Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review - (2019) Volume 11, Issue 4
Metabolic Engineering of Microorganisms to Produce Isoprene
Piyush Sethia, Manmeet Ahuja and Vidhya Rangaswamy*Received: 02-Apr-2018 Published: 24-Apr-2019
Abstract
Isoprene is an industrially important five carbon compound primarily used for production of high quality synthetic rubber. Two major pathways are involved in isoprene synthesis. The mevalonate pathway is present in eukaryotes, archaebacteria and cytosol of higher plants whereas the non-mevalonate pathway exists in many eubacteria and plastids in algae/plants. There have been continuous efforts to study and understand the phenomenon of biological production of isoprene for more than half a century. Although, the current feasibility and cost advantage of chemical processes leading to production of isoprene seems to be far from being dominated by a suitable biological substitute, the fear of extinction of non-renewable resources (raw material for chemical processes) in the near future prompts for a colossal expectation from the synthetic biology community. Technological advances in the field of metabolic engineering have made it possible to vigorously modify and swap genes among different organisms and push the limits for microorganisms to over-produce isoprene to an enormous extent. This review touches upon the limitations faced while improving isoprene titres and the meticulous strategies used to overcome them. It analyzes recent approaches that have resulted in significant improvement of biologically produced isoprene, summarizes the lessons learned from them, and compiles an exhaustive list of potential gene targets that could facilitate prospective research in this widespread arena.
Keywords
Isoprene; MEP/DXP pathway; MVA pathway; Metabolic engineering; Flux
Introduction
Isoprene, a naturally produced cell metabolite, is a volatile compound emitted from the leaves of many plant species. A brief history about the discovery of isoprene as a cell metabolite during the early second half of the twentieth century has been exquisitely described by Professor Guivi Sanadze, the person who published the first report of emission of isoprene from plants [1]. Speculations regarding the native role of isoprene dictates its function as a thermoprotectant and a potential plant defence mechanism against the invading parasites [2,3]. Isoprene is an important chemical used in the production of synthetic rubber, medicines and pesticides. The commercially valuable isoprenoid family of organic compounds are produced using isoprene as a monomeric building block. Chemical synthesis of such isoprenoids is hindered by factors which include depletion of fossil fuels and the complexity of the molecules. Harvesting of isoprene which is gaseous above 34°C from plants is not feasible and therefore isoprene is exclusively produced through chemical synthesis from petrochemicals [4,5]. With the recent advances in synthetic biology/metabolic engineering, isoprene production by microorganisms is a feasible and attractive alternative. Anticancer compound taxol and antimalarial drug artemisinin are both commercially produced by microorganisms with engineered/ modified isoprenoid pathways [6,7]. Several studies and patents in recent times demonstrating genetic modifications enhancing production of isoprene from microorganisms have been published.
Mechanism of Isoprene Production
Biosynthesis of isoprenoids is from the basic building blocks isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) which are synthesised from two naturally occurring pathways-methylerythritol 4-phosphate (MEP) pathway and mevalonate (MVA) pathway. The removal of pyrophosphate from DMAPP results in generation of isoprene and is catalysed by an enzyme, isoprene synthase. It is considered as a key enzyme involved in biosynthesis of isoprene and is usually found in the chloroplasts of various plant species [8-10]. Several variants of isoprene synthases have been identified and tested towards improving isoprene production [11-13]. Moreover, certain bacterial species such as Bacillus subtilis are also known to produce isoprene naturally despite lacking a homolog of isoprene synthase from plant sources [14-16].
Metabolic Engineering of MEP Pathway
The MEP pathway is of prokaryotic origin and present in most bacterial species, including Escherichia coli and B. subtilis, as well as plastids in plants and blue green algae [17,18]. Intricate regulatory control of this pathway has given rise to a prodigious demand for its extensive in-depth exploration, particularly in heterologous hosts. Optimizations of the MEP pathway are worth pursuing since the calculated theoretical yields of isoprene from glucose are higher with MEP as compared to the MVA pathway. IPP and DMAPP synthesis via this pathway requires glyceraldehyde-3-phosphate and pyruvate as initial substrates. The common bacterial host in metabolic engineering viz. E. coli, uses the MEP pathway and it consists of seven enzymatic reactions (Figure 1). The genes identified as bottlenecks in various studies are dxs, dxr and idi [19-21]. Other studies have identified overexpression of IspD, IspF and IspE which resulted in increased production of isoprenoids [22-24]. The observed efflux of methylerythritol cyclodiphosphate (MEC) has also been noted as a rate limiting step in isoprenoid production. Overexpression of IspG, which is an iron-sulfur cluster protein in the MEP pathway, led to diminished MEC efflux thereby bypassing the bottleneck and enhancing the production of isoprenoids [25]. Bacillus subtilis
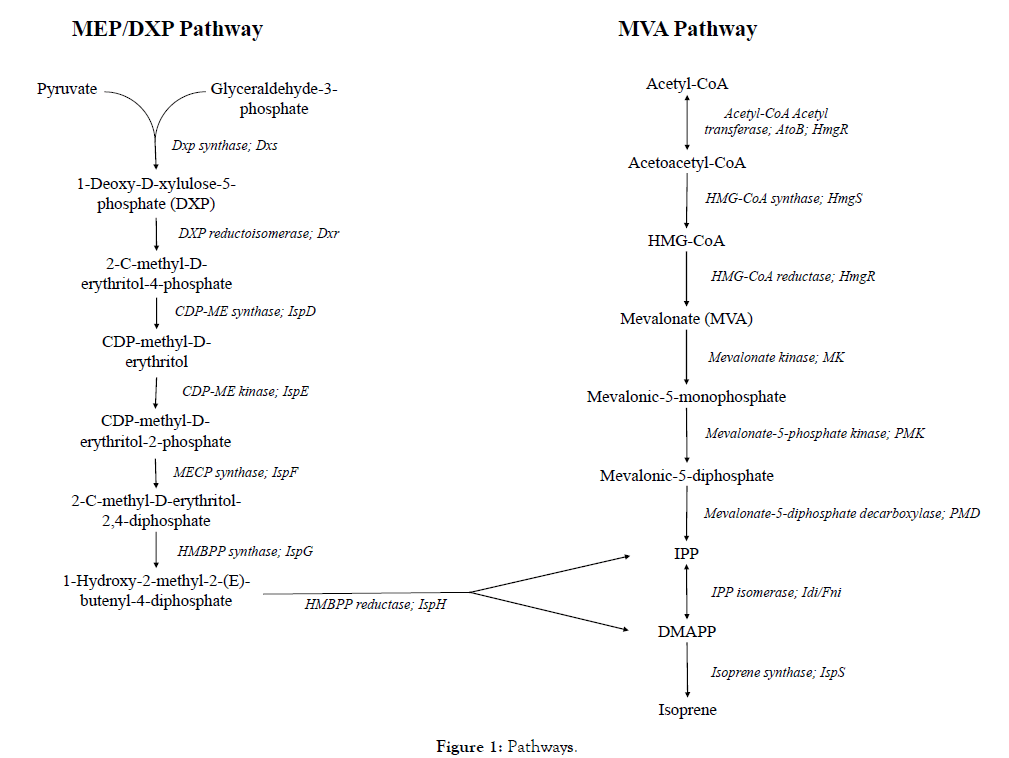
Figure 1. Pathways.
