Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2025) Volume 16, Issue 5
Incidence of Red Blood Cell Alloimmunisation in Transfused Myelodysplastic Syndromes Patients: A Systematic Review and Meta-Analysis
Vera Abba and Denise E. Jackson*Received: 20-Nov-2025, Manuscript No. JBDT-25-30334; Editor assigned: 24-Nov-2025, Pre QC No. JBDT-25-30334 (PQ); Reviewed: 08-Dec-2025, QC No. JBDT-25-30334; Revised: 15-Dec-2025, Manuscript No. JBDT-25-30334 (R); Published: 22-Dec-2025, DOI: 10.35248/2155-9864.25.16.630
Abstract
Background: Red Blood Cell (RBC) alloimmunisation represents a major complication in transfused Myelodysplastic Syndrome (MDS) patients. Frequent transfusion dependency increases the risk of alloantibody formation, while Hypomethylating Agent (HMA) therapy may have immunomodulatory effects that influence this outcome. This meta-analysis aimed to evaluate the incidence of RBC alloimmunisation in transfused MDS patients and assess the impact of HMA therapy.
Methods: A systematic review and meta-analysis was conducted according to PRISMA guidelines. Relevant studies were identified through PubMed, Scopus, Embase, and Google Scholar databases. Study quality was assessed using the STROBE checklist. Pooled Odds Ratios (ORs) with 95% Confidence Intervals (CIs) were calculated for alloimmunisation outcomes using a random-effects model in RevMan 5.4.1.
Results: Seven studies published between 2001 and 2024 met the inclusion criteria. The pooled analysis demonstrated a significantly higher risk of RBC alloimmunisation in transfused MDS patients compared with non-MDS controls (OR=1.68; 95% CI 1.13-2.51; P=0.01). A higher transfusion burden was associated with an even greater risk (OR=3.43; 95% CI 1.83-6.43; P=0.0001). HMA-treated MDS patients showed a lower risk compared with untreated controls, though this trend did not reach statistical significance (OR=0.42; 95% CI 0.17-1.03; P=0.06).
Conclusion: Transfused MDS patients are at increased risk of RBC alloimmunisation, particularly with higher transfusion exposure. Although not statistically significant, HMA therapy showed a trend toward reducing alloimmunisation, suggesting a potential immunomodulatory effect that requires further investigation. These findings may inform transfusion strategies and guide future research.
Keywords
Myelodysplastic syndrome; RBC alloimmunisation; Hypomethylating agents; Transfusion; Meta-analysis.
Introduction
Haematological malignancies
Haematological malignancies are clonal neoplasms of myeloid or lymphoid lineages arise from disrupted haematopoiesis, frequently resulting in peripheral blood cytopenias requiring transfusion support [1]. They are broadly classified as leukaemia, lymphoma, or multiple myeloma and are characterised by uncontrolled proliferation of abnormal cell clones, leading to systemic complications and impaired blood cell production [2]. Among myeloid disorders, Acute Myeloid Leukaemia (AML) and Chronic Myelomonocytic Leukaemia (CMML) often necessitate transfusion, while lymphoid malignancies, including Acute Lymphoblastic Leukaemia (ALL) and Multiple Myeloma (MM) also commonly require red cell or platelet support [3,4]. Each neoplasm exhibits distinct genetic, clinical, and prognostic features, highlighting their significance for transfusion management during therapy and supportive care [5].
Myelodysplastic syndrome
Myelodysplastic Syndrome (MDS) is a diverse group of clonal myeloid neoplasms driven by somatic mutations in Hematopoietic Stem Cells (HSC), resulting in accumulation of immature blood cells in the Bone Marrow (BM) [6]. It is characterised by ineffective haematopoiesis, dysplasia of one or more myeloid lineages, and persistent cytopenias that manifest as anaemia, leukopenia, or thrombocytopenia [7]. MDS is among the most commonly diagnosed myeloid neoplasms, predominantly affecting individuals over 70 years of age [8].
As disease progress, there is a high risk of transformation to AML or marrow failure, complicating the clinical course [9]. Patients are classified into Lower-Risk (LR) and Higher-Risk (HR) categories, each with varied therapeutic objectives: LR-MDS management aims to improve cytopenias through supportive interventions, whereas HR-MDS measures focus on controlling cytopenias and delaying progression to AML [10].
Management and therapeutic approaches
Management of haematological malignancies, including MDS, primarily involves lifelong supportive care transfusion with platelets or RBC units to improve symptoms and prolong survival [11,12]. As most MDS patients present with symptomatic anaemia, transfusions remain a fundamental therapy. Studies report that around 82% of MDS patients require blood support, with up to 42% developing long-term transfusion dependence [6,13]. Allogeneic transplantation of stem cell or bone marrow is the only curative option but is often limited by patient age [8,11]. Beyond transfusion support, Disease-Modifying Therapies (DMTs) are also employed, including Hypomethylating Agents (HMAs), immunomodulatory agents, erythropoiesis-stimulating agents, and intensive chemotherapy [14]. Despite differences in disease pathophysiology and prognosis among AML, CMML, and MDS therapeutic options for older patients remain restricted, often leading to reliance on HMAs such as azacitidine or decitabine [3].
