Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 1
Low-Dose Palliative Chemotherapy Compared to Standard-Dose Chemotherapy for Poor-Prognosis Cancers Improves Overall Survival - A Single Institution Retrospective Study
Andrew Dind1,2, Rebecca Kannourakis3, George Kannourakis1,3* and Jarmila Sterbova42Department of Medical Oncology, University of New South Wales, Sydney, Australia
3Ballarat Oncology and Haematology Services, Ballarat, Australia
4Department of Anaesthesia, Flinders Medical Centre, Adelaide, Australia
Received: 16-Jan-2023, Manuscript No. JCM-22-19591; Editor assigned: 18-Jan-2023, Pre QC No. JCM-22-19591 (PQ); Reviewed: 01-Feb-2023, QC No. JCM-22-19591; Revised: 08-Feb-2023, Manuscript No. JCM-22-19591 (R); Published: 17-Feb-2023, DOI: 10.35248/2157-2518.23.14.405
Abstract
Background: Palliative oncology is a balance between maximising quality and quantity of life. Whilst aggressive chemotherapy is associated with severe side effects, low-dose chemotherapy now plays a role in treating many advanced malignancies with palliative intent. There is a need to compare the survival of patients receiving low-dose with standard-dose chemotherapy.
Methods: Data collected from Ballarat Oncology and Haematology Services (BOHS) records was retrospectively assessed for patients diagnosed between 2004-2010 with advanced ovarian, lung, colorectal and pancreatic cancers. 166 patients were assessed for their chemotherapy doses, classed as low-dose chemotherapy (n=69) or standard-dose chemotherapy (n=97). Survival was assessed using the Kaplan-Meier method and the difference between the groups assessed using log rank tests with hazard ratios created using the Cox proportional hazards model.
Findings: Across all cancers, low-dose chemotherapy patients had a survival advantage (log rank=33•76, p<0•00001, HR 0•38, 95% CI 0•38-0•54, p<0•00001). There was a survival benefit for low-dose therapy in ovarian cancer (log rank=9•91, p=0•0016, HR 0•15, 95% CI 0•04-0•54, p=0•0047), pancreatic cancer (log rank=7•47, p=0•0063, HR 0•2, 95% CI 0•057-0•71, p<0•0001) and lung cancer (log rank=24•72, p<0•0001, HR 0•3, 95% CI 0•18-0•50, p<0•0001). There was no significant survival benefit for colorectal cancer patients receiving low-dose chemotherapy (log rank=1•16, p=0•28, HR 0•72, 95% CI 0•39-1•33, p=0•30), although there was a trend to improved survival.
Interpretation: Low-dose chemotherapy was associated with longer survival compared to standard doses of chemotherapy in this group. This novel study found a survival benefit with low dose chemotherapy in patients with advanced ovarian, pancreatic and lung cancers. However this study was not powered to find benefit in individual cancer groups. Large randomised controlled trials are required in order to adequately assess this effect without the presence of confounders.
Funding: This project was performed as a MBBS (Honours) project and as such did not have funding associated with it. RK and JS volunteered their time to the project to provide assistance, ensuring that data collected was accurate.
Keywords
Cancer; Radiotherapy; Chemotherapy; Toxicity; Malignancy
INTRODUCTION
In the setting of advanced malignancies where life expectancy is limited, control of cancer-related symptoms, prevention of cancer-related complications and avoidance of treatment-related side effects are often what is preferred by patients as opposed to maximising overall survival [1]. Yet the idea of “more is better” in chemotherapy dosing is one that has been at the forefront of oncology research and practice for many decades. However, there is a lack of high level evidence for a dose-cure or dose-palliation relationship for chemotherapy in solid cancers [2]. Rather, the evidence suggests a threshold dose-effect relationship for survival and palliation where additional doses provide little increases in effect but great increases in cost and toxicity.
