Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 11, Issue 3
MMT, a High Promising, Cost Effective Micro-carrier for Gene Delivery
Sai Krishna Repalli*, Chaitanya kumar Geda and Rao GJNReceived: 11-Mar-2019 Published: 01-May-2019
Abstract
As an alternative to the conventional but expensive gold micro carriers regularly employed in micro-projectile bombardment method, this study had utilized, novel, promising but cheaper clay particles-MMT (montmorillonite) as micro-carriers for gene transfer. Gene expression with novel micro carriers was reported through the universally employed GUS expression system at both transient and stable expression levels in comparison to the gold and tungsten.
Results suggest that GUS expression levels are higher with MMT than tungsten carriers but lower to gold carriers. It is interesting to note that the GUS expression zones are larger (>1 mm) with MMT carriers in comparison to tungsten (0.6 mm) and gold (0.4 mm). Molecular assays on the transformed cells suggest proper gene delivery by MMT carriers. The results suggest that novel micro carriers can be a viable alternative to gold for gene transfer with high promise in minimizing the costs without compromising the transformation efficiency.
Keywords
Rice; Transformation; Biolistics; Micro-carriers; Montmorillonite
Introduction
The biolistic or micro-projectile bombardment method, introduced in the late 1980’s (Sanford, 1988), was as efficient method of plant transformation. This method is more versatile and allows transformation of plants and other organisms that are not amenable through other modes of gene transfer like Agrobacterium mediated transformation [1]. The Agrobacterium-mediated transformation, the other popular method, cannot be applied to all plant species/ genotypes due to poor infectivity or other related physiological parameters. Biolistic system has become the method of choice because it alleviates the need for preparing protoplasts, reduces the time needed to regenerate transgenic plants, and results in transgenic plants with higher fertility [2]. Employing this approach, pure linear transgene sequences can be transferred without any vector backbone interference. To achieve higher levels of transformation efficiency, biolistic method is preferable as large number of cells can be treated and transformed at a single time [3]. In addition, particle-mediated gene delivery is the only method reported so far to introduce foreign genes into cell organelles such as chloroplasts [4].
The first generation of this technique for gene transfer into plant cells was developed by plant virologist [5]. Particle bombardment has been widely exploited to produce tissues and plants expressing traits with agronomic value and has a major impact on basic plant science research and biotechnology [6-9]. Transgenic plants generated by this method have been reported for monocotyledonous such as Kentucky bluegrass [10], jute [11], rice [12,13] and dicotyledonous species including moth bean [14], cowpea [15], sunflower [16] soybean [17] and wheat [18].
Application of biolistic has demonstrated transient gene expression in plant studies by production of genetically transformed plants and tissues [7,19-22]. The universality of application through cell types, size, shape, presence or absence of cell wall and direct introduction of biological material into the cell has very high delivery efficiency to enhance the transformation rates [23]. This physical method of gene transfer is very often exclusively employed for DNA delivery in transient gene expression studies like analysis of tissue specific promoters [24] or to deliver DNA molecules carrying a marker gene and a chemical that is needed for transgene expression into plant cells simultaneously and release the encapsulated chemical in a controlled manner to trigger the expression of co-delivered transgenes in the cell [24]. Biolistic is highly adaptable and may be used in other fields as well. For instance, the use of biolistic as a method to administer vaccines to humans is currently in preclinical trials [25]. The use of this technology for human vaccination is not unreasonable, as it is already being used to inoculate plant tissue with viruses for study [26].
Despite of these advantages, biolistic procedure is tedious, cumbersome and expensive. Moreover, introduced DNA will be expressed in the plant cell only if it is integrated into plant chromosomal DNA [27]. Hence integration of gene into chromosomal DNA is a chance factor and this is one of the reasons why the transformation efficiencies of biolistic are comparatively lower than that of Agrobacterium mediated transformation, but the transient expression levels are always higher than that of stable expression as evidenced by GUS expression studies [28], which can be explained by the stabilization process during various steps of selection after bombardment till regeneration. These drawbacks can be avoided by following well established tissue culture protocols and stringent selection methods to come out with positive transgenic plants. In addition to the utilization of effective protocols and selection strategies, improvement of biolistic procedure is also quite essential for effective gene transfer. Micro-carriers play an important role in gene delivery to the explants. Depending upon genotype, size of micro-carrier is also an important factor that governs the transformation efficiency of monocots [29]. A very small micro-carrier will have a lower penetration force and a bigger one will increase tissue/cell damage [30].