Escherichia coli
Efforts to improve isoprene and isoprenoid production in E. coli have focused on the overexpression of (a) endogenous MEP pathway genes as well as (b) heterologous genes [26,27]. In case of the native MEP genes, the pathway constructs were cloned as one super operon into a suitable expression plasmid where transcription was driven from a strong promoter and a translation initiation region placed in front of each gene. In this study, isoprene production titre with modifications of the MEP pathway improved up to 3.04 mg/L [26]. Overexpression of dxs/ dxr/idi in the specific order consistent with that of the metabolic pathway resulted in a production yield of 2.7 mg/g/h [19]. In case of heterologous expression, overexpression of dxs and dxr genes from B. subtilis in E. coli resulted in an enhancement of isoprene production giving a yield of 314 mg/L [27]. Besides, Type II idi when expressed in E. coli was found to enhance the production of lycopene in comparison with the Type I idi [28]. Yang et al. reported an approach with hybrid MVA pathway utilizing upper pathway genes from Enterococcus faecalis possessing mvaS A110G mutant and achieved 6.3 g/L isoprene titre [29]. Combinatorial approaches towards combining MVA and MEP pathways have also yielded promising results producing 24 g/L isoprene [30].
Bacillus subtilis
This organism’s fast growth rate and GRAS (generally recognised as safe) status makes it a promising microbial host for the production of isoprenoids. Overexpression of DXS resulted in production of 3.73 ng/ml/OD600 while overexpression of both DXS and DXR gave nearly identical yields but resulted in the loss of diauxic growth of the strain [31]. Amorphadiene, the precursor of the anti-malarial drug Artemisinin, was produced at ~20 mg/L with overexpression of DXS, IDI and ADS (Amorpha-4,11-diene Synthase) coupled with protein translation engineering and systematic media optimization [32].
Saccharomyces cerevisiae
Replacing the endogenous MVA pathway with a synthetic bacterial MEP pathway while using an integration based approach was unsuccessful and growth could not be restored. However, bacterial MEP genes on an expression plasmid were able to sustain S. cerevisiae growth in the presence of very well-known mevalonate pathway inhibitor lovastatin [33]. Amorphadiene, the precursor of the anti-malarial drug Artemisinin has been successfully produced in S. cerevisiae with manipulations of the MVA pathway with yields of 40 g/L [34]. Directed evolution of isoprene synthase coupled with perturbation of gal regulon in S. cerevisiae resulted in 3.7 g/L of isoprene [35].
Synechocystis sp. PCC 6803
Recently, cyanobacteria have been explored as “green” environment friendly alternate hosts for production of isoprene [36]. Heterologous overexpression of fni from S. pneumoniae along with the overexpression of ispS gene as a fusion construct with the highly expressed cyanobacterial cpcB gene encoding the β-subunit of phycocyanin resulted in more than 60 fold increase in isoprene production in the type strain Synechocystis sp. PCC 6803 [37]. A benchmark level of 12.3 mg isoprene per gram dry cell weight was achieved using the autotrophic photosynthetic route. In anticipation of freshwater becoming a limiting factor for autotrophic mass production of biofuels in future, research on cultivation of these freshwater cyanobacteria at different concentrations of NaCl has also been instigated [38-40].
The metabolic regulatory constraints of the MEP pathway restrict it to a confined boundary despite the multidimensional efforts in diverse arenas; some of which are listed above. There exists an immediate requirement for better understanding of cellular functioning and concomitant development of advanced molecular engineering tools that could precisely predict and bypass the possible bottlenecks to fast-track the scientific progress made so far in the field.
Metabolic Engineering of MVA Pathway
The predominant metabolic pathway engineering attempts for the microbial production of isoprenoid family of compounds have focused on the MVA pathway due to the obvious advantage of not being subject to as tight regulation as for the MEP pathway. It is further classified as upper pathway that leads to synthesis of mevalonate followed by the lower pathway that consumes mevalonate to synthesize IPP/DMAPP. In one attempt, the upper pathway was cloned from E. faecalis and the lower pathway from S. pneumoniae along with the addition of an extra thiolase (atoB) which resulted in the increased yield of isoprene [26,41].