The idea of maximum tolerated dose has long been used without considering the minimum effective dose of chemotherapy, where treatment effects occur with minimal side effects. Compared with maximum tolerated dose, low-dose chemotherapy provides an attractive therapeutic option for palliative stage patients due to a lower toxic burden and lower treatment-related toxicity [3]. Low- dose chemotherapy may therefore have a particularly effective role in those who are too elderly or frail for maximum tolerated dose chemotherapy due to residual toxicity from previous treatment.
Low-dose chemotherapy is thought to induce mainly anti-angiogenic and immunomodulatory effects, in contrast to the direct cytotoxic of traditional maximum tolerated dose chemotherapy [4].
Currently, there is evidence for the safety, efficacy and cost effectiveness of low-dose chemotherapy over best supportive care [5]. However there has not yet been a comparison between the survival of low-dose and standard maximum tolerated dose chemotherapy in palliative patients. The aim of this investigation is to compare the survival outcomes of palliative oncology patients receiving low-dose versus standard dose chemotherapy.
Materials and Methods
Study design
This study was a retrospective cohort study with recruitment from the Ballarat Oncology and Haematology Services (BOHS) electronic medical records. The study was approved by the University of Notre Dame Human Research Ethics Committee (018021S) and the Ballarat Health Services and St John of God Hospital Ballarat Human Research Ethics Committee (LNR/17/BHSSJOG/78). Site-specific approval was obtained by Ballarat Oncology and Haematology Services (LNRSSA/17/BHSSJOG/79).
Study sample
Inclusion criteria included: patients of BOHS, aged >18 years old at diagnosis, cancer diagnosis between 1 January 2004 and 31 December 2009 (inclusive), initial diagnosis of stage III or IV ovarian, stage III or IV lung cancer, stage IV colorectal cancer or any stage pancreatic cancer and treated with chemotherapy. Patients not treated for their malignancy at BOHS were excluded. Previous adjunct therapy for malignancy, including surgery, chemotherapy or radiotherapy did not render participants ineligible.
Data collection
Baseline characteristics including age, cancer type, stage, postcode and health insurance status were collected at diagnosis. The date of diagnosis was the date of definitive diagnosis from a pathology report. Where only a month of diagnosis was available, patients were given a date of diagnosis of the fifteenth day of that month. If only a year of diagnosis was available, the patient was excluded. Staging was checked with radiological reports. Overall survival was considered from date of diagnosis to date of death for all causes of mortality. Where date of death was not available and no follow up had occurred after 1st January 2016, death certificates from the Births, Deaths and Marriages Victoria were retrieved. For those whom a death certificate was not available, they were assumed to be alive and their survival censored to 30th September 2018.
Data analysis
Power calculations using the log rank test suggested 63 patients are required in each group (total 126 patients) to attain adequate power to detect a 20% difference in median survival time between the two groups (α=0•05, α=0•80). Sample size calculations were performed using Power and Sample Size Program.
Low-dose chemotherapy was considered to be less than or equal to 50% of the standard dose of chemotherapy for their specific malignancy. Where patients were assessed as receiving greater than 50% of the standard dose, they were assigned to the standard dose group.
Survival analyses in the two groups were conducted using the Kaplan-Meier method and compared using the log rank test. Two sample t-tests and chi square tests were performed to assess for differences in baseline characteristics in the two groups. The Cox proportional hazards model was then used to create hazard ratios to calculate the size of the treatment difference between each group, which was adjusted for age and sex. Statistical analyses were performed using the SAS statistics software version 9•4 [6].
Results
228 patients were assessed for eligibility with 166 patients included in the study. Reasons for exclusion are summarised in Figure 1. Baseline characteristics for all patients are summarised in Table 1.
