Benjamin RG, Nmom FW. Antimycotic potential of alum on postharvest deterioration of tomato. Microbiol Res J Int. 2019;29(3):1-10.
- Ade GV, Makode PM. ICH and White eye disease management in Fishes using novel components. Int. J Res Biosci Agri Technol.2021;17:551-558.
- Forbes BA, Sahm DE, Weissfeld AS. Bailey and Scott’s Diagnostic Microbiology. International edition. (12th edn). Mosby, Inc., an affiliate of Elsevier, Inc., USA. 2007.
- Cheesbrough M. District laboratory practices in tropical countries. 2006.
- Kirby WMM, Bauer AW, Sherris JC, Truck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1966;45(4):493 - 496.
- Lee S, Park MS, Lim YW. Diversity of marine-derived Aspergillus from tidal mudflats and sea sand in Korea. Mycobiol. 2016;44(4):237-247.
- Hubka V, Kolařik M, Kubatova A, Peterson SW. Taxonomic revision of Eurotium and transfer of species to Aspergillus. Mycologia. 2013;105(4):912-937.
- Maldonado MC, Cáceres S, Galli E, Navarro AR. Regulation of the production of polygalacturonase by Aspergillus niger. Folia Microbiol. 2002;47(4):409-412.
- Jefferey SB, Daniel PR, Estelle RC. Microbial community structure and function in the spermosphere as affected by soil and seed type. Can J Microbiol. 1999;45(2):138-144.
- Liu Y, Cheng C, Yao S, Xu P, Cao Y. Composition and diversity of endophytic bacterial communities in noni (Morinda citrifoliaL.) seeds. Int J Agric Pol Res. 2014;2(3):98-104.
- Brown S. Effect of climate on plant diseases. J Plant Pathol Microbiol. 2021;12(7)No.1000:305.
- Amadi LO. A review of antimicrobial properties of alum and sundry applications. Acta Scientific Microbiology. 2020;3(4):109-117.
Citation: Lawrence OA, Mercy OS (2021) Mycological Quality of Noni (Morinda citrifolia) Foliar Infection and Susceptibility to a Novel agent. J Plant Pathol Microbiol 12:591.
Copyright: © 2021 Lawrence OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