Several researchers used different types of micro-carriers for gene delivery viz. gold, tungsten, platinum, glass etc. Platinum and gold are expensive, while tungsten and glass have limitations in their usage. Gold particles are also preferred due to their high density, low toxicity and lack of chemical reactivity [31-33]. Moreover, gold particles, which are homogenous in size, biologically inert, nontoxic and do not degrade DNA bonds, are often preferred for particle bombardment Tungsten particles are highly heterogeneous in size and shape, toxic, affects growth of calli and regeneration can also acidify solutions and catalyse plasmid DNA degradation and moreover they are non-biocompatible [34-36]. There is evidence that tungsten toxicity can reduce the recovery of stable transformants in some plant species [37]. Low density, biocompatibility and particle impaction are important parameters to be considered in this area of research because cell damage may occur through particle impaction [21,38]. Hence there is search for alternate micro-carriers. MMT carriers have prominent advantage as they have low density, and particle impaction. Moreover clay particles are more biocompatible, making them a good carrier material for gene transfer. In this study, the potential of MMT as a micro carrier vis-a-vis gold and tungsten was reported. The assumption that clay minerals played an important role in the prebiotic formations of biomolecules that are basic to life, has paved the way for a series of studies on the adsorption of various organic molecules, including nucleic acids [39-41], DNA molecules have a persistent ability to transform competent cells when bound to clay minerals and other particles [42-44]. Much research has used 2:1 type layer phyllosilicates (e.g., montmorillonite) as adsorptive particles to understand DNA adsorption by soils. The phosphate group at the end of the DNA molecule binds directly to the OH groups present on the edges of phyllosilicates such as montmorillonite (Figure 1) [45,46].
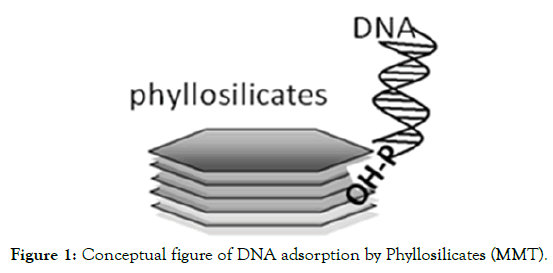
Figure 1. Conceptual figure of DNA adsorption by Phyllosilicates (MMT).
DNA retention can occur on negatively charged phyllosilicates through the formation of bridges by cations. Ca2+ binds to the SiOH groups of minerals such as silica and depresses the negative charges on the mineral surfaces, resulting in increased DNA adsorption [47]. This can be co-related to one of the important steps during preparation of micro-carriers where Cacl2, when added, enable DNA to bind onto the micro-carriers. As it is evident that clay particles like montmorillonite could adsorb DNA, the present study reports an assessment of MMT with gold and tungsten as controls for delivery of DNA in biolistic mode of transformation.
Materials and Methods
Genotypes
The rice genotypes selected for the study were three elite indica rice cultivars Swarna, Gayatri and Samba Mahsuri. Swarna, a widely grown indica rice variety in eleven states of India, is highly popular with a yield potential of 8.0 t/ha [48]. It is also being extensively grown in Bangladesh and Myanmar suggesting its wide adaptability [49]. Gayatri, a high yielding cultivar released from CRRI, is widely grown in shallow and medium low land ecology in Eastern India. Samba Mahsuri (BPT 5204) is one of India’s most popular and highly prized rice varieties because of its high yield 4.5 to 5.0 t/ha and excellent cooking quality [50].
Callus induction and proliferation
Mature dehusked grains of the three cultivars were washed with sterile distilled water and were surface sterilized successively with, 70% ethanol for two min, sodium hypochlorite (contains 4% (v/v) active chlorine) for 15 min and with 0.1% (w/v) aqueous mercuric chloride solution for 5 min with intermittent repeated washings with sterile distilled water [51]. The kernels were inoculated in culture tubes containing semisolid callus induction (CI) medium [MS medium supplemented with 2, 4-dichlorophenoxy acetic acid (2, 4-D) (2 mgl-1), maltose (30 gl-1) and solidified with gel-rite (2.6 gl-1)] [52] and the cultures were incubated in dark at 25 ± 1ºC for three weeks. The scutellum-derived calli were excised and sub cultured on the same CI medium for another four days and clusters of highly embryogenic compact calli (3-5 mm in diameter) were selected and arranged (80-100 no/dish) side up in the center of petridishes (90 X 15 mm) containing 20 ml of semi-solid modified MS medium (MS salts and vitamins, maltose (30 gl-1) L-proline (500 mg l-1), casein hydrolysate (300 mg l-1) and 2,4-D (2.0 mgl-1), Myo Inositol (100 mgl-1), Mannitol (36.4 gl-1), Sorbitol, (20 g l-1) Gelrite, (2.6 g l-1) pH 5.8) ready for bombardment.