Azacitidine (AZA) is a demethylating agent and first-line therapy for MDS and may serve as an alternative to intensive chemotherapy in older AML patients [15]. It modifies DNA methylation in haematopoietic stem and progenitor cells, improves peripheral blood counts, and delays AML progression in a subset of MDS patients [16]. AZA is approved for HR-MDS, AML with >20% blasts in transplantation-ineligible patients, and CMML with 10-29% blasts, based on clinical trial efficacy [3]. While HMAs have improved outcomes in elderly patients, these malignancies remain incurable. Clinical responses to AZA are often unpredictable; however, emerging evidence suggests HMAs may reduce alloimmunisation risk in MDS, potentially improving transfusion outcomes [14,15].
Transfusion-associated complications
Patients with long-term transfusion dependence are at risk of complications including RBC alloimmunisation, iron overload, and delayed haemolytic transfusion reactions, contributing to adverse clinical outcomes [6,13]. Among these, RBC alloimmunisation stand out as the most clinically significant, particularly in haematological malignancies and MDS patients receiving repeated transfusions [17]. Alloimmunisation complicate transfusion therapy by inducing antibodies against donor RBC antigens or can even drive RBC autoantibodies, limiting the availability of compatible blood [18]. These challenges lead to extensive compatibility testing, often delaying access to blood products, reducing supply, and increasing healthcare costs [13,18]. Leukaemia patients frequently require regular transfusions due to persistent anaemia, which increase the risk of RBC alloantibody formation [19]. Jawish, et al. reported an alloimmunisation incidence of 11% in a cross-sectional study of 100 multi-transfused leukaemia patients, with anti-Kell antibodies being the most frequent [4]. Similarly, Leisch, et al. found a 10.8% incidence in 184 patients with myeloid neoplasms receiving AZA [3]. Rates in transfused MDS patients range from 15-32%, though recent studies from Australia and Western countries report broader variability of 9-59% [13,20]. Most alloantibodies target Rh and Kell antigen systems [18,20].
Scope of the review
Current literature has investigated the risk of RBC alloimmunisation in patients with haematological malignancies, with particular focus on transfused MDS patients. Reported incidence rates vary considerably, and risk factors such as treatment with azacitidine, and the cumulative number of RBC Units (RBCU) transfused may contribute to this variability. However, findings across studies are inconsistent with limited literature reporting effect of azacitidine therapy on alloimmunisation. Some report reduced alloimmunisation in azacitidine-treated patients [14,15]. Others demonstrate similar or higher incidence compared to untreated cohorts [3]. Several studies indicate a correlation with transfusion burden, showing higher rates with increasing RBCU exposure, whereas others found no clear association [21]. In contrast, some conclude that MDS patients are not at greater risk than non-MDS transfused patients, while a few suggest higher overall rates in MDS [22]. These conflicting findings highlight the need for a systematic review to evaluate the rate of RBC alloimmunisation in haematological malignancy patients, focusing on transfused MDS patients. Particularly, this review will compare the incidence in transfused MDS patients versus transfused non-MDS recipients as it remains unclear whether the high rate of alloantibodies is due to repeated transfusions in haematological malignancies or whether MDS itself represents an independent risk factor [22]. Comparisons will include AML, CMML, and other myeloid neoplasms to assess the impact of transfusion burden and estimate alloimmunisation rates after multiple RBC transfusions. Additionally, the review will examine whether HMAs therapy reduces alloimmunisation risk in MDS patients compared to untreated individuals receiving supportive care. By clarifying these associations, this research aims to provide strong evidence base for transfusion management and risk mitigation in MDS.
Primary objective
In this research article, it is hypothesised that transfused MDS patients may have a higher incidence of RBC alloimmunisation compared with transfused non-MDS recipients. The primary research question, framed according to the Patient, Intervention, Comparison and Outcome (PICO) framework, is: Do patients with haematological malignancies (population) including transfused MDS patients (intervention) have an increased incidence risk of RBC alloimmunisation (Outcome) compared to transfused non-MDS recipients (comparator)? [23]
Secondary objective
Applying the same PICO framework, the secondary research question is: Do patients with haematological malignancies (population) including transfused MDS patients (intervention) have an increased risk of alloimmunisation per number of RBC units transfused (outcome) compared to transfused non-MDS recipients (comparator)?
Tertiary objective
It is further hypothesised that HMA therapy may reduce the incidence of RBC alloimmunisation in MDS patients compared with those untreated. Do patients with haematological malignancies (population) including MDS patients receiving HMA therapy (intervention) reduce RBC alloimmunisation incidence rate (outcome) compared to those untreated with HMA therapy receiving supportive care (comparator)?
Methods
Study design
This systematic review and meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24]. The research article aimed to identify and analyse studies investigating the risk of RBC alloimmunisation among patients with haematological malignancies, including MDS. It compared the incidence and transfusion burden in transfused MDS patients with non-MDS populations comprising other haematological malignancies, particularly AML and CMML. Additionally, the analysis assessed the impact of HMA therapy on alloimmunisation rates in MDS patients.
Search strategy
Electronic searches were performed in PubMed, Embase, Scopus, and Google Scholar, without restrictions on publication year. Search terms included: “alloimmunization” OR “alloimmunisation” AND “red blood cell” OR “RBC” AND “myelodysplastic syndrome” OR “MDS” OR “haematological malignancies” OR “hematological malignancies” AND “transfusion”. Both British and American spelling variants were included to maximise coverage. Reference lists of eligible studies were manually reviewed to identify any additional relevant articles.