The efficiency of MVA pathway was improved in order to increase MVA production; the source of the “upper pathway” which contains HMG-CoA synthase, acetyl-CoA acetyltransferase and HMG-CoA reductase to convert acetyl-CoA into MVA was changed from S. cerevisiae to E. faecalis [29]. Replacing the S. cerevisiae MVA upper pathway genes with those from Staphylococcus aureus resulted in doubling of production titres of Amorpha-4,11-diene [42]. Comparison of upper and lower MVA pathway genes from S. pneumoniae, E. faecalis, S. aureus, Streptococcus pyogenes and S. cerevisiae was carried out and the highest production of β-carotene was seen where the upper pathway was from E. faecalis and lower pathway from S. pneumoniae [43]. In the case of isoprene production, yields of up to 60 g/L were achieved with upper and lower pathway from S. cerevisiae with an additional copy of the mvk gene from Methanosarcina mazei [5]. Control systems for heterologous metabolic pathways predominantly rely on swapping of promoters [6,44]. The pmk and mk genes, previously identified as the bottlenecks were placed under a much stronger promoter as compared to the other genes [45]. To further enhance the production of isoprene, mvaS gene was modified replacing an alanine 110 with glycine. With these modifications, isoprene was produced up to 6.3 g/L after 40 h of fed-batch cultivation [29].
Strategies Based on Plasmids and Chromosomal Integrations
The metabolic burden from DNA, RNA and protein synthesis of the cell is increased if it has to maintain multiple plasmids [46]. It is further increased due to the total number of antibiotic resistance proteins that the cell has to produce [47]. This often leads to low yields of the desired metabolite, therefore endeavours to have all the genes on a single plasmid have proved more efficient [45]. Successful isoprene production was seen with some genes of the pathway integrated into the chromosome while the remaining pathway plus additional accessory genes were expressed on two different plasmids [5].
Plasmid based expression systems have several drawbacks which include segregational instability or allele segregation and possible structural instability which may reduce the amount of production of compound of interest [48]. Additionally, antibiotics required for selecting and maintaining plasmids in the host during fermentation result in increased costs. A more stable and reliable approach is integration of heterologous genes or multiple copies of the host genes using suitable integration vector into the bacterial attachment (attB) site of E. coli using helper plasmids which express the phage integrase, by direct transformation [5,22,49]. Another strategy available for chromosomal integration is the Lambda-Red recombinase system in combination with the Flp/FRT site-specific recombination system for marker excision [50,51].
To achieve the high copy numbers for the production of metabolites, the desired pathway genes are first integrated into the genome and then can be evolved to the desired gene copy numbers by the process of chemical induction resulting in chemically induced chromosomal evolution (CIChE) [50]. To further remove the drawback of the presence of the antibiotic selection marker, existing variants of the CIChE technique could be readily employed [49].
Enhancing Flux Towards Isoprene
Distinct studies have been attempted to increase the flux of substrates towards the relevant pathway and to prevent the efflux of intermediates from them. Some of the examples include attempts to increase the amount of acetyl CoA substrate for the MVA pathway. atoB overexpression has also been shown to be effective to an extent [26]. Overexpression of aceto-acetyl transferase (pho) from R. eutropha was found to be effective in increasing acetyl CoA substrate flux [43]. In certain host strains such as E. coli BL21, which has low phosphogluconolactonase (PGL) activity resulting in low carbon flux through the pentose phosphate pathway, constitutive overexpression of PGL along with the rest of the MVA and ispS gave titres of 60 g/L [5].
Overexpression of certain MEP pathway enzymes resulted in the efflux of MEC indicating the existence of a novel competing pathway branch in DXP metabolism. To overcome this, overexpression of ispG was found to effectively reduce the efflux of MEC outside the cells [25]. Proteins encoded by ispG and ispH are metalloproteins with Fe-S clusters. Overexpression of fpr (flavodoxin reductase) and fldA (flavodoxin I), iron-sulfur cluster-interacting redox polypeptide along with ispG and ispH increased isoprene productivity to 600 μg/L/h [52-55]. A list of potential overexpression targets compiled from several references is summarized in Table 1 [52-66].
| Sr. no. | Gene name | Process / Molecule targeted | Reference |
|---|---|---|---|
| 1 | galP; glk | Metabolism | [52] |
| 2 | gld | Isoprene | [53] |
| 3 | ompF, ompE, ndk, cmk, fbaA, fbaB, ompC, adk, pfkA, pfkB, pgi, pitA, tpiA, ompN | Lycopene | [54] |
| 4 | gapB, fbp, pckA | Riboflavin | [55] |
| 5 | ppc, pck | Succinate | [56] |
| 6 | PEPCK | Succinate | [57] |
| 7 | yhfR, nudF | IPP, DMAPP, Isopentenol | [58] |
| 8 | pck, pps, rpoS, appY, yjiD, ycgW, wrbA, atpE | Lycopene | [49] |
| 9 | appY, crl, rpoS | Lycopene | [59] |
| 10 | yggV + lpxH + hisL + ppa + cdh | Isoprenol/prenol | [60] |
| 11 | nuo, cyoABCD, cyAB, sucAB, talB, tktA, gltA, sdhABCD | β-Carotene | [61] |
| 12 | yajO, rib | Terpene | [62] |
| 13 | zwf, gnd | Riboflavin | [63] |
| 14 | pps | Lycopene | [64] |
| 15 | glf, glk | Shikimic acid | [65] |
| 16 | erpA, fldA, fpr, iscA | Fe-S cluster | [66] |
| 17 | TpiA; OmpN | Lycopene | [54] |
Table 1: List of potential targets to be overexpressed.