Plasmid preparation
A single colony of E.coli strain DH5α/pCAMBIA1301 carrying a GUS reporter gene and hygromycin selectable marker gene, under control of 35S promoter was picked from a freshly streaked selection plate and inoculated into 5 ml of LB medium [53] supplemented with the appropriate selective antibiotic to initiate a starter culture (Figure 2). The starter culture was incubated for approximately 8 h at 37°C with vigorous shaking (~220rpm). The starter culture was diluted (100 μl of starter culture was added to 100 ml LB medium supplemented with selective antibiotics) and the cells are grown at 37°C for 12-16 h with vigorous shaking (~ 200 rpm). The bacterial cells were harvested by centrifugation at 6000 g for 15 min at 4°C. Further plasmid extraction steps were followed using Quiagen Plasmid extraction kit as per manufacturer’s instructions.
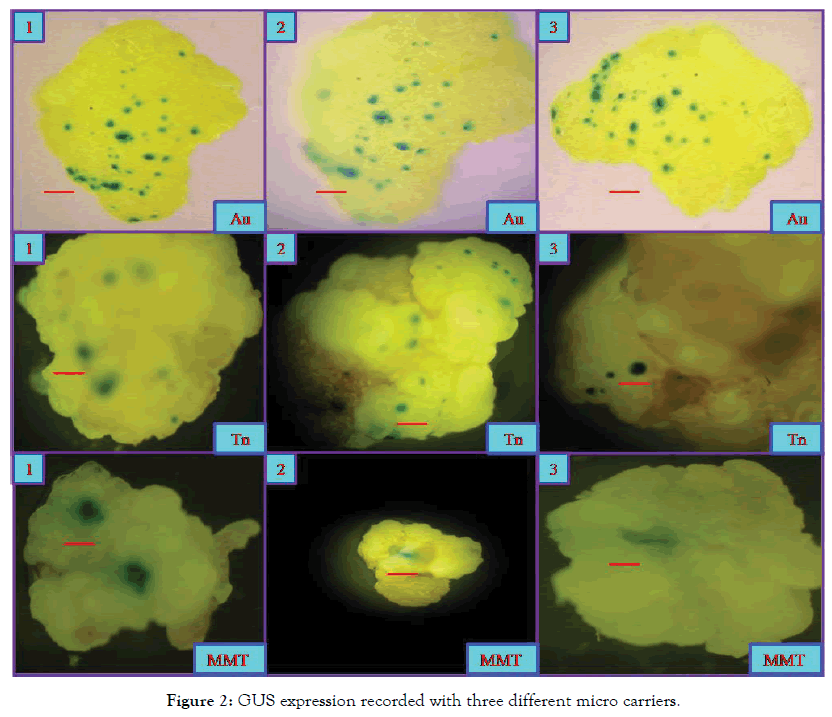
Figure 2. GUS expression recorded with three different micro carriers.
Transformation
Micro-carriers were prepared as per standard protocol using gold (1 μ), tungsten (>1 μ) and MMT (<1 μ) particles. Embryogenic calli were bombarded at 1100 psi helium pressure with the transformation vector pCAMBIA1301 (contaning both GUS and Hpt genes) coated on three different micro carriers in three individual experiments using the particle gun PDC-1000/He system (BIORAD) following manufacturer’s instructions.
Selection
After the bombardment, the calli are plated on media supplemented hygromycin (30 mgl-1 for Swarna and 40 mgl-1 for Gayatri and Samba Mahsuri) and the selection continued for four selection cycles and GUS expression was studied in the first (transient) and last (stable) selection cycles.