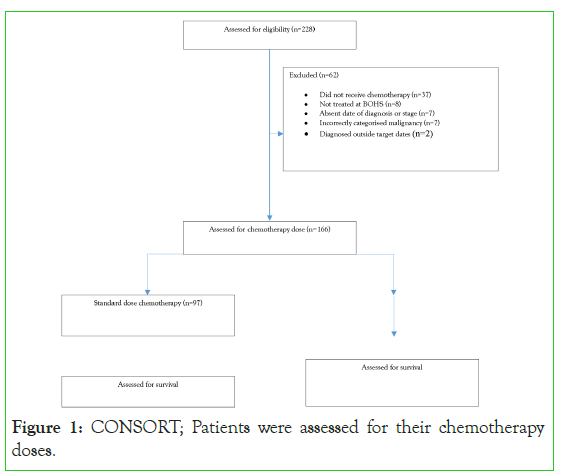
Figure 1:CONSORT; Patients were assessed for their chemotherapy doses.
|
Standard dose | Low dose | P-value |
|---|---|---|---|
Number of patients |
97 | 69 | N/A |
Mean age |
69+/-11·5 | 70+/-11·1 | 0·71 |
Sex:
|
50 47 |
35 34 |
0·92 |
Cancer
|
11 20 24 42 |
7 7 22 33 |
0·29 |
Insurance
|
39% 58% |
24% 45% |
0·48 |
Table 1: Comparison between standard dose and low dose patient baseline characteristics.
A total of 97 (58%) patients received standard-dose chemotherapy while 69 (42%) received low-dose chemotherapy with the two groups well matched for age, sex, cancer type and insurance status (Table 1 and Graph 1).
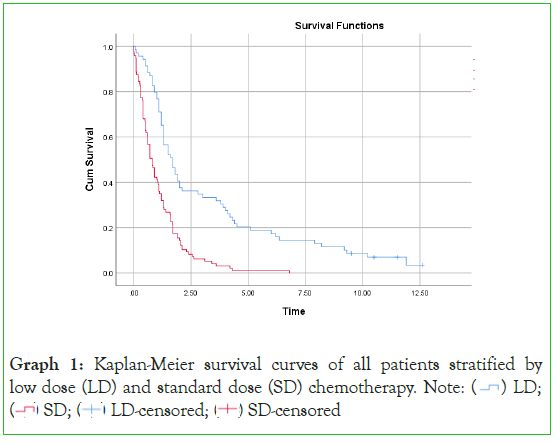
Graph 1: Kaplan-Meier survival curves of all patients stratified by
low dose (LD) and standard dose (SD) chemotherapy.
Graph 2 provides a survival comparison of all patients between the two treatment groups. There was a significant survival benefit of low-dose chemotherapy Graph (log rank=33•76, p<0•00001) with a significant hazard ratio of 0•38 (95% CI 0•38-0•54, p<0•00001) in favour of low-dose therapy. Low-dose chemotherapy provided a median survival of 1•70 years and five-year survival of 20%. Standard dose chemotherapy provided a median survival of 0•80 years and five-year survival of 1%. This data highlights a group of long-term survivors in the low-dose chemotherapy group of 7% who reached greater than ten years survival post diagnosis. There were no survivors in the standard chemotherapy group beyond 6•8 years.
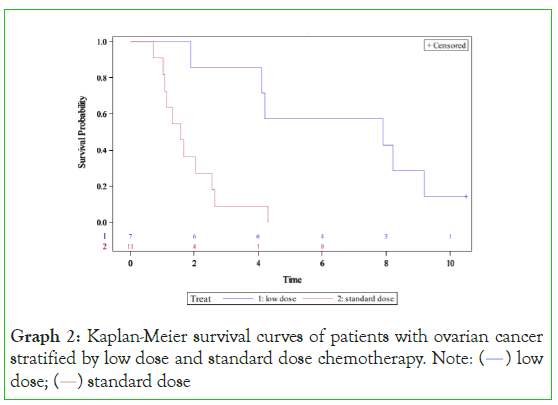
Graph 2: Kaplan-Meier survival curves of patients with ovarian cancer
stratified by low dose and standard dose chemotherapy. Note: (  ) low
dose; (
) low
dose; ( ) standard dose
) standard dose
When stratified by cancer type, most cancers showed a survival benefit with low-dose chemotherapy. In ovarian cancer (Graph 3), low-dose chemotherapy provided a survival benefit when compared to the standard dose group. Low-dose therapy patients had a median survival of 7•90 years compared to a median survival of 1•58 years for standard-dose chemotherapy (p=0•0016). The hazard ratio was 0.15 (95% CI 0•04-0•54, p=0•0047) in favour of low dose therapy. Furthermore, 14% survived at least 10 years in the low-dose group compared to 0% in the standard dose group. This highlights a group of patients with ovarian cancer that survive long term.