Systematic and combinatorial analysis to ascertain potential gene knockout targets for improving lycopene production in E. coli led to the identification of three genes viz. glutamate dehydrogenase (gdh), pyruvate dehydrogenase (aceE) and formate dehydrogenase (fdh) [61]. A combinatorial knockout of all the three genes resulted in 40% improved yield [67,68]. A list of potential knockout targets compiled from several references is summarized in Table 2 [69-78].
| Sr. no. | Gene Name | Process / Molecule targeted | Reference |
|---|---|---|---|
| 1 | iclr; arcA | Metabolism | [69] |
| 2 | cra | Sugars | [70] |
| 3 | atpFH; adhE; sucA; poxB; ldhA; frdBC; pflB; ackA | Pyruvate | [71] |
| 4 | ldhA; pflB | Metabolism | [72] |
| 5 | pts1 | Metabolism | [52] |
| 6 | pts; pgi; zwf; gnd; pyk; ppc; pckA; lpdA, pfl | Metabolism | [73] |
| 7 | cyaA; pts1; crr; pfkA; pgi; ptsG; ihfA; ihfB; fis; pstH; atpCDEF; sucA; sucB; lpdA; sdhCDAB | Metabolism | [74] |
| 8 | maeB; frdA; pta; poxB; ldhA; zwf; ndh; mdh; sfcA | Ethanol | [75] |
| 9 | galK | Isoprene | [53] |
| 10 | tdh; tdC; sst; rhtA23 | Threonine | [76] |
| 11 | deoB; yhfW; yahI; pta; eutD; arcC; yqeA; gdhA; ppc; pta; serA; thrC | Lycopene | [54] |
| 12 | sr1; gapB; pckA; gapA; ccpN | Metabolism | [77] |
| 13 | ldhA; pflB; ptsG; pepCK | Succinate | [57] |
| 14 | ppsA; poxB; aceBA | Metabolism | [78] |
| 15 | iclR; gdhA; aceE | Lycopene | [49] |
| 16 | cra; edd; iclR | Metabolism | [79] |
| 17 | hnr; yliE | Lycopene | [80] |
| 18 | arcA | Metabolism | [81] |
| 19 | nudF | Isoprenol/Prenol | [60] |
| 20 | ptsHIcrr operon | Isoprenoids | [82] |
| 21 | pgi; gnd | Isoprene | [83] |
| 22 | gdhA; gpmA; gpmB; aceE; fdhF; talB; fdhF |
Lycopene | [67] |
| 23 | eno | Lycopene | [67] |
| 24 | gapA; mgsA; gapB; pgk; zwf; edd; eda | Metabolism | [84] |
| 25 | pgm | Metabolism | [85] |
| 26 | gapA | Coenzyme Q10 | [86] |
| 27 | ptsG | Metabolism | [87] |
| 28 | thrA | Metabolism | [88] |
| 29 | glnA | Bio-fuels | [89] |
| 30 | serA | Succinate | [90] |
| 31 | fabD | Metabolism | [91] |
| 32 | rpiA | Metabolism | [92] |
| 33 | talA; talB | Metabolism | [93] |
| 34 | tktB | Metabolism | [94] |
| 35 | rpe | Metabolism | [95] |
| 36 | gntK | Metabolism | [96] |
Table 2: List of potential targets to be knocked out.
Moreover, computational analysis performed using genome scale modelling (data not shown) suggested certain DNA-binding transcriptional regulators as targets that could largely improve the isoprene titres [69,70]. These targets include global regulators like cra (Catabolite Repressor Activator), fis (Factor for Inversion Stimulation), arcA (Regulator for respiratory and fermentative metabolism under microaerobic/anaerobic conditions) and iclR (Isocitrate Lyase Regulator); the expression of which could be delicately modulated in combination with the above mentioned overexpression and knockout targets accordingly [71-73].
Rational Perturbations of Metabolic Pathways for Yield Enhancement
Increasing copy numbers of heterologous or homologous genes for the desired product will result in increased titres only up to a certain point. Beyond this, to improve the yields, other strategies have to be employed. The efficiency of expression will also be affected as the cell has to maintain many copies within the cell. By chromosomally integrating the desired genes, the problem of vector load and maintenance are possibly bypassed. However, this does not rule out the cells own expression apparatus or precursor limitation. Various strategies proposed to optimize yields include:
Precursor balancing: Precursor balancing is an indispensable tool towards achieving increased yield of desired metabolites. A few examples and strategies applied in the past for precursor balancing are described. Isoprenoid production via MEP pathway requires equimolar quantities of G3P and pyruvate [74-76]. The imbalanced supply of G3P and pyruvate precursors persists to be the main bottleneck of the MEP pathway. One possible way to manipulate the ratio between G3P and pyruvate, is to alter the flux of the phosphoenolpyruvate (PEP) to pyruvate interconversion, which is controlled by the enzymes pyruvate kinase (Pyk) and PEP synthase (Pps). Pps converts pyruvate to PEP and thus overexpression of pps resulted in a five-fold increase in lycopene yield over the wild type strain (25 mg/g dried cell weight). Moreover, the deletion of pyk also increased lycopene production with similar enhancements as observed with overexpression of other gluconeogenic and glycolytic enzymes [77-97]. In a separate study, the deletion of competing phosphotransferase system which otherwise consumes PEP also resulted in enhanced lycopene production [82]. It has also been shown that mere deletion of gap A gene which prevents conversion of G3P to glycerate 1,3-bisphosphate makes more G3P available for funnelling into the MEP pathway [77-80]. On the other side, overexpression of gapB (NADPH-dependent glyceraldehyde-3- phosphate dehydrogenase) and fbp (fructose-1,6-bisphosphatase) resulted in increased yield of riboflavin [81,82]. It is imperative to remember that the relative regulation of metabolic flux through the glycolytic and the gluconeogenic pathways play an important role in central carbon metabolism.
There are four main glycolytic pathways that serve as feeding modules which generate pyruvate and G3P from sugar substrates:
• EMP (Embden–Meyerhof–Parnas)
• ED (Entner-Doudoroff)
• PP (Pentose phosphate)
• Dhams
These pathways were investigated as feeding modules for increasing isoprene production. Highest isoprene production was seen with overexpression of the EDP in which pyruvate and G3P were generated simultaneously in contrast to EMP. In terms of precursor generation and energy/reducing-equivalent supply, overexpression of both EDP and PPP was found to be the ideal feeding module for MEP. Blocking EDP by knocking out pgi almost completely channelled the glucose through PPP and resulted in a significantly increased isopentanol production [83].