DNA Extraction and PCR assay
Actively growing calli on the selection media were selected and from a half portion of the callus, DNA was extracted following the mini prep method [54] while, GUS histochemical assay was conducted on the second half of the callus as per [55]. The presence of GUS gene was examined by polymerase chain reaction (PCR) with the help of specific primers to give an amplification product of ~1.2-kb size. The plasmid DNA (pCAMBIA1301) was used as the positive control and non-transformed callus DNA is taken as negative control. The PCR mix contained 1 μl of plant DNA (20 ng), 0.8 μl of 2.5 mM dNTPs (Fermentas), 1.0 μl of 10X PCR buffer (10 mM Tris, pH 8.4, 50 mM KCl, and 15 mM MgCl2; Sigma), 0.2 μl of Taq DNA Polymerase (5 U/μl Sigma), 1 μl each of both forward and reverse primers (5 pico moles/μl Sigma) and 5 μl of autoclaved sterile distilled water in a total volume of 10 μl. The amplification was done in a thermal cycler (Eppendorf Vapo protect) under following conditions: An initial denaturation of template DNA at 94°C for 2 min followed by 36 cycles of amplification i.e., 30 sec denaturation at 94°C, 30 sec primer annealing at 55°C, 1 min primer extension at 72°C and 10 min final primer extension at 72°C. Isolated DNA was also examined for the presence of Hpt gene which was used as the antibiotic selectable marker as per the conditions similar to that of GUS gene amplification mentioned above but with the different PCR program as per the following conditions: an initial denaturation of template DNA at 94°C for 5 min followed by 40 cycles of amplification i.e., 1 min denaturation at 94°C, 1 min primer annealing at 58°C, 1 min primer extension at 72°C and 5 min final primer extension at 72°C. PCR products were separated in 1.2% agarose gel (in 1X TBE electrophoresis buffer) containing 0.5 g/ml ethidium bromide. Separated PCR products were visualized under UV light and photographed by gel documentation system (Alpha innotech) to examine the size of the product.
GUS assay
The GUS gene expression analysis was performed on calli within 2-4 days after bombardment before subjecting the tissues for antibiotic selection [56] and also at the end of the 4th selection cycle. For the histo chemical detection, segments (5 mm in length) of rice tissues were incubated in a reaction mixture of 50 mM phosphate buffer (pH 6.8), 1% Triton X-100, 20% methanol and 1 mM 5-bromo-4- chloro-3-indolyl-β-D-gluronide (X gluc). The reaction was initiated under a mild vacuum for few min and carried out overnight at 37°C. The frequency of transient transformation is expressed as the ratio between the number of calli showing GUS expression and the total number of calli kept for staining. For qualitative assay, the area of GUS expression and intensity of the blue colour at each spot were given due weightage so as to give a holistic picture of the effects of the various micro-carriers used. Through visual observations, distinction could be made between small spots (<0.5 mm in diameter) representing one or few GUS expressing cells, and large spots (>1 mm in diameter), representing a complete cell cluster expressing the GUS gene [57].
Results and Discussion
The results suggest that gold carriers were ranked first both in transient (8.75%) and stable (20%) assays and MMT, was ranked 2nd in transient (7.84%) but 3rd in stable assays (16.6%) while tungsten was ranked last in transient expression (7.36%) but 2nd (20%) in stable expression assays (Tables 1 and 2). From the results it is evident that gene transfer is feasible with MMT carriers at reasonable frequency in all the genotypes tested.
| Variety | Particle | No. of calli tested | No of GUS positive calli | Transformation Efficiency (%) |
Rank |
|---|---|---|---|---|---|
| Swarna | Gold | 190 | 26 | 13.68 | 1 |
| Gayatri | Gold | 80 | 7 | 8.75 | 2 |
| Swarna | MMT | 102 | 8 | 7.84 | 3 |
| Samba Mahsuri | Tungsten | 190 | 14 | 7.36 | 4 |
Table 1: Transient GUS expression observed with three different micro carriers.
| Variety | Particle | No. of calli tested | No of GUS positive calli | Transformation Efficiency (%) |
Rank |
|---|---|---|---|---|---|
| Gayatri | Gold | 5 | 1 | 20.0 | 1 |
| Samba Mahsuri | Tungsten | 20 | 4 | 20.0 | 2 |
| Swarna | MMT | 18 | 3 | 16.6 | 3 |
| Swarna | Gold | 18 | 2 | 11.1 | 4 |
Table 2: Stable GUS expression observed with three different micro carriers.
When the GUS expression zones were measured, the area (diameter) of the zones varied for different types of micro-carriers. It is interesting to note that the diameter for MMT carriers is more (>1 mm) compared to that of both tungsten (0.6 mm) and gold (0.4 mm) carriers though for the number of GUS expression zones category, gold is ranked first followed by tungsten and MMT (Table 3 and Figure 3). Though gold carriers can induce higher number of expression zones, it is interesting to note that area of GUS expression zone is large in the case of MMT carriers followed by tungsten and gold.
| Type of Micro-carrier | No of GUS expression zones per callus | Area of GUS expression (dia) | ||
|---|---|---|---|---|
| Swarna | Gayatri | Samba mahsuri | ||
| Gold(Au) | 28 | 25 | 26 | 0.4 mm |
| Tungsten(Tn) | 6 | 12 | 4 | 0.6 mm |
| Montmorillonite (MMT) | 2 | 1 | 1 | 1.0 mm |
Table 3: GUS expression zones (in dia) observed with the three micro carriers.