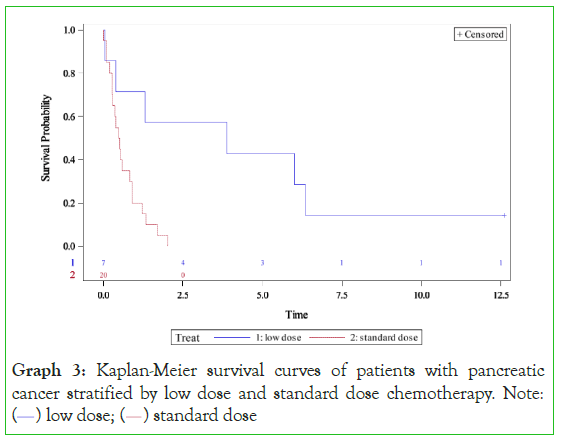
Graph 3: Kaplan-Meier survival curves of patients with pancreatic
cancer stratified by low dose and standard dose chemotherapy. Note:
( ) low dose; (
) low dose; ( ) standard dose
) standard dose
Pancreatic cancer (Graph 4) also illustrated improved survival with low-dose chemotherapy. Patients receiving low-dose treatment had a median survival of 3•90 years compared to a median survival of 0•50 years in standard-dose chemotherapy (p=0•0063). The hazard ratio of 0•2 (95% CI 0•057-0•71, p<0•0001) was in favour of low-dose therapy. Patients receiving low-dose chemotherapy had a five-year survival of 43% and a ten-year survival of 14% versus 0% five-year survival and 0% ten-year survival for the standard chemotherapy group. Although the number of patients was small, the implications of low-dose chemotherapy were dramatic in this poor outcome cancer. The data also indicates that patients receiving standard chemotherapy initially as adjuvant therapy did not have a good long-term survival.
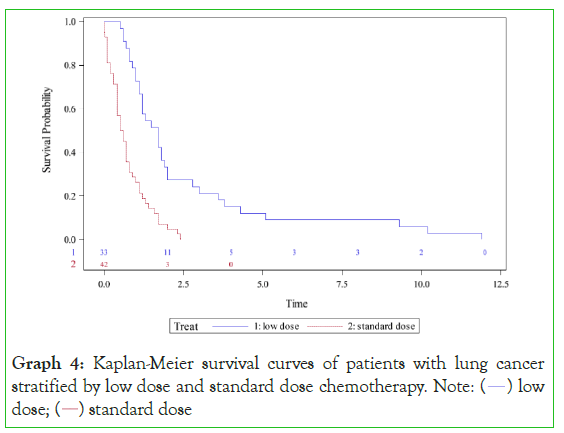
Graph 4: Kaplan-Meier survival curves of patients with lung cancer
stratified by low dose and standard dose chemotherapy. Note: ( ) low
dose; (
) low
dose; ( ) standard dose
) standard dose
Low-dose chemotherapy provided a survival benefit in lung cancer (Graph 5) with a median survival of 1•70 years compared with a median survival of 0•55 years in standard dose chemotherapy (p<0•0001). A significant hazard ratio of 0•3 (95% CI 0•18- 0•50, p<0•0001) was in favour of low-dose therapy. Patients receiving low-dose chemotherapy had a five-year survival of 12% and a ten-year survival of 6% versus 0% five-year survival and 0% ten-year survival for the standard chemotherapy group.