Eligibility criteria
Records retrieved from the database searches were exported to EndNote and imported into Covidence for duplicate removal and screening. Titles and abstracts were assessed to exclude irrelevant articles, followed by full-text screening based on inclusion and exclusion criteria. Articles were eligible if they reported alloimmunisation, with or without RBCU data, in transfused MDS patients and included a comparator group of other haematological malignancies or assessed the effect of HMA therapy. Only adult populations (≥ 16 years) and observational or interventional study designs were considered. Studies consisting of case reports, reviews, and abstracts, non-English or inaccessible publications were excluded, as were studies that lacked relevant outcome data or a comparator group.
Data extraction
Data from eligible articles was independently extracted using a standardized form. Extracted information included primary author, year of publication, country, study design, study period, sample size and incidence of the measured outcomes. Key parameters collected for subsequent meta-analysis included Alloimmunisation (Al) events, Red Blood Cell Unit (RBCU) transfusion data, and Hypomethylating Agent (HMA) use.
Quality assessment
The quality of eligible studies was assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [25]. Publication bias was evaluated visually in the meta-analysis.
Statistical analysis
Using Review Manager (RevMan) version 5.4.1, Odds Ratios (OR) and 95% Confidence Intervals (CI) for dichotomous outcomes were estimated via the Mantel-Haenszel method within a random-effects model framework [26]. Heterogeneity was evaluated using the I2 statistic and Chi2 test, with P<0.05 considered significant. Forest plots were generated for each outcome, including alloimmunisation in MDS versus non-MDS controls, per RBC unit, and in HMA-treated versus untreated MDS patients. Risk of bias was visually assessed using colour-coded tables in RevMan software.
Results
Study selection
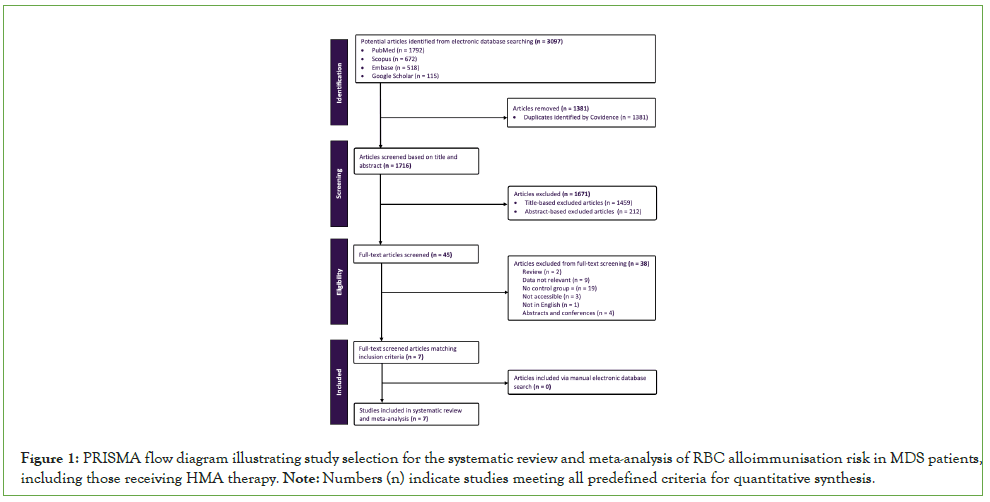
The database search retrieved 3,097 articles across PubMed, Scopus, Embase, and Google Scholar as shown in (Figure 1). After removing 1,381 duplicates in Covidence, 1,716 articles were subjected to title and abstract screening, of which 1,671 were excluded based on eligibility criteria. Reasons for exclusion at the full-text review included studies with insufficient outcome data, irrelevant patient populations, or lack of comparison groups relevant to MDS and alloimmunisation. The remaining 45 full-text articles were assessed for eligibility, and seven articles met all inclusion criteria and were included in the meta-analysis. No additional studies were identified through manual searching.
Figure 1: PRISMA flow diagram illustrating study selection for the systematic review and meta-analysis of RBC alloimmunisation risk in MDS patients, including those receiving HMA therapy. Note: Numbers (n) indicate studies meeting all predefined criteria for quantitative synthesis.
Study characteristics
As demonstrated, the seven studies analysed in this review were published between 2001 and 2024 and conducted across Europe (Austria, Spain, and Netherlands), Israel, and China (Table 1). Most studies employed retrospective cohort designs, with one multicentre case-control study and one observational study. Sample sizes varied widely, ranging from small cohorts, such as in Stiegler, et al. with 42 patients, to larger cohorts, such as Zalpuri, et al. with 24,063 patients) [12,19]. Patient demographic details, including age and sex, were variably reported, limiting pooled analyses of these characteristics. Follow-up periods were inconsistently reported, preventing detailed comparisons of observation duration. Of the seven studies, five assessed RBC alloimmunisation risk in transfused MDS patients compared with non-MDS controls, three specifically assessed incidence based on the number of RBC units transfused, and three examined the impact of HMAs therapy on alloimmunisation risk among MDS patients [12-14]. Collectively, these studies allowed comparisons of alloimmunisation risk between MDS and non-MDS transfused patients, as well as between HMA-treated and untreated MDS patients [15,19,22].
| Study | Country | Design | Period | Sample size | Parameter measured |
|---|---|---|---|---|---|
| Ortiz, et al. 2017 [15] | Spain | Retrospective | 1995-2014 | 209 | Al, RBCU, HMA |
| Leisch, et al. 2017 [3] | Austria | Retrospective | 2009-2015 | 184 | Al |
| Rozovski, et al. 2015 [22] | Israel | Retrospective | 2012-2015 | 112 | Al, RBCU |
| Evers, et al. 2017 [19] | Netherlands | Case-control | 2005-2013 | 24,063 | Al |
| Rozema, et al. 2022 [14] | Netherlands | Observational | 2005-2017 | 233 | HMA |
| Wang, et al. 2024 [13] | China | Retrospective | 2012-2022 | 103 | HMA |
| Stiegler, et al. 2001 [12] | Austria | Retrospective | - | 42 | Al, RBCU |
Note:“-” indicates data not reported in the study. Abbreviations: Al: Alloimmunisation; RBCU: Red Blood Cell Unit; HMA: Hypomethylating Agent.