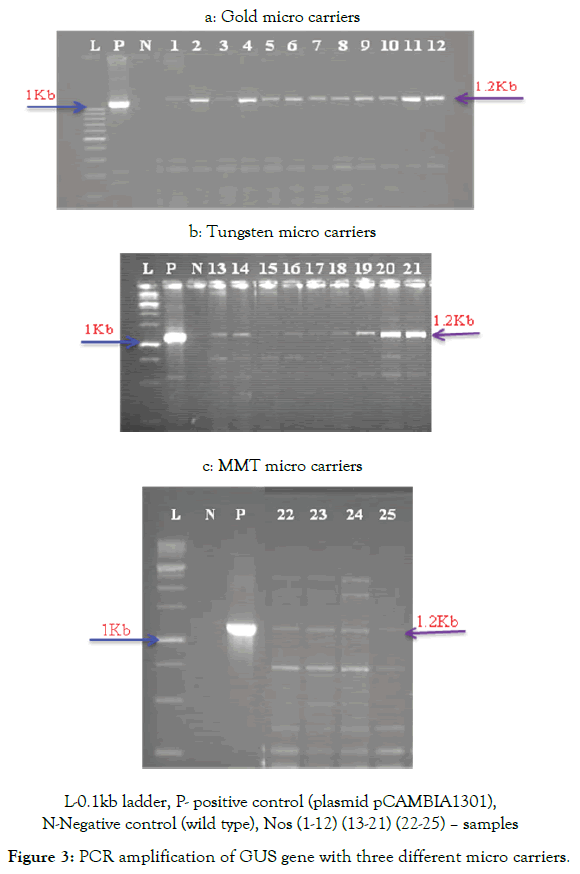
N-Negative control (wild type), Nos (1-12) (13-21) (22-25) – samples
The results on the amplification of GUS and Hpt gene sequences through PCR assays suggest that the presence of both GUS and Hpt genes was detected with all the three micro-carriers with varying frequencies. While 12 positives were recorded with gold, 9 positives were recorded with tungsten while 4 positives were recorded with MMT for both the genes while amplification was not observed in the wild types (Figure 4a-c). The stable integration of the GUS gene was corroborated by molecular analysis and the same is case with Hpt gene. It is clear from the results that with all the three micro-carriers, stable transformation is possible.

N-Negative control (wild type) Nos (1-25) – samples
However, one of the important factors to be taken into consideration is the presence of regions with dark brownish colour in the calli in case of both tungsten and MMT. However, these dark regions are in higher frequency in tungsten than in MMT and in case of tungsten, the origin of these regions is attributed to toxicity of the tungsten to the cells [37]. In addition, from this study, it is evident that genotypes vary in the transformation rates and influenced by the type of carriers employed. These results are in accordance with our earlier report on the variation in genotypes for transformation rates [28].
As in the other transformation experiments, the transient gene expression rates are more in this study also. Since the transient gene expression is temporary as it occurs almost immediately after gene transfer, its rate was higher which require stable integration of the transgene. Transient gene expression is a rapid and useful method for analysing the function of gene of interest [58] and the transient expression frequency provides the most convenient measure of the frequency of introduction of DNA into explants during the optimization of bombardment conditions [59]. In contrast, although the stable expression occurs with the lower frequency, the expression was maintained for long term as the DNA incorporate into the chromosome of the recipient cell [60,61].
Introduction and expression of an exogenous gene into cells does not always involve stable integration of the gene into the genome of the recipient cell. This may be because of the tissue culture conditions and the regeneration capability of the genotypes used for transformation. It is evident in our previous experiments that the cultivars with good callus induction abilities may not have good regeneration potential and vice versa [28] and also due to stabilization in gene integration process. Frequently, plasmid DNA may be introduced into host cells and may express in a transient fashion. This activity declines over time and eventually disappears. Even though measuring levels of such transient activity may be useful in specific cases, when it comes to the creation of stable transgenic phenotypes, only transformation events leading to integration of the foreign gene into the genome of the host cell are useful [62-65].
Though more experiments are needed to standardize the protocols and to improve its efficiency in gene delivery, the present results suggest that MMT can be viewed as a viable alternative to the highly popular but expensive gold micro-carriers [66,67]. Since MMT constitutes only clay particles, major toxicity issues associated with the metal micro carriers may not be issue with MMT and being inexpensive, the experimental costs to develop new transgenic can be minimized and more number of transgenic can be generated.