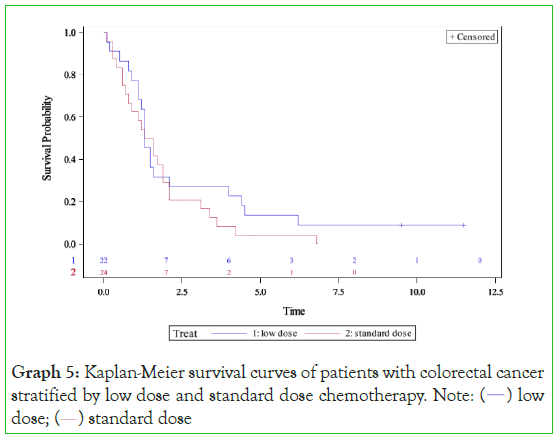
Graph 5: Kaplan-Meier survival curves of patients with colorectal cancer
stratified by low dose and standard dose chemotherapy. Note: (  ) low
dose; (
) low
dose; ( ) standard dose
) standard dose
Colorectal cancer showed similar survival for each group with low- dose chemotherapy providing a median survival of 1•30 years compared to a median survival of 1•76 years with standard-dose chemotherapy (p=0•28). Low-dose chemotherapy provided a five- year survival of 14% and ten-year survival of 9% compared to a five-year survival of 4%, ten-year survival of 0% with standard- dose chemotherapy (p=0•28). A non-significant hazard ratio of 0•72 (95% CI 0•39-1•33, p=0•30) was in favour of low-dose chemotherapy.
Discussion
This novel study is the first to compare overall survival between cohorts of patients receiving low-dose and standard-dose chemotherapy with palliative intent. Across the entire cohort, the results suggest that the use of low-dose chemotherapy is associated with a longer survival compared to standard doses of chemotherapy. This suggests the possibility that reduced myelosuppression in low- dose chemotherapy patients may allow the immune system to play a role in controlling tumour burden, whereby improving survival in advanced disease. Furthermore, these results suggest that the improved treatment tolerability and lower rates of chemotherapy associated side effects with low-dose therapy may result in reduced complications and patients accepting an increased length of palliative therapy.
When comparing five-year survival in low-dose patients from the current study to previously published data from comparable dates, it is evident that the low-dose treatment is at least as effective as what has been published. Our study, when compared to standard studies of advanced ovarian cancer, pancreatic cancer, stage IV colorectal cancer and stage IV lung cancer, illustrated improvements in rates of five-year survival when compared to these cancer groups from the United Kingdom, United States of America and Australia [7- 9]. Furthermore, a cohort of our low-dose chemotherapy patients demonstrated survival beyond 10 years in these groups which is rarely reported in the literature. This indicates the possibility of long-term immune modulating effects of these regimes [7].
Ovarian cancer is the most lethal of the gynaecological malignancies with most patients diagnosed with advanced staging [10]. Survival benefit over supportive care has been shown with low dose combination of decitabine, carboplatin and paclitaxel in recurrent ovarian cancer and multiple case studies have shown extended remission of ovarian cancer using metronomic chemotherapy [11,12]. Low-dose chemotherapy has shown to maintain the quality of life of patients with metastatic ovarian cancer and provide significant symptom improvement [13,14]. Studies have shown the presence of tumour invading lymphocytes in ovarian tumours provide an improved survival in these patients, suggesting the role of the host immune system in tumour management [15]. In the present study, low-dose chemotherapy significantly improved survival when compared to that receiving standard-dose therapy. These results suggest that this particularly vascular malignancy is an ideal candidate for low-dose chemotherapy due to its combined immunomodulatory and anti-angiogenic effect, reducing growth and spread of the tumour [16]. A recent study by Garsed et al. reported that long-term survivors of ovarian cancer (greater than ten years), were more likely to have multiple alterations in genes associated with DNA repair and increased immune reponse genes, futher indicating a role for the immune system in long-term survivors [17].