Table 1: Study characteristics included in the systematic review and meta-analysis of RBC alloimmunisation in MDS patients.
The data included in this meta-analysis are presented in (Table 2). Categorical outcomes were reported as the number of alloimmunisation events relative to the study population (n), with proportional rates calculated to allow cross-study comparisons. The table summarises both absolute counts and proportional rates of alloimmunisation across the included studies, including subgroup analyses of patients receiving multiple RBC unit transfusions and those treated with HMA therapy.
| Study | Al events MDS /total | Al events non-MDS /total | allo MDS /RBCU | allo non-MDS /RBCU | HMA treated /total | HMA untreated /total |
|---|---|---|---|---|---|---|
| Ortiz, et al. 2017 [15] | 19/151 | 5/58 | 19/106 | 5/106 | 1/43 | 23/166 |
| Leisch, et al. 2017 [3] | 11/ 70 | 9/95 | - | - | - | - |
| Rozovski, et al. 2015 [22] | 15/56 | 7/56 | 15/54 | - | - | - |
| Evers, et al. 2017 [19] | 18/64 | 62/279 | - | 7/58 | - | - |
| Rozema, et al. 2022 [14] | - | - | - | - | 3/63 | 18/170 |
| Wang, et al. 2024 [13] | - | - | - | - | 2/29 | 6/74 |
| Stiegler, et al. 2001 [12] | 9/42 | 3/28 | 9/96 | - | - | - |
| Pooled incidence | 18.8% | 16.7% | 16.8% | 3/97 | 4.4% | 11.5% |
Note: Values originally reported as median (number of units {range}) were converted to mean values for consistency [27]; “-” indicates data not reported in the study. Abbreviations: Al: Alloimmunisation; MDS: Myelodysplastic Syndrome; Non-MDS: Other Haematological Malignancies; Allo: Alloimmunised; RBCU: Red Blood Cell unit; HMA: Hypomethylating Agent.
Table 2: Incidence of RBC alloimmunisation in transfused MDS vs. Non-MDS patients: Data extracted from included studies.
Study quality assessment
The reporting quality of the included studies was assessed using the STROBE checklist in (Table 3). Overall, most studies fulfilled the majority of STROBE criteria, reflecting strong reporting standards and supporting their inclusion as high-quality evidence. Some studies partially met certain items, such as incomplete reporting of eligibility criteria, statistical methods, or handling of missing data [12]. In a few cases, key elements of study design, including participant characteristics and discussion of limitations were not fully described or were reported only indirectly or outside the main text [12,19].
| Ortiz, et al. 2017 [15] | Leisch, et al. 2017 [3] | Rozovski, et al. 2015 [22] | Evers, et al. 2017 [19] | Rozema, et al. 2022 [14] | Wang, et al. 2024 [13] | Stiegler, et al. 2001 [12] |
|---|---|---|---|---|---|---|
| Title and Abstract: Provides informative and balanced summary | ||||||
| Y | Y | Y | Y | Y | Y | Y |
| Introduction: Explains scientific background with rationale of the study | ||||||
| Y | Y | Y | Y | Y | Y | Y |
| Methods: Eligibility criteria described | ||||||
| Y | Y | Y | Y | Y | Y | N |
| Methods: Statistical methods described | ||||||
| Y | Y | Y | Y | Y | Y | N |
| Results: Participant characteristics reported | ||||||
| Y | Y | Y | Y | Y | Y | N |
| Results: Outcomes and main results reported | ||||||
| Y | Y | Y | Y | Y | Y | Y |
| Discussion: Summarises key results and discusses limitations or bias | ||||||
| Y | Y | Y | P | P | Y | P |
Notes: Y: Criterion fully met; P: Criterion partially met or information unclear; N: Criterion not met or not reported.
Table 3: Assessment of reporting quality of included studies using STROBE checklist.
Meta-Analysis
Transfused MDS vs. Non-MDS
For the meta-analysis of RBC alloimmunisation incidence in transfused MDS patients compared with non-MDS controls, 5 studies were included, presented in (Figure 2A). The pooled OR was 1.68 (95% CI: 1.13 to 2.51; P=0.01), indicating that MDS patients have a significantly higher risk of RBC alloimmunisation. The absence of heterogeneity (I2=0%) supports the reliability and consistency of the pooled results.
Figure 2: Forest plots of meta-analysis comparing the incidence of RBC alloimmunisation in different patient groups. Note: A) Transfused MDS vs. non-MDS patients; B) Alloimmunised MDS vs. alloimmunised non-MDS patients per RBCU; C) HMA-treated vs. untreated MDS patients. Data were analysed using two-way proportion data, with odds ratios calculated via Mantel-Haenszel statistics under a random-effects model. Statistical significance was assessed using overall P-values, and heterogeneity was evaluated by I².