Acknowledgment
The authors are thankful to Director, CRRI for the facilities and encouragement. The author’s express their sincere thanks to Dr. R.A. Jeffrerson, CAMBIA, Australia for providing pCAMBIA plasmid for their work at CRRI. The first two authors are also thankful to ICAR-NPTC for providing them Senior Research Fellowship.
Authors Contribution Statement
Idea to utilize MMT micro-carriers was the brain child of GJN Rao, He had supervised the entire work, corrected the manuscript and is the driving force for the success of this work. Sai Krishna Repalli had done the bombardment experiments, generated putative transgenic and compiled the manuscript. Chaitanya Kumar Geda had done the GUS expression analysis.
Compliance with Ethical Standards
This manuscript is in compliance with ethical standards.
Consent for Publication
That is applicable for this work.
Conflict of Interest
The authors have no conflict of interest to declare.
REFERENCES
- Cheng M, Lowe BA, Spencer TM, Ye XD, Armstrong CL. Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell Dev Biol Plant. 2004;40(1):31-45.
- Rakszegi M, Tamas C, Szucs P, Tamas T, Bedo Z. Current status of wheat transformation. J Plant Biotechnol. 2001;3:67-81.
- Dalton SJ, Bettany AJE, Timms E, Morris P. Co-transformed, diploid Lolium perenne (perennial ryegrass) Lolium multiflorum (Italian ryegrass) and Lolium temulentum (darnel) plants produced by microprojectile bombardment. Plant Cell Rep. 1999;18(9):721-726.
- Grevich JJ, Daniell H. Chloroplast genetic engineering: Recent advances and future perspectives. Crit Rev Plant Sci. 2005;24(2):83-107.
- Birch RG, Bower R. Principles of gene transfer using particle bombardment. In: Yang SN, Christou P. Particle bombardment technology for gene transfer. New York: Oxford University Press. 1994;3-37.
- Altpeter F, Baisakh N, Beachy R, Bock R, Capell T, Christou P, et al. Particle bombardment and the genetic enhancement of crops: myths and realities. Mol Breeding. 2005;15(3):305-327.
- Taylor NJ, Fauquet CM. Micro particle bombardment as tool in plant science agricultural biotechnology. DNA Cell Biol. 2002;21(12):963-977.
- Zuraida AR, Rahiniza K, Hafiza MRN. Factors affecting delivery and transient expression of gusa gene in Malaysian indica rice MR 219 callus via biolistic gun system. Afr J Biotechnol. 2010;9:8810-8818.
- Kuriakose B, Toit ES, Jordaan A. Transient gene expression assays in rose tissues using a Bio-Rad Helios hand-held gene gun. S Afr J Bot. 2012;78:307-311.
- Gao C, Jiang L, Folling M, Han L, Nielsen KK. Generation of large numbers of transgenic Kentucky bluegrass (Pao pratensis L.) plants following biolistic gene transfer. Plant Cell Rep. 2005;25(1):19-25.
- Ghosh M, Saha T, Nayak P, Sen SK. Genetic transformation by particle bombardment of cultivar jute Corchorus capsularis L. Plant Cell Rep. 2002;20(10):936-942.
- Jain RK, Jain S, Wang B, Wu R. Optimization of biolistic method for transient gene expression and production of agronomically useful transgenic Basmati rice plants. Plant Cell Rep. 1996;15(12):963-968.
- Li L, Qu R, Kochko A, Fauquet C, Beachy RN. An improved rice transformation system using the biolistic method. Plant Cell Reports. 1993;12(5):250-255.
- Kamble S, Misra HS, Mahajan SK, Eapen S. A protocol for efficient biolistic transformation of mothbean Vigna aconitifolia L. Jacq. Marechal. Plant Mol Biol. 2003;21(4):457-458.
- Ikea J, Inglebrecht I, Uwaifo A, Thottappily G. Stable gene transformation in cowpea (Vigna unguiculata L.walp) using particle gun method. Afr J Biotechnol. 2003;2(8):211-218.
- Molinier J, Thomas C, Brignou M, Hahne G. Transient expression of ipt gene enhances regeneration and transformation rates of sunflower shoot apices (Helianthus annuus L.). Plant Cell Rep. 2002;21(3):251-256.
- Moore PJ, Moore AJ, Collins GB. Genotypic and developmental regulation of transient expression of a reporter gene in soybean zygotic cotyledons. Plant Cell Rep. 1994;13(10):556-560.
- Zhang L, Rybczynski JJ, Langenberg WG, Mitra A, French R. An efficient wheat transformation procedure: transformed calli with long-term morphogenic potential for plant regeneration. Plant Cell Reports. 2000;19:241-251.