Pancreatic cancer has a high mortality rate and as such small improvements in survival are important [18]. The current standard of care in non-resectable disease is associated with a significant side effect profile [19]. Numerous preclinical studies have shown the efficacy of metronomic chemotherapy in counteracting growth in pancreatic cancer [18]. However there have been limited and non-definitive clinical studies, demonstrating the strategy to be moderately active and well tolerated with mild toxicity [18]. In the current cohort, low-dose chemotherapy provided a significantly improved survival compared to standard-dose in pancreatic cancer. It is conceivable that with the increased tolerability of low-dose chemotherapy, patients may have been more likely to continue this treatment, allowing greater length of treatment and therefore better control of tumour burden.
Lung cancer is the most common cause of cancer related death with many presentations occurring with locally advanced or metastatic disease [20]. For patients without directly targetable mutations, palliative chemotherapy is associated with a significant side effect profile. Numerous studies have demonstrated the efficacy, safety and low side effect profile of low-dose chemotherapy in lung cancer [21]. In our study, lung cancer patients treated with low-dose chemotherapy experienced significantly increased survival with low-dose therapy compared to standard dose-therapy. Similar to pancreatic cancer, due to the poor prognosis of lung cancer without immunotherapy, we hypothesise that patients in the standard group were more likely to suffer from chemotherapy-related adverse effects and therefore refuse further treatment, resulting in their poor survival compared to their low dose counterparts. The improved survival of stage IV non-small cell lung cancer with immunotherapy appears similar to the survival of patients receiving low-dose chemotherapy, indicating the importance of an active immune system in these patients [22].
Metastatic colorectal cancer showed a non-significant survival benefit for patients with low dose treatment. Despite no significant change in survival, we hypothesise that patients in this group with high levels of microsatellite instability may have improved survival with low dose chemotherapy. These patients, making up 15% of the total number of all colorectal cancers, have improved prognosis [23]. It is only in MSI-High patients that immunotherapy has been shown to provide a mortality benefit [24]. Given one of the major effects of low dose chemotherapy is improved antigen presentation and cellular immunity; it is conceivable that low dose chemotherapy would provide a survival benefit in this subset of patients.
A limitation of this study is that it lacked adequate power to assess the two regimens in individual malignancies although trends demonstrating a survival benefit for the low dose group are evident. Further, while overall the two cohorts were well matched for their baseline characteristics, individual cancers showed some significant differences in these characteristics due to their low sample sizes. We believe these differences may be confounders in the analysis of these individual groups. While hazard ratios were adjusted for patient age and cancer stage, other confounders such as sex and insurance status could conceivably act as effect modifiers in this study.
The retrospective and observational nature of this study provides numerous limitations, namely the inability to assess treatment related side effects and complications. A main benefit of low-dose chemotherapy is its tolerability compared to standard-dose therapy. Furthermore, we did not assess the use of other treatment modalities in these patients. The use of radiotherapy and surgery would be of great significance to patient survival, particularly in surgically resectable malignancies. Future prospective studies would provide insights into this, including if the effect of low-dose chemotherapy is similar in the adjuvant or neoadjuvant setting perioperatively, and if there is a synergistic effect with radiotherapy.
A future direction for this study might be to calculate specific chemotherapy doses received by the patients in each group. At present the use of 50% as an arbitrary Graph for low-dose therapy means that doses close to the 50% Graphs are separated. Theoretically, a dose of 49% and 51% of the standard dose are placed in separate groups while they are clinically equivalent, although many patients in the low-dose group received much less than the 50% cut-off. Calculation of specific chemotherapy doses would allow the titration of doses in the future and an attempt to calculate specific dosages where the balance of survival benefit and treatment tolerability is maximal.