Reducing carbon loss: It is well established that bacterial growth using sugar substrates lead to production of secondary metabolites such as acetic acid, lactic acid, formic acid and ethanol; hence it is desirable to redirect this wasteful carbon towards the MEP pathway for higher production of isoprenoids. The genes which control the production of these secondary metabolites include ackA, pta, ldh, pflB, poxB and adhE. Reducing expression or knockout of these genes can result in making more carbon available for enhanced production of the desired metabolite. One such example was demonstrated for the production of pyruvate from glucose. Multiple gene deletions (ackA, pflB, ldh, adhE) resulted in an increase in pyruvate while acetate production was reduced by 85% [84]. For the production of ethanol from glycerol, knockout of the ldh gene resulted in up to 90% of its theoretical yield while lactate was reduced [85-88].
Optimizing growth rate: Isoprene production is growth associated. Manipulation of global gene regulators affects cellular growth. For example, deletion of iclR and arcA genes resulted in 47% increase in biomass (in glucose abundant conditions). Modulation of the NADH: Ubiquinone oxidor-eductase (nuo), cytochrome bd-I oxidase (cyd), cytochrome b oxidase (cyo) and ATP synthase (atp) gene operons resulted in 20%, 16%, 5% and 21% increase in β carotene production respectively. Modulation of the nuo operon resulted in 29-40% decrease in cell mass [61].
Growth Conditions and Media Standardizations
An effective method to increase cell density and production of metabolites is optimization of growth medium. The organic carbon supplement has an impact on the isoprene yield. Maximum production of isoprene was seen with glycerol as compared to other sources such as fructose and xylose [26]. Also, glycerol resulted in maximum production of β-carotene and lycopene in discrete studies and was shown to be superior to other carbon sources such as glucose, galactose, xylose and maltose [43,82]. Pyruvate and dipotassium phosphate as supplements were found to be beneficial for isoprenoid production possibly indicating enhanced pathway flux [32]. However, monopotassium phosphate was found to be dominant factor for the production of lycopene [82].
The source of nitrogen in the growth medium also plays an important role is improving biosynthesis of the product. Beef extract from a particular source was found to significantly increase α-pinene production as compared to other nitrogen sources [44]. Carbon and nitrogen restrictions during the process of fermentation resulted in significant enhancement of amorpha- 4,11-diene production [42,101-104].
Conclusion
If we look back and attempt to estimate the progress achieved in terms of basic research on the subject, despite more than sixty years of intense research, the exact reason behind how evolutionary selection pressure has rendered some plants capable of synthesizing isoprene and others not still remains to be unveiled. This understanding could help us to predict how the future environmental changes would affect the capacity of plants to produce isoprene. The MEP pathway in particular seems to be yet in its nascent stage and demands a lot more effort to understand the overall fine-tuned regulation of the entire pathway and its significance. In terms of production titres, metabolic engineering of MEP and MVA pathways have significantly aided an upsurge in the production of isoprene from microbial sources and still has a lot more potential for further improvement with an optimal control guided in tactful manner. It is a continuous learning and development process. The design-build-test-debug approach for driving isoprenoid production in E. coli described recently by Wang et al. exemplifies one such continuous process towards achieving maximally improved production titres rapidly approaching the theoretical limits. An interesting synergistic approach combining the MVA and MEP pathways was recently pursued to utilize the merits of both the pathways in a well-balanced manner towards producing isoprene. Looking at the current societal consumption of non-renewable resources, there exists a huge demand for isoprene based alternate biofuel candidates. Scientists are developing novel strategies to explore current know-how about the biological functioning of the microorganisms and expand the limits further.
REFERENCES
- Sanadze GA. Biogenic isoprene (A review). Russ J Plant Physiol. 2004;51:729-741.
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781-1787.
- Laothawornkitkul J, Paul ND, Vickers CE, Possell M, Taylor JE, Mullineaux PM, et al. Isoprene emissions influence herbivore feeding decisions. Plant Cell Environ. 2008;31:1410-1415.
- Sharkey TD, Singsaas EL. Why plants emit isoprene. Nature. 1995;374:769.
- Whited GM, Feher FJ, Benko DA, Cervin MA, Chotani GK. Technology update: Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Ind Biotechnol. 2010;6:152-163.
- Ajikumar PK, Xiao WH, Tyo KEJ, Wang Y, Simeon F, Leonard E, et al. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science. 2010;330:70-74.
- Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528-532.
- Lichtenthaler HK. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47-65.
- Gou Y, Liu Z, Wang G. Advances in isoprene synthase research. Sheng Wu Gong Cheng Xue Bao Chin J Biotechnol. 2017;33:1802-1813.
- Ye L, Lv X, Yu H. Engineering microbes for isoprene production. Metab Eng. 2016;38:125-138.
- Cervin MA, Whited GM, Miasnikov A, Peres CM, Weyler W. Isoprene synthase variants for improved microbial production of isoprene. 2010;US20100003716A1.
- Sharkey TD, Gray DW, Pell HK, Breneman SR, Topper L. Isoprene synthase genes form a monophyletic clade of acyclic terpene synthases in the TPS-B terpene synthase family. Evol Int J Org Evol. 2013;67:1026-1040.
- Ilmén M, Oja M, Huuskonen A, Lee S, Ruohonen L, Jung S. Identification of novel isoprene synthases through genome mining and expression in Escherichia coli. Metab Eng. 2015;31:153-162.
- Kuzma J, Nemecek-Marshall M, Pollock WH, Fall R. Bacteria produce the volatile hydrocarbon isoprene. Curr Microbiol. 1995;30:97-103.
- Julsing MK, Rijpkema M, Woerdenbag HJ, Quax WJ, Kayser O. Functional analysis of genes involved in the biosynthesis of isoprene in Bacillus subtilis. Appl Microbiol Biotechnol. 2007;75:1377-1384.
- Wagner WP, Helmig D, Fall R. Isoprene biosynthesis in Bacillus subtilis via the methylerythritol phosphate pathway. J Nat Prod. 2010;63:37-40.
- Lichtenthaler HK. Non-mevalonate isoprenoid biosynthesis: Enzymes, genes and inhibitors. Biochem Soc Trans. 2000;28:785-789.