Alloimmunised MDS vs. Non-MDS per RBCU
For the meta-analysis evaluating the association between transfusion burden and alloimmunisation risk in MDS versus non-MDS patients, 3 studies were included, presented in (Figure 2B). The pooled OR was 3.43 (95% CI, 1.83 to 6.43, P=0.0001), indicating a significantly increased risk of alloimmunisation with higher numbers of RBCU transfused. The I2 value of 0% indicates minimal heterogeneity among the included studies.
HMA-treated vs. HMA-untreated MDS
For the meta-analysis assessing the effect of HMA therapy on alloimmunisation incidence in MDS patients, 3 studies were included, presented in (Figure 2C). The pooled OR was 0.42 (95% CI, 0.17 to 1.03, P=0.06), indicating no statistically significant difference in alloimmunisation risk between HMA-treated and untreated patients. Consistency across studies was high, with I2 of 0%, indicating minimal variability in effect estimates.
Risk of bias
The overall risk of bias across all studies was moderate. Performance bias was high in most studies due to a lack of blinding, while detection bias was largely unclear due to inconsistent reporting of antibody screening procedures and outcome assessment methods. Allocation concealment and handling of missing data varied across studies. Reporting and other biases were generally low. Despite these limitations, the consistent direction of effect and absence of heterogeneity (I2=0%) support the reliability of the pooled findings.
Discussion
Systematic review and meta-analysis summary
This systematic review and meta-analysis evaluated the risk of RBC alloimmunisation in haematological malignancy groups, focusing on transfused MDS patients versus non-MDS controls for overall incidence and transfusion burden, with an additional analysis assessing the effect of HMA therapy in treated versus untreated MDS patients. The meta-analysis revealed a significantly higher risk of alloimmunisation in transfused MDS patients, with risk further increasing in those receiving higher transfusion burdens. However, there was a non-significant trend toward reduced alloimmunisation with HMA therapy.
Alloimmunisation in MDS vs. non-MDS
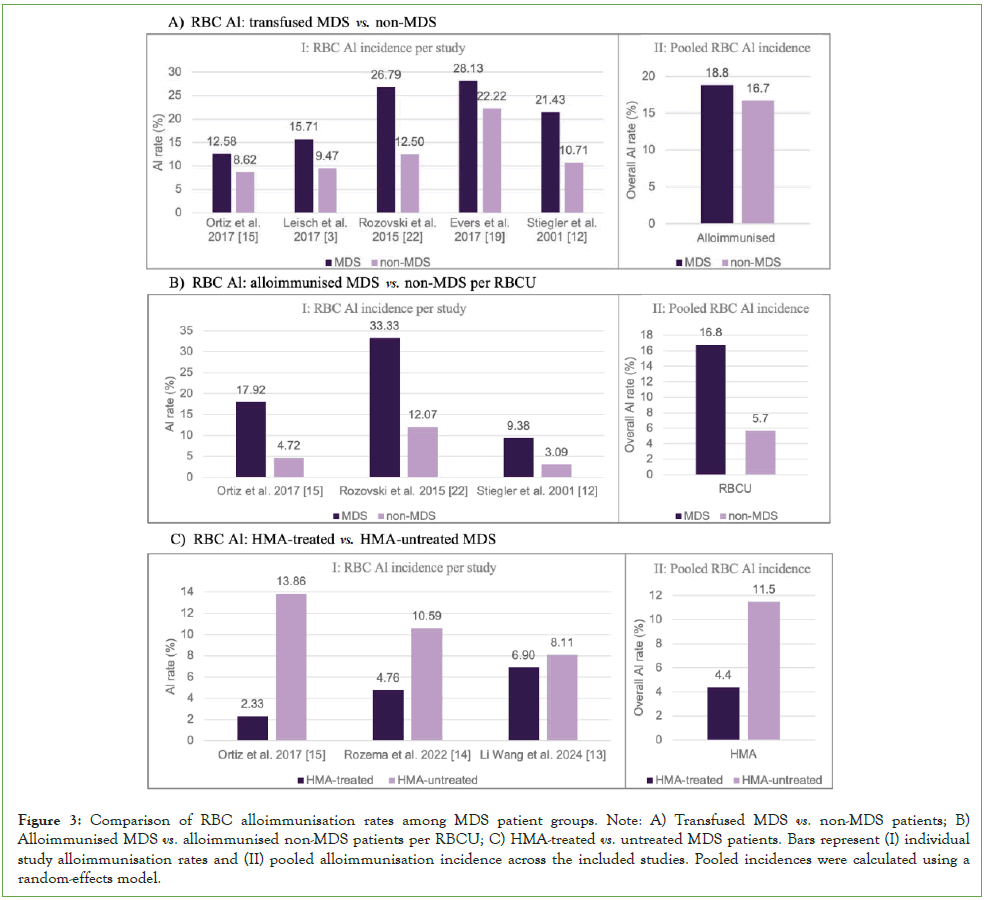
As shown, the overall incidence of RBC alloimmunisation was 18.8% in MDS patients compared to 16.7% in non-MDS controls (Figure 3A). The meta-analysis yielded an odds ratio of 1.68 (95% CI of 1.13 to 2.51, P=0.01), demonstrating a significantly higher risk of alloimmunisation in MDS patients than in non-MDS individuals. This increased risk may be explained by immune dysregulation associated with the disease and cumulative antigenic exposure resulting from chronic transfusion dependence.