Even if the survival benefit is discounted, the lower treatment toxicity of this treatment is significant in the journey of patients with advanced malignancies that have a life limiting effect. Standard dose chemotherapy is associated with a significant side effect profile and this perception is a common reason for patients to refuse treatment in advanced cancers. Patients often associate chemotherapy with a poorer quality of life. In this cohort, 38% of patients, after discussion with their oncologist, decided to follow a plan of low-dose chemotherapy. This highlights a significant proportion of patients who valued a perceived greater quality of life with reduced treatment related side effects over a possibly increased overall survival. Treatment discussions with patients should involve the opportunity to receive palliative low-dose chemotherapy. This may result in many patients who would previously have refused chemotherapy, choosing to have a lower dose treatment associated with a lower toxicity and potentially leading to increased overall survival.
It is interesting to postulate that low-dose chemotherapy may have similar survival benefits to immune check-point inhibitor therapy, especially in metastatic lung cancer. This study was undertaken in the period between 2006-2010, at a time when immunotherapy for lung cancer was not available but the survival benefits for both groups appear to be similar. The expense of immunotherapy is a significant cost especially for developing countries and further larger studies will need to be undertaken to determine if low-dose chemotherapy may offer similar long-term survival in this cohort of patients, at a much reduced cost.
Conclusion
This study has addressed the paucity in the literature surrounding the use of low dose chemotherapy for palliative stage cancer patients. In this cohort, the provision of low dose chemotherapy was associated with improved survival compared to standard doses of chemotherapy. This novel study specifically suggests a survival benefit with low dose chemotherapy in advanced ovarian, pancreatic and lung cancers. Large randomised controlled trials to adequately assess the effect of low-dose chemotherapy on survival without the presence of confounders. Further studies on the immune system of patients receiving low-dose versus standard doses of chemotherapy, may shed some insights as to the mechanism of improved survival in patients receiving low-dose chemotherapy.
References
- Delgado-Guay MO, Rodriguez-Nunez A, De la Cruz VJ, Frisbee-Hume S. Advanced cancer patients' reported wishes wishes at the end of life: A randomized controlled trial. Support Care Cancer. 2016; 24(10):4273.
[Crossref], [Google Scholar], [Pub Med]
- Berry DA, Ueno NT, Johnson MM, Lei X, Caputo J, Rodenhuis S. High-dose chemotherapy with autologous stem-cell support as adjuvant therapy in breast cancer: overview of six randomized trials. J Clin Oncol. 2011; 29(24):3224-31.
[Crossref], [Google Scholar], [Pub Med]
- Lien K, Georgsdottir S, Sivanathan, L, Chan K, Emmenegger U. Low-dose metronomic chemotherapy: A systematic literature analysis. Eur J Cancer. 2013;49(16):3387-3395.
[Crossref], [Google Scholar], [Pub Med]
- Bertolini F, Paul S, Mancuso P. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63(15): 4342-4346.
[Google Scholar], [Pub Med]
- Bocci G, Tuccori M, Emmenegger U. Cyclophosphamide-methotrexate ‘metronomic’chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol. 2005; 16(8):1243-1252.
[Crossref], [Google Scholar], [Pub Med]
- SAS. SAS 9.4 for Windows. Cary, NC, USA: 2012.
- Menon U, Gentry-Maharaj A, Hallett R. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009; 10(4):327-340.
[Crossref], [Google Scholar], [Pub Med]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64(1):9-29.
[Crossref], [Google Scholar], [Pub Med]
- Thursfield VFH, Karahalios E, Giles G. Cancer in Survival Victoria 2012: Estimates of survival for 2006-2010 (and comparisons with earlier periods). 2012, Melbourne: Cancer Council Victoria. 2012.
- Zhang Y, Mei Q, Liu Y, Li X, Brock MV, Chen M, et al. The safety, efficacy, and treatment outcomes of a combination of low-dose decitabine treatment in patients with recurrent ovarian cancer. Oncoimmunology. 2017; 6(9): e1323619.