- Kuzuyama T. Mevalonate and non-mevalonate pathways for the biosynthesis of isoprene units. Biosci Biotechnol Biochem. 2010;66:1619-1627.
- Lv X, Xu H, Yu H. Significantly enhanced production of isoprene by ordered coexpression of genes dxs, dxr, and idi in Escherichia coli. Appl Microbiol Biotechnol. 2013;97:2357-2365.
- Kajiwara S, Fraser PD, Kondo K, Misawa N. Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J. 1997;324:421-426.
- Kim SW, Keasling JD. Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol Bioeng. 2001;72:408-415.
- Yuan LZ, Rouvière PE, Larossa RA, Suh W. Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metab Eng. 2006;8:79-90.
- Heider SAE, Wolf N, Hofemeier A, Peters-Wendisch P, Wendisch VF. Optimization of the IPP precursor supply for the production of lycopene, decaprenoxanthin and astaxanthin by Corynebacterium glutamicum. Front Bioeng Biotechnol. 2014;2:28.
- Xue D, Abdallah II, de Haan IEM, Sibbald MJJB, Quax WJ. Enhanced C30 carotenoid production in Bacillus subtilis by systematic overexpression of MEP pathway genes. Appl Microbiol Biotechnol. 2015;99:5907-5915.
- Zhou K, Zou R, Stephanopoulos G, Too HP. Metabolite profiling identified methylerythritol cyclodiphosphate efflux as a limiting step in microbial isoprenoid production. PLoS ONE. 2012;7:e47513.
- Zurbriggen A, Kirst H, Melis A. Isoprene production via the mevalonic acid pathway in Escherichia coli (Bacteria). BioEnergy Res. 2012;5:814-828.
- Zhao Y, Yang J, Qin B, Li Y, Sun Y, Su S, et al. Biosynthesis of isoprene in Escherichia coli via methylerythritol phosphate (MEP) pathway. Appl Microbiol Biotechnol. 2011;90:1915-1922.
- Rad SA, Zahiri HS, Noghabi KA, Rajaei S, Heidari R, Mojallali L. Type 2 IDI performs better than type 1 for improving lycopene production in metabolically engineered E. coli strains. World J Microbiol Biotechnol. 2012;28:313-321.
- Yang J, Xian M, Su S, Zhao G, Nie Q, Jiang X, et al. Enhancing production of bio-isoprene using hybrid MVA pathway and isoprene synthase in E. coli. PLoS ONE. 2012;7:e33509.
- Yang C, Gao X, Jiang Y, Sun B, Gao F, Yang S. Synergy between methylerythritol phosphate pathway and mevalonate pathway for isoprene production in Escherichia coli. Metab Eng. 2016;37:79-91.
- Xue J, Ahring BK. Enhancing isoprene production by genetic modification of the 1-deoxy-D-xylulose-5-phosphate pathway in Bacillus subtilis. Appl Environ Microbiol. 2011;77:2399-2405.
- Zhou K, Zou R, Zhang C, Stephanopoulos G, Too HP. Optimization of amorphadiene synthesis in bacillus subtilis via transcriptional, translational, and media modulation. Biotechnol Bioeng. 2013;110:2556-2561.
- Maury J, Asadollahi MA, Møller K, Schalk M, Clark A, Formenti LR, et al. Reconstruction of a bacterial isoprenoid biosynthetic pathway in Saccharomyces cerevisiae. FEBS Lett. 2008;582:4032-4038.
- Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci U S A. 2012;109:E111-E118.
- Wang C, Zada B, Wei G, Kim SW. Metabolic engineering and synthetic biology approaches driving isoprenoid production in Escherichia coli. Bioresour Technol. 2017;241:430-438.
- Lindberg P, Park S, Melis A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab Eng. 2010;12:70-79.
- Chaves JE, Melis A. Biotechnology of cyanobacterial isoprene production. Appl Microbiol Biotechnol. 2018;102:6451-6458.
- Chisti Y. Constraints to commercialization of algal fuels. J Biotechnol. 2013;167:201-214.
- Pade N, Hagemann M. Salt acclimation of cyanobacteria and their application in biotechnology. Life. 2014;5:25-49.
- Pade N, Erdmann S, Enke H, Dethloff F, Dühring U, Georg J, et al. Insights into isoprene production using the Cyanobacterium Synechocystis sp. PCC 6803. Biotechnol Biofuels. 2016;9:89.
- Melis A, Zurbriggen A. Constructs and methods for improved isoprene biosynthesis. 2013;WO2013096863A1.
- Tsuruta H, Paddon CJ, Eng D, Lenihan JR, Horning T, Anthony LC, et al. High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS one 4:e4489.
- Yoon SH, Lee SH, Das A, Ryu HK, Jang HJ, Kim JY, et al. Combinatorial expression of bacterial whole mevalonate pathway for the production of beta-carotene in E. coli. J Biotechnol. 2009;140:218-226.
- Yang J, Nie Q, Ren M, Feng H, Jiang X, Zheng Y, et al. Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol Biofuels. 2013;6:60.
- Alonso-Gutierrez J, Chan R, Batth TS, Adams PD, Keasling JD, Petzold CJ, et al. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab Eng. 2013;19:33-41.
- Bentley WE, Mirjalili N, Andersen DC, Davis RH, Kompala DS. Plasmid-encoded protein: The principal factor in the “metabolic burden” associated with recombinant bacteria. Biotechnol Bioeng. 1990;35:668-681.
- Rozkov A, Avignone-Rossa CA, Ertl PF, Jones P, O’Kennedy RD, Smith JJ, et al. Characterization of the metabolic burden on Escherichia coli DH1 cells imposed by the presence of a plasmid containing a gene therapy sequence. Biotechnol Bioeng. 2004;88:909-915.
- Noack D, Roth M, Geuther R, Müller G, Undisz K, Hoffmeier C, et al. Maintenance and genetic stability of vector plasmids pBR322 and pBR325 in Escherichia coli K12 strains grown in a chemostat. Mol Gen Genet MGG. 1981;184:121-124.