- Svarovsky S, Borovkov A, Sykes K. Cationic gold micro particles for biolistic delivery of nucleic acids. Biotechniques. 2008;45(5):535-540.
- Huang PH, Chen PY. Design of a two-stage electromagnetic impulse force circuit for gene gun. J Mar Sci Technol. 2011;19(6):686-692.
- O’Brien JA, Lummis SCR. Nano-biolistics: A method of biolistic transfection of cells and tissues using a gene gun with novel nanometer-sized projectiles. BMC Biotechnol. 2011;11:66.
- Manjila SB, Baby JN, Bijin EN, Constantine I, Pramod K, Valsalakumari J. Novel gene delivery system. Int J Pharm Invest. 2013;3(1):1-7.
- Sanford JC, Klein TM. Delivery of substances into cells and tissues using a particle bombardment process. Sci Tech. 1987;5:27-37.
- Torney F, Trewyn BG, Lin VSY, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nature Nanotechnol. 2007;2:295-300.
- Liu Y. Impact studies of high-speed micro-particles following biolistic delivery. IEEE Trans Biomed Eng.2007;54:1507-1513.
- Yamagishi N, Terauchi H, Kanematsu S, Hidaka S. Biolistic inoculation of soybean plants with soybean dwarf virus. J Virol Methods.2006;137(1):164-167.
- Wong JR. The PDS-1000/He, a helium shock wave device. In: Yang SN, Christou P. Particle bombardment technology for gene transfer. New York: Oxford Univ. Press. 1994;Pp:46-51.
- Saikrishna R, Chaitanya KG, Rao GJN. Efficacy of different transformation methods in rice (Oryza sativa L.). J Exp Biol Agri Sci. 2013;1:(2S).
- Sood P, Bhattacharya A, Sood A. Problems and possibilities of monocot transformation. Plant Bio. 2011;55(1):1-15.
- Klein TM, Fromm M, Weissinger A, Tomes D, Schaaf S, Sletten M, et al. Transfer of foreign genes into intact maize cells with high-velocity microprojectiles. Proc Natl Acad Sci USA. 1988;85(12):4305-4309.
- Macklin MD, Drape RJ, Swain WF. Preparation for particle mediated gene transfer using the accell® gene gun. Methods Mol Med. 2000;29:297-303.
- Rosi NL, Giljohann DA, Thaxton CS. Oligonucleotide modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027-1030.
- Valenstein JC. Developing nanotechnology for biofuel and plant science applications, Thesis for the degree of Doctor of Philosophy, Iowa State University, USA. 2012.
- Sanford JC, Smith FD, Russell JA. Optimizing the biolistic process for different biological applications. Methods Enzymol. 1993;217:483-509.
- Bastian S, Busch W, Kuhnel D, Springer A, Meissner T, Holke R, et al. Toxicity of tungsten carbide and cobalt-doped tungsten carbide nanoparticles in mammalian cells in vitro. Environ Health Perspect. 2009;117(4):530-536.
- Yoshimitsu Y, Tanaka K, Tagawa T. Improvement of DNA/metal particle adsorption in tungsten-based biolistic bombardment, alkaline pH is necessary for DNA adsorption and suppression of DNA degradation. J Plant Biol. 2009;52(6):524-532.
- Russell JA, Roy MK, Sanford JC. Physical trauma and tungsten toxicity reduce the efficiency of biolistic transformation. Plant Physiol. 1992;98:1050-1056.
- Sato H, Hattori S, Kawamoto S. In vivo gene gun-mediated DNA delivery into rodent brain tissue. Biochem Biophys Res Commun. 2000;270(1):163-170.
- Bernal JD. The Physical Basis of Life. London: Routledge and Kegan Paul. 1951.
- Greaves MP, Wilson MJ. The adsorption of nucleic acids by montmorillonite. Soil Biol Biochem. 1969;1(4):317-323.
- Franchi M, Bramanti E, Bonzi LM, Orioli PL, Vettori C, Enzo Gallori. Clay-nucleic acid complexes: Characteristics and implications for the preservation of genetic material in primeval habitats. Orig Life Evol Biospheres. 1999;29(3):297-315.
- Khanna M, Stotzky G. Transformation of Bacillus subtilis by DNA bound on montmorillonite and effect of DNase on the transforming ability of bound DNA. Appl Environ Microbiol. 1992;58(6):1930-1939.
- Gallori E, Bazzicalupo M, Dal Canto L, Fani R, Nannipieri P, Vettori C, et al. Transformation of Bacillus subtilis by DNA bound on clay in non‐sterile soil. FEMS Microbiol Eco. 1994;15(1-2):119-126.