[Crossref], [Google Scholar], [Pub Med]
- Aigner J, Bischofs E, Hallscheidt P. Long-term remission in a patient with heavily pretreated, advanced ovarian cancer achieved by bevacizumab and metronomic cyclophosphamide treatment. Anti-cancer Drugs. 2011; 22(10):1030-1033.
[Crossref], [Google Scholar], [Pub Med]
- Rose PG, Roma A. Evidence of extended (> 7 years) activity of bevacizumab and metronomic cyclophosphamide in a patient with platinum-resistant low-grade serous ovarian carcinoma. Anti-cancer Drugs. 2013; 24(9):986-988.
[Crossref], [Google Scholar], [Pub Med]
- Friedlander M, Butow P, Stockler M, Gainford C, Martyn J, Oza A, et al. Symptom control in patients with recurrent ovarian cancer: Measuring the benefit of palliative chemotherapy in women with platinum refractory/resistant ovarian cancer. Int J Gynecol Cancer. 2009; 19(11):S44-S48.
[Crossref], [Google Scholar], [Pub Med]
- Perroud HA, Alasino CM, Rico MJ, Queralt F, Pezzotto SM, Rozados VR, et al . Quality of life in patients with metastatic breast cancer treated with metronomic chemotherapy. Future Oncol. 2016; 12(10):1233-1242.
[Crossref], [Google Scholar], [Pub Med]
- Hwang W-T, Adams SF, Tahirovic E. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol Oncol. 2012; 124(2):192-198.
[Crossref], [Google Scholar], [Pub Med]
- Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998; 153(4):1249-1256.
[Crossref], [Google Scholar], [Pub Med]
- Garsed DW, Pandey A, Fereday S, Kennedy CJ, Takahashi K, Alsop K, et al. The genomic and immune landscape of long-termmsurvivors of high-grade serous ovarian cancer. Nat Genet. 2022; 54:1853-1864.
[Crossref], [Google Scholar], [Pub Med]
- Romiti A, Falcone R, Roberto M, Marchetti P. Tackling pancreatic cancer with metronomic chemotherapy. Cancer Lett. 2017; 394: 88-95.
[Crossref], [Google Scholar], [Pub Med]
- Gupta R, Amanam I, Chung V. Current and future therapies for advanced pancreatic cancer. J Surg Oncol. 2017; 116(1):25-34.
[Crossref], [Google Scholar], [Pub Med]
- Dehua Z, Mingming C, Jisheng W. Meta-analysis of gemcitabine in brief versus prolonged low-dose infusion for advanced non-small cell lung cancer. PloS One. 2018; 13(3):e0193814.
[Crossref], [Google Scholar], [Pub Med]
- Guetz S, Tufman A, Von Pawel J. Metronomic treatment of advanced non-small-cell lung cancer with daily oral vinorelbine–a Phase I trial. OncoTargets Ther. 2017; 10:1081.
[Crossref], [Google Scholar], [Pub Med]
- Mok TSK, Wu Y-L, Kudaba I. Pembroluzimab versus chemotherapy for previously untreated, PD-L-1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019; 393:1819-1830.
[Crossref], [Google Scholar], [Pub Med]
- Kim SH, Shin SJ, Lee K. Prognostic value of mucinous histology depends on microsatellite instability status in patients with stage III colon cancer treated with adjuvant FOLFOX chemotherapy: a retrospective cohort study. Ann Surg Onc. 2013; 20(11):3407-3413.
[Crossref], [Google Scholar], [Pub Med]
- Le DT, Uram JN, Wang H. PD-1 blockade in tumors with mismatch-repair deficiency. N Eng J Med. 2015; 372(26):2509-2520.
[Crossref], [Google Scholar], [Pub Med]
Citation: Dind A, Kannourakis R, Kannourakis G, Sterbova J (2023) Low-Dose Palliative Chemotherapy Compared to Standard-Dose Chemotherapy for Poor-prognosis Cancers Improves overall Survival - a Single Institution Retrospective Study. J Carcinog Mutagen. 14:405
Copyright: © 2023 Dind A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