- Chen YY, Shen HJ, Cui YY, Chen SG, Weng ZM, Zhao M, et al. Chromosomal evolution of Escherichia coli for the efficient production of lycopene. BMC Biotechnol. 2013;13:6.
- Tyo KEJ, Ajikumar PK, Stephanopoulos G. Stabilized gene duplication enables long-term selection-free heterologous pathway expression. Nat Biotechnol. 2009;27:760-765.
- Chotani GK, McAuliffe JC, Miller MC, MUIR RE, Vaviline DV, Weyler W. Isoprene production using the DXP and MVA pathway. 2013;US8507235B2.
- Lu J, Tang J, Liu Y, Zhu X, Zhang T, Zhang X. Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl Microbiol Biotechnol. 2012;93:2455-2462.
- Ramos KRM, Valdehuesa KNG, Liu H, Nisola GM, Lee WK, et al. Combining De Ley-Doudoroff and methylerythritol phosphate pathways for enhanced isoprene biosynthesis from D-galactose. Bioprocess Biosyst Eng. 2014;37:2505-2513.
- Wang J, Meng H, Xiong Z, Zhang S, Wang Y. Identification of novel knockout and up-regulated targets for improving isoprenoid production in E. coli. Biotechnol Lett. 2014;36:1021-1027.
- Wang G, Bai L, Wang Z, Shi T, Chen T, Zhao X. Enhancement of riboflavin production by deregulating gluconeogenesis in Bacillus subtilis. World J Microbiol Biotechnol. 2014;30:1893-1900.
- Zhang X, Jantama K, Shanmugam KT, Ingram LO. Reengineering Escherichia coli for succinate production in mineral salts medium. Appl Environ Microbiol. 2009;75:7807-7813.
- Singh A, Cher Soh K, Hatzimanikatis V, Gill RT. Manipulating redox and ATP balancing for improved production of succinate in E. coli. Metab Eng. 2011;13:76-81.
- Withers ST, Gottlieb SS, Lieu B, Newman JD, Keasling JD. Identification of isopentenol biosynthetic genes from Bacillus subtilis by a screening method based on isoprenoid precursor toxicity. Appl Environ Microbiol. 2007;73:6277-6283.
- Kang MJ, Lee YM, Yoon SH, Kim JH, Ock SW, Jung KH, et al. (2005) Identification of genes affecting lycopene accumulation in Escherichia coli using a shot-gun method. Biotechnol Bioeng. 2005;91:636-642.
- Zheng Y, Liu Q, Li L, Qin W, Yang J, Zhang H, et al. Metabolic engineering of Escherichia coli for high-specificity production of isoprenol and prenol as next generation of biofuels. Biotechnol Biofuels. 2013;6:57.
- Zhao J, Li Q, Sun T, Zhu X, Xu H, Tang J, et al. Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab Eng. 2013;17:42-50.
- Kirby J, Nishimoto M, Chow RWN, Baidoo EEK, Wang G, Martin J, et al. Enhancing terpene yield from sugars via novel routes to 1-deoxy-D-xylulose 5-phosphate. Appl Environ Microbiol. 2015;81:130-138.
- Wang Z, Chen T, Ma X, Shen Z, Zhao X. Enhancement of riboflavin production with Bacillus subtilis by expression and site-directed mutagenesis of zwf and gnd gene from Corynebacterium glutamicum. Bioresour Technol. 2011;102:3934-3940.
- Farmer WR, Liao JC. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat Biotechnol. 2000;18:533-537.
- Martínez JA, Bolívar F, Escalante A. Shikimic acid production in Escherichia coli: From classical metabolic engineering strategies to omics applied to improve its production. Front Bioeng Biotechnol. 2015;3:1-16.
- Partow S, Siewers V, Daviet L, Schalk M, Nielsen J. Reconstruction and evaluation of the synthetic bacterial MEP pathway in Saccharomyces cerevisiae. PLoS ONE. 2012;7:e52498.
- Alper H, Jin YS, Moxley JF, Stephanopoulos G. Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab Eng. 2005;7:155-164.
- Alper H, Miyaoku K, Stephanopoulos G. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nat Biotechnol. 2005;23:612-616.
- Waegeman H, Beauprez J, Moens H, Maertens J, De Mey M, Foulquié-Moreno MR, et al. Effect of iclR and arcA knockouts on biomass formation and metabolic fluxes in Escherichia coli K12 and its implications on understanding the metabolism of Escherichia coli BL21 (DE3). BMC Microbiol. 2011;11:70.
- Yao R, Kurata H, Shimizu K. Effect of cra gene mutation on the metabolism of Escherichia coli for a mixture of multiple carbon sources. Adv Biosci Biotechnol. 2013;04:477-486.
- Causey TB, Shanmugam KT, Yomano LP, Ingram LO. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci U S A. 2004;101:2235-2240.
- Van Dien S. From the first drop to the first truckload: commercialization of microbial processes for renewable chemicals. Curr Opin Biotechnol. 2013;24:1061-1068.
- Matsuoka Y, Shimizu K. Importance of understanding the main metabolic regulation in response to the specific pathway mutation for metabolic engineering of Escherichia coli. Comput Struct Biotechnol J. 2013;3:e201210018.
- Baptist G, Pinel C, Ranquet C, Izard J, Ropers D, de Jong H, et al. A genome-wide screen for identifying all regulators of a target gene. Nucleic Acids Res. 2013;41:e164.
- Trinh CT, Srienc F. Metabolic engineering of Escherichia coli for efficient conversion of glycerol to ethanol. Appl Environ Microbiol. 2009;75:6696-6705.
- Lee JH, Sung BH, Kim MS, Blattner FR, Yoon BH, Kim JH, et al. Metabolic engineering of a reduced-genome strain of Escherichia coli for L-threonine production. Microb Cell Factories. 2009;8:2.