- Crecchio C, Stotzky G. Binding of DNA on humic acids: effect on transformation of Bacillus subtilis and resistance to DNase. Soil Biol Biochem. 1998;30(8-9):1061-1067.
- Khanna M, Yoder M, Calamai L, Stotzky G. X-ray diffractometry and electron microscopy of DNA from Bacillus subtilis bound on clay minerals. Sci Soils. 1998;3(1):1-10.
- Pietramellara G, Franchi M, Gallori E, Nannipieri P. Effect of molecular characteristics of DNA on its adsorption and binding on homoionic montmorillonite and kaolinite. Biol Fertil Soils. 2001;33:402-409.
- Nguyen TH, Elimelech M. Adsorption of plasmid DNA to a natural organic matter coated silica surface: Kinetics, conformation, and reversibility. Langmuir. 2007;23:3273-3279.
- Rao VR, Reddy PS, Murthy N, Rao I, Rao PS. Swarna (MTU 7029)-a new stable hybrid with wide adaptation. Oryza. 1983;20:240-242.
- Baisakh N, Datta K, Oliva N, Ona I, Rao GJN, Mew T, et al. Rapid development of homozygous transgenic rice using anther culture harbouring rice chitinase gene for enhanced sheath blight resistance. Plant Biotechnol. 2001;18(2):101-108.
- Reddi MV, Prasad SSNDB, Reddy BM, Rao LVS. BPT 5204: A new rice variety for kharif season for coastal districts of Andhra Pradesh. Andhra Agri J. 1979;26:66-67.
- Vijayachandra K, Palanichelvam K, Veluthambi K. Rice scutellum induces Agrobacterium tumefaciens vir genes and T-strand generation. Plant Mol Biol. 1995;29:125-133.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962;15:473-497.
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293-300.
- Dellaporta SL, Wood J, Hicks JB. A plant DNA mini preparation: Version II. Plant Mol Biol Rep. 1983;14:19-21.
- Jefferson RA, Kacanagh TA, Bevan MW. b-glucouronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci USA. 1986;83(22):8447-8451.
- Rueb S, Hensgens LAM. An improved histochemical staining for B-D-glucuronidase activity in monocotyledonous plants. Rice Genet Newsl. 1989;6:168-169.
- De Clercq J, Zambre M, Van Montagu M, Dillen W, Angenon G. An optimized Agrobacterium-mediated transformationprocedure for Phaseolus acutifolius A. Gray. Plant Cell Rep. 2002;21(4):333-340.
- Lincoln JE, Folmer S, Bostock RM, Gilchrist DG. Construction of plant transient expression vector which co-express the marker β-glucuronidase. Pl Mol Biol. 1998;16:1-4.
- Birch RG, Bower R. Principles of gene transfer using particle bombardment. In: YangSN, Christou, P. Particle bombardment technology for gene transfer. New York: Oxford University Press. 1994; pp: 3-37.
- Datta KS, Torrizo LB, Tu J, Oliva NP, Datta K. Production and molecular evaluation of transgenic plants. Philiphines: Int. Rice Research Institute Discussion Paper Series No.21. 1997.
- Mitrovic T. Gene Transfer System. Med Bio. 2003;10(3):101-105.
- Christou P, Yang NS. Applications to Plants in Particle bombardment technology for gene transfer. (Ed) Oxford University Press, New York. 1994.
- Zhang G, Gurtu V, Kain SR. An enhanced green fluorescent protein allows sensitive detection of gene transfer in mammalian cells. Biochem Biophys Res Commun. 1996;227(3):707-711.
- Stotzky G. Persistence and biological activity in soil of insecticidal proteins from Bacillus thuringiensis and of bacterial DNA bound on clays and humic acids. J Environ Qual. 2000;29:691-705.
- Saeki K, Sakai M. The influence of soil organic matter on DNA adsorptions on andosols. Microbes and Environments. 2009;24(2):175-179.
- Rogers SG. Free DNA methods for plant transformation. Curr Opin Biotechnol. 1991;2:153-157.
- Chaitanya KG, Sai Krishna R, Mohan Dev T, GJN Rao. Genetic variation in in vitro response of elite aromatic and non-aromatic rice varieties. Oryza. 2003;50:329-333.
Citation: Repalli SK, Geda CK, Rao GJN (2019) MMT, a High Promising, Cost Effective Micro-carrier for Gene Delivery. J Microb Biochem Technol 11: 417. doi: 10.4172/1948-5948.1000417
Copyright: © 2019 Repalli SK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