Rozovski, et al. reported the largest difference in alloimmunisation rates (26.8% vs. 12.5%), followed by Zalpuri, et al. (28.1% vs. 22.2%) and Stiegler, et al. (21.4% vs. 10.7%) [12, 19, 22]. Smaller differences were reported by Leisch, et al. (15.7% vs. 9.5%) and Ortiz, et al. (12.6% vs. 8.6%), likely reflecting variations in transfusion protocols, antibody screening frequency, and follow-up duration [3, 15]. Despite these variations, the overall direction of effect remained consistent, confirming a higher alloimmunisation risk in MDS patients.
Figure 3: Comparison of RBC alloimmunisation rates among MDS patient groups. Note: A) Transfused MDS vs. non-MDS patients; B) Alloimmunised MDS vs. alloimmunised non-MDS patients per RBCU; C) HMA-treated vs. untreated MDS patients. Bars represent (I) individual study alloimmunisation rates and (II) pooled alloimmunisation incidence across the included studies. Pooled incidences were calculated using a random-effects model.
Impact of transfusion frequency
The overall incidence of RBC alloimmunisation relative to the number of transfused units was 16.8% in MDS patients compared to 5.7% in non-MDS controls (Figure 3B). The meta-analysis produced an odds ratio of 3.43 (95% CI, 1.83 to 6.43; P=0.0001), demonstrating a strong and statistically significant association between higher transfusion exposure and increased alloimmunisation risk. Although the confidence interval indicates some variability across studies, the association remained reliable, suggesting that alloimmunisation risk increases with the number of transfused RBCU.
Rozovski, et al. observed the highest rates (27.8% vs. 12.1%), followed by Ortiz, et al. reporting 17.9% in MDS compared to 4.7% in non-MDS, showing a marked difference consistent with the overall analysis [15, 22]. Stiegler, et al. also reported higher rates in MDS patients (9.4% vs. 3.1%), although the absolute values were lower due to smaller cohort size. These differences may reflect variations in transfusion thresholds, antibody testing sensitivity, and patient characteristics. Despite these minor discrepancies, the consistent direction of effect across studies supports the association between transfusion frequency and RBC alloimmunisation.
Impact of HMA therapy
The overall incidence of RBC alloimmunisation was 4.4% in HMA-treated MDS patients compared to 11.5% in untreated controls (Figure 3C). The meta-analysis produced an odds ratio of 0.42 (95% CI, 0.17 to 1.03; P=0.06), indicating no statistically significant difference. However, a clear trend toward reduced alloimmunisation with HMA therapy was observed, suggesting a possible protective effect. The borderline P-value may reach statistical significance with larger cohorts or additional studies.
Rozema, et al. reported a lower alloimmunisation rate in HMA-treated patients (4.8%) compared with untreated controls (10.6%). Similarly, Wang, et al. observed reduced rates among those receiving HMA therapy (6.9% vs. 8.1%) [13]. Ortiz, et al. also found a lower incidence (2.3% vs. 13.9%), although this finding was likely influenced by the small size of the treated cohort [15]. The consistency in effect direction across studies and minimal heterogeneity suggest that the observed trend is reliable supporting a potential immunomodulatory role of HMA therapy in reducing alloantibody formation, although low event counts limit definitive conclusions.
Variations in reported rates
The differences in alloimmunisation rates observed between studies may reflect variations in study design, patient characteristics, and transfusion practices. For instance, Rozovski, et al. reported substantially higher rates among MDS patients (27-33%) compared to non-MDS controls (12-13%), possibly due to higher transfusion dependence and cumulative antigen exposure [22]. Differences in red cell antigen-matching policies, antibody screening frequency, and follow-up duration may have further influenced detection rates. In studies evaluating the impact of HMA therapy, Ortiz, et al. (2.33% vs. 13.86%) and Wang, et al. (6.60% vs. 8.11%) observed differing effects, potentially due to cohort size, disease severity, and the immune modulatory properties of HMA therapy, which may reduce alloantibody formation [15]. Untreated patients, receiving only supportive care, were generally more transfusion-dependent, increasing their risk. Regional and ethnic differences in antigen distribution between donors and recipients may also contribute to interstudy variability.
Immunopathogenesis of RBC alloimmunisation
The increased risk of RBC alloimmunisation in MDS patients is likely driven by underlying immune dysregulation, ineffective haematopoiesis, and chronic inflammation. Ineffective haematopoiesis and clonal expansion impair immune tolerance, while abnormal function of T-cell and antigen-presenting cell promotes excessive immune activation against transfused donor antigens [28]. Impaired regulatory T-cell activity and elevated pro-inflammatory cytokines further enhance antigen presentation and antibody production [29]. Additionally, chronic transfusion dependence exposes MDS patients to multiple foreign RBC antigens, increasing the risk of sensitisation and alloantibody formation. Individual differences in immune response intensity may also contribute to the variability in alloimmunisation risk observed among patients.
In contrast, HMAs such as azacitidine and decitabine modulate immune function through epigenetic reprogramming, including changes in DNA methylation that influence T-cell activation, cytokine secretion, and antigen presentation [30]. These agents have been shown to expand regulatory T-cell populations and suppress pro-inflammatory pathways, potentially reducing production of alloantibody [30]. However, these immunosuppressive effects may temporarily suppress immune activity, which may complicate interpretation of their true impact on alloimmunisation risk [31]. A clearer understanding of these mechanisms is critical for optimising transfusion strategies and identifying patients who may benefit from targeted antigen-matching or immunomodulatory approaches.