- Tännler S, Fischer E, Le Coq D, Doan T, Jamet E, Sauer U, et al. CcpN controls central carbon fluxes in Bacillus subtilis. J Bacteriol. 2008;190:6178-6187.
- Son YJ, Phue JN, Trinh LB, Lee SJ, Shiloach J. The role of Cra in regulating acetate excretion and osmotic tolerance in E. coli K-12 and E. coli B at high density growth. Microb Cell Factories. 2011;10:52.
- Sarkar D, Siddiquee KAZ, Araúzo-Bravo MJ, Oba T, Shimizu K. Effect of cra gene knockout together with edd and iclR genes knockout on the metabolism in Escherichia coli. Arch Microbiol. 2008;190:559-571.
- Alper H, Stephanopoulos G. Uncovering the gene knockout landscape for improved lycopene production in E. coli. Appl Microbiol Biotechnol. 2008;78:801-810.
- Jeong JY, Kim YJ, Cho N, Shin D, Nam TW, Ryu S, Seok YJ. Expression of ptsG encoding the major glucose transporter is regulated by ArcA in Escherichia coli. J Biol Chem. 2004;279:38513-38518.
- Zhang C, Chen X, Zou R, Zhou K, Stephanopoulos G, Too HP. Combining genotype improvement and statistical media optimization for isoprenoid production in E. coli. PLoS one. 2013;8:e75164.
- Liu H, Sun Y, Ramos KRM, Nisola GM, Valdehuesa KNG, Won–Keun L, et al. Combination of entner-doudoroff pathway with MEP increases isoprene production in engineered Escherichia coli. PLoS one. 2013;8:e83290.
- Lin PP, Jaeger AJ, Wu T-Y, Xu SC, Lee AS, Gao F, et al. Construction and evolution of an Escherichia coli strain relying on nonoxidative glycolysis for sugar catabolism. Proc Natl Acad Sci U S A. 2018;115:3538-3546.
- Blank LM, Ebert BE, Bühler B, Schmid A. Metabolic capacity estimation of Escherichia coli as a platform for redox biocatalysis: constraint-based modeling and experimental verification. Biotechnol Bioeng. 2008;100:1050-1065.
- Huang M, Wang Y, Liu J, Mao Z. Multiple strategies for metabolic engineering of Escherichia coli for efficient production of Coenzyme Q10. Chin J Chem Eng. 2011;19:316-326.
- Ohno S, Furusawa C, Shimizu H. In silico screening of triple reaction knockout Escherichia coli strains for overproduction of useful metabolites. J Biosci Bioeng. 2013;115:221-228.
- Kumar VS, Maranas CD. Growmatch: An automated method for reconciling in silico/in vivo growth predictions. PLoS Comput Biol. 2009;5:e1000308.
- Choi KY, Wernick DG, Tat CA, Liao JC. Consolidated conversion of protein waste into biofuels and ammonia using Bacillus subtilis. Metab Eng. 2014;23:53-61.
- Bodor Z, Fazakas A, Kovacs E, Lanyi S, Albert B. Systems biology and metabolic engineering for obtaining E. coli mutants capable to produce succinate from renewable resources. Romanian Biotechnol Lett. 2014;19:9625-9636.
- Peng L, Shimizu K. Effect of fadR gene knockout on the metabolism of Escherichia coli based on analyses of protein expressions, enzyme activities and intracellular metabolite concentrations. Enzyme Microb Technol. 2006;38:512-520.
- Long CP, Gonzalez JE, Sandoval NR, Antoniewicz MR. Characterization of physiological responses to 22 gene knockouts in Escherichia coli central carbon metabolism. Metab Eng. 2016;37:102-113.
- Jin L, Zhang H, Chen L, Yang C, Yang S, Gu Y. Combined overexpression of genes involved in pentose phosphate pathway enables enhanced D-xylose utilization by Clostridium acetobutylicum. J Biotechnol. 2014;173:7-9.
- Maciąg M, Nowicki D, Janniere L, Szalewska-Pałasz A, Węgrzyn G. Genetic response to metabolic fluctuations: correlation between central carbon metabolism and DNA replication in Escherichia coli. Microb Cell Factories. 2011;10:19.
- Chemler JA, Fowler ZL, McHugh KP, Koffas MAG. Improving NADPH availability for natural product biosynthesis in Escherichia coli by metabolic engineering. Metab Eng. 2010;12:96-104.
- Shin JH, Lee SY. Metabolic engineering of microorganisms for the production of L-arginine and its derivatives. Microb Cell Factories. 2014;13:166.
- Farmer WR, Liao JC. Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol Prog. 2001;17:57-61.
- Jiang L-Y, Zhang Y-Y, Li Z, Liu JZ. Metabolic engineering of Corynebacterium glutamicum for increasing the production of L-ornithine by increasing NADPH availability. J Ind Microbiol Biotechnol. 2013;40:1143-1151.
- Hwang GH, Cho JY. Implication of gluconate kinase activity in L-ornithine biosynthesis in Corynebacterium glutamicum. J Ind Microbiol Biotechnol. 2012;39:1869-1874.
- Jiang LY, Chen SG, Zhang YY, Liu JZ. Metabolic evolution of Corynebacterium glutamicum for increased production of L-ornithine. BMC Biotechnol. 2013;13:47.
- Sharkey TD, Monson RK. Isoprene research - 60 years later, the biology is still enigmatic. Plant Cell Environ. 2017;40:1671-1678.
- Banerjee A, Sharkey TD. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat Prod Rep. 2014;31:1043-1055.
- Niu FX, Lu Q, Bu YF, Liu JZ. Metabolic engineering for the microbial production of isoprenoids: Carotenoids and isoprenoid-based biofuels. Synth Syst Biotechnol. 2017;2:167-175.
- https://www.caister.com/hsp/abstracts/biofuels/06.html
Citation: Sethia P, Ahuja M, Rangaswamy V (2019) Metabolic Engineering of Microorganisms to Produce Isoprene. J Microb Biochem Technol 11: 419.
Copyright: © 2019 Sethia P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