Clinical significance
These findings have important clinical implications for transfusion management in MDS patients. The higher risk of RBC alloimmunisation highlights the need for strategic transfusion planning, as alloantibody formation can delay treatment, complicate crossmatching, and increase the risk of haemolytic reactions. The observed link between cumulative transfusion exposure and increased alloimmunisation risk highlights the value of minimising unnecessary transfusions and adopting preventive measures. Approaches such as phenotype or genotype matching, consistent antibody monitoring, and careful documentation of transfusion history may substantially reduce sensitisation risk. While HMA therapy did not show statistically significant reductions, the consistent trend toward lower incidence suggests a potential protective role of HMAs therapy that require further investigations. This trend aligns with the principles of precision transfusion medicine, integrating clinical factors to guide donor selection and antibody monitoring, thereby improving compatibility and patient outcomes in MDS.
Future scope
Further research should aim to validate the potential protective role of HMA therapy and to identify underlying mechanisms linking immune modulation to alloantibody development. Well-designed prospective, multicentre studies with standardised definitions of alloimmunisation and transfusion load are needed to strengthen the evidence base. Future investigations should also explore additional risk factors such as disease subtype, gender, immune profile, and treatment duration to enable better risk stratification and tailored transfusion management. A deeper understanding of the interaction between disease biology, transfusion exposure, and therapy will help establish refined transfusion guidelines for MDS and other transfusion-dependent haematological disorders. If confirmed, this trend may inform future transfusion strategies, potentially leading to reduced sensitisation rates and improved long-term outcomes for chronically transfused patients.
Conclusion
This meta-analysis demonstrated that MDS patients undergoing chronic transfusion therapy experience a significantly higher risk of RBC alloimmunisation than non-MDS recipients. The risk appears closely linked to transfusion intensity, highlighting the need for early intervention and preventive transfusion strategies. Although the potential association between HMA therapy and reduced alloimmunisation did not reach statistical significance, the consistent direction of effect across studies suggests a biologically meaningful trend requiring further investigation. These findings emphasise the need for implementing targeted transfusion practices, including extended antigen matching and regular antibody monitoring to minimise sensitisation risk. Incorporating these strategies into standard haematology and transfusion practices may help optimise patient safety and inform future policy development aimed at achieving precision- based transfusion care in MDS.
Limitations
Several limitations need to be considered when interpreting these results. The small number of included studies in some analyses, particularly for HMA-treated cohorts, along with variability in study design, sample size, and data quality, may have introduced bias. Differences in reporting of transfusion load, antibody specificity, and alloimmunisation definitions further contributed to heterogeneity. The conversion of medians to means for pooled analysis represents an approximation that may affect quantitative accuracy, therefore, derived means and total RBCU estimates should be interpreted with caution. Most studies were retrospective, potentially underestimating true alloimmunisation incidence. Performance and detection biases were frequently unclear due to the lack of blinding and inconsistent antibody follow-up protocols. Publication bias cannot be excluded, as smaller or negative studies may be underrepresented. Despite these limitations, acknowledging them provides valuable guidance for designing reliable, prospective, multicentre investigations in the future.
Acknowledgement
Mrs Sue Quiring is gratefully acknowledged for providing guidance of this meta-analysis.
Conflict of Interest
The authors declare no conflicts of interest in relation to this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Karagianni P, Giannouli S, Voulgarelis M. From the (Epi) genome to metabolism and vice versa; Examples from hematologic malignancy. Int J Mol Sci. 2021;22(12):6321.
[Crossref] [Google Scholar] [PubMed]
- Vakili-Samiani S, Jalil AT, Abdelbasset WK, Yumashev AV, Karpisheh V, Jalali P, et al. Targeting Wee1 kinase as a therapeutic approach in Hematological Malignancies. DNA Repair (Amst). 2021;107:103203.
[Crossref] [Google Scholar] [PubMed]
- Leisch M, Weiss L, Lindlbauer N, Jungbauer C, Egle A, Rohde E, et al. Red blood cell alloimmunization in 184 patients with myeloid neoplasms treated with azacitidine-A retrospective single center experience. Leuk Res. 2017;59:12-19.
[Crossref] [Google Scholar] [PubMed]
- Jawish TA, Hessen HM, Alshafeea MA, Mohamedahmed KA, Ahmed EA, Modawe GA, et al. Red cell alloimmunization in repeatedly transfused Sudanese patients with leukemia in northern Sudan. Asian Pac J Cancer Prev. 2023;24(1):21.
[Crossref] [Google Scholar] [PubMed]
- Stanworth SJ, Estcourt LJ. When to transfuse and how much in hematologic malignancies. Hematol Educ. 2014;8(3):421-426.
- Singhal D, Kutyna MM, Chhetri R, Wee LY, Hague S, Nath L, et al. Red cell alloimmunization is associated with development of autoantibodies and increased red cell transfusion requirements in myelodysplastic syndrome. Haematologica. 2017;102(12):2021.
[Crossref] [Google Scholar] [PubMed]
- Platzbecker U, Fenaux P, Adès L, Giagounidis A, Santini V, Van De Loosdrecht AA, et al. Proposals for revised IWG 2018 hematological response criteria in patients with MDS included in clinical trials. Blood. 2019;133(10):1020-1030.
[Crossref] [Google Scholar] [PubMed]
- Gupta P, LeRoy SC, Luikart SD, Bateman A, Morrison VA. Long-term blood product transfusion support for patients with myelodysplastic syndromes (MDS): Cost analysis and complications. Leuk Res. 1999;23(10):953-959.
[Crossref] [Google Scholar] [PubMed]
- Dotson JL, Lebowicz Y. Myelodysplastic syndrome.2022.
[Google Scholar] [PubMed]
- Scalzulli E, Pepe S, Colafigli G, Breccia M. Therapeutic strategies in low and high-risk MDS: What does the future have to offer?. Blood Rev. 2021;45:100689.
[Crossref] [Google Scholar] [PubMed]
- Hellstrom-Lindberg E. Management of anemia associated with myelodysplastic syndrome. Semin Hematol. 2005;42(2):S10-S13.
[Crossref] [Google Scholar] [PubMed]
- Stiegler G, Sperr W, Lorber C, Fabrizii V, Hocker P, Panzer S. Red cell antibodies in frequently transfused patients with myelodysplastic syndrome. Ann Hematol. 2001;80(6):330-333.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Xu X, Wang S, Li R, Zhang P. Red blood cell alloimmunization in transfused patients with myelodysplastic syndromes: A retrospective study from northern China. Lab Med. 2025;56(1):22-30.
[Crossref] [Google Scholar] [PubMed]
- Rozema J, Slim CL, Veeger NJ, Kibbelaar RE, de Wit H, van Roon EN, et al. A clinical effect of disease-modifying treatment on alloimmunisation in transfused patients with myelodysplastic syndromes: Data from a population-based study. Blood Transfus. 2020;20(1):18.
[Crossref] [Google Scholar] [PubMed]
- Ortiz S, Orero MT, Javier K, Villegas C, Luna I, Pérez P, et al. Impact of azacitidine on red blood cell alloimmunisation in myelodysplastic syndrome. Blood Transfus. 2016;15(5):472.
[Google Scholar] [PubMed]
- Thoms JA, Yan F, Hampton HR, Davidson S, Joshi S, Saw J, et al. Clinical response to azacitidine in MDS is associated with distinct DNA methylation changes in HSPCs. Nat Commun. 2025;16(1):4451.
[Crossref] [Google Scholar] [PubMed]
- Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96(10):3369-3373.
[Crossref] [Google Scholar] [PubMed]
- Sanz C, Nomdedeu M, Belkaid M, Martinez I, Nomdedeu B, Pereira A. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013;53(4):710-715.
[Crossref] [Google Scholar] [PubMed]
- Zalpuri S, Evers D, Zwaginga JJ, Schonewille H, de Vooght KM, le Cessie S, et al. Immunosuppressants and alloimmunization against red blood cell transfusions. Transfusion. 2014;54(8):1981-1987.
[Crossref] [Google Scholar] [PubMed]
- Lin Y, Saskin A, Wells RA, Lenis M, Mamedov A, Callum J, et al. Prophylactic RhCE and Kell antigen matching: Impact on alloimmunization in transfusion-dependent patients with myelodysplastic syndromes. Vox Sang. 2017;112(1):79-86.
[Crossref] [Google Scholar] [PubMed]
- Kim HY, Cho EJ, Chun S, Kim KH, Cho D. Red blood cell alloimmunization in Korean patients with myelodysplastic syndrome and liver cirrhosis. Ann Lab Med. 2019;39(2):218-222.
[Crossref] [Google Scholar] [PubMed]
- Rozovski U, Ben-Tal O, Kirgner I, Mittelman M, Hareuveni M. Increased Incidence of Red Blood Cell Alloantibodies in Myelodysplastic Syndrome. Isr Med Assoc J. 2015;17(10):624-627.
[Google Scholar] [PubMed]
- Eriksen MB, Frandsen TF. The impact of Patient, Intervention, Comparison, Outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J Med Libr Assoc. 2018;106(4):420.
[Crossref] [Google Scholar] [PubMed]
- Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372.
[Crossref] [Google Scholar] [PubMed]
- Vandenbroucke JP, Von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int J Surg. 2014;12(12):1500-1524.
[Crossref] [Google Scholar] [PubMed]
- From the (Epi) genome to metabolism and vice versa; Examples from hematologic malignancy
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
[Crossref] [Google Scholar] [PubMed]
- Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+ CD25high Foxp3+ regulatory T cells in Myelodysplastic Syndrome (MDS). Blood. 2007;110(3):847-850.
[Crossref] [Google Scholar] [PubMed]
- Rodriguez-Sevilla JJ, Colla S. T-cell dysfunctions in myelodysplastic syndromes. Blood. 2024;143(14):1329-1343.
[Crossref] [Google Scholar] [PubMed]
- Wong KK, Hassan R, Yaacob NS. Hypomethylating agents and immunotherapy: Therapeutic synergism in acute myeloid leukemia and myelodysplastic syndromes. Front Oncol. 2021;11:624742.
[Crossref] [Google Scholar] [PubMed]
- Kannan S, Vedia RA, Molldrem JJ. The immunobiology of myelodysplastic neoplasms: A mini-review. Front Immunol. 2024;15:1419807.
[Crossref] [Google Scholar] [PubMed]
Citation: Abba V, Jackson DE. (2025). Incidence of Red Blood Cell Alloimmunisation in Transfused Myelodysplastic Syndromes Patients: A Systematic Review and Meta-Analysis. J Blood Disord Transfus. 16:630.
Copyright: © 2025 Abba V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.