Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Commentary - (2020) Volume 11, Issue 2
Impact of Insulin on Memory Recall
Yuki Totani1, Junko Nakai1 and Etsuro Ito2*2Graduate Institute of Medicine, Kaohsiung Medical University, Kaohsiung 80756, Japan
Received: 15-Jun-2020 Published: 06-Jul-2020
Abstract
Recent studies revealed that an injection of insulin into the central nervous system ameliorates deficits in memory formation and recall in snails. A spontaneous increase in the insulin levels in snails is also associated with improved memory recall. Thus, insulin is thought to be a critical factor for memory recall in snails. In the present study, we describe the production and function of insulin in the snail central nervous system for memory recall and expand this scenario to other animals. These findings provide a new avenue for studying the mechanisms underlying learning and memory.
Keywords
Conditioned taste aversion; Insulin; Lymnaea; Memory recall; Snail
Introduction
In 1988, a mini-review titled “Invertebrate neuroendocrinology. Insulin found at last?” in Nature astounded researchers in the field of invertebrate neuroscience [1]. This review highlighted the article by Smit and colleagues who found molluscan insulinrelated peptides (MIPs) in the central nervous system (CNS) of the pond snail Lymnaea stagnalis [2]. Lymnaea MIPs were the first established insulin-like peptides in invertebrates, and since then Smit and colleagues have clarified various aspects of MIPs, such as their involvement in growth control and egg-laying [3]. Eighteen years later, Azami et al. provided evidence demonstrating that MIPs are involved in learning and memory in Lymnaea [4]. They used the conditioned taste aversion (CTA) protocol, in which a sucrose solution is used as the conditioned stimulus (CS) and a KCl solution is used as the unconditioned stimulus (US) [5,6]. Application of the CS to the lips increases the feeding response, whereas application of the US inhibits it. After repeated temporal pairings of the CS and US, the CS no longer elicits feeding [5]. Using a cDNA microarray system, Azami and colleagues found that MIP gene expression is upregulated during CTA formation [4].
The CTA response persists for at least a month [5], and is classified as long-term memory (LTM) according to the temporal classification of memory [6]. LTM should include a de novo protein-synthesis process [7], and a protein synthesis-dependent period during the consolidation of Lymnaea CTA to LTM has been demonstrated by pharmacologic inhibition of transcription or translation [8]. The robustness for CTA-LTM was confirmed by extinction trials, because repeated presentations of the CS alone after establishing a CTA fails to extinguish the CTA. Furthermore, the CTA is formed even when the presentation of the US is delayed. Thus, CTA in Lymnaea is thought to be similar to CTA in mammals.
In the present study, we review the production of insulin, MIPs, in the snail CNS and then describe the effects of insulin on memory formation and memory recall. Finally, we expand the scenario of the involvement of insulin in learning and memory to other animals.
MIPs IN THE SNAIL CNS
Five types of MIPs, MIP I, II, III, V, and VII, are produced in Lymnaea [9-14]. The neurons that synthesize MIPs are the growth controlling neuroendocrine light green cells (LGCs) and canopy cells in the snail CNS [15-17]. In situ hybridization experiments for MIP II, the major MIP, clarified that the signals are localized in the LGCs [18]. On the other hand, only one MIP receptor gene is reported for these different MIPs [16]. The cDNA structure of this MIP receptor is a putative tyrosine kinase receptor [19], and In situ hybridization signals for the MIP receptor are observed in many neurons throughout the CNS.
Murakami and colleagues demonstrated that applying MIPs to an isolated Lymnaea CNS preparation evokes long-lasting synaptic enhancement at synapses involved in the feeding response [18]. This synaptic enhancement is blocked by simultaneous application of an anti-insulin receptor antibody. They used a human, not snail, antibody because there was evidence that the human antibody would block the binding between MIP and the MIP receptor (for a detailed explanation, see the Murakami study). Direct injection of this anti-insulin receptor antibody into the snail body also blocks LTM formation at the behavioral level. Because Lymnaea has an open bloodvascular system, this blockade is thought to occur in the CTArelated neural circuits in the CNS.
MIPs For Memory Recall In Snail
A relation between the food-deprivation state and memory recall for CTA is observed in Lymnaea [20-23]. Mildly food-deprived snails (i.e., 1-day food deprivation) can form a strong CTA, whereas heavily food-deprived snails (i.e., 5-day food deprivation) do not seem to form a CTA. When the heavily food-deprived snails are injected with insulin, however, they, too, exhibit CTALTM [21-24]. This effect of insulin for attenuating the deficiency of CTA-LTM in heavily food-deprived snails can be abolished by simultaneously injecting the anti-insulin receptor antibody described above [21-24].
From the above results, we hypothesized that the heavily fooddeprived snails form a CTA, but do not exhibit memory recall, because very hungry snails eat whatever is in front of them, as if ‘necessity knows no law’ [25,26]. The results obtained support this hypothesis. Moreover, the effect of insulin injection on memory recall in the heavily food-deprived snails, as described above, was mimicked by mild food deprivation as described below. After snails were heavily food-deprived (e.g., 5 days), CTA training was performed. At this point, the snails did not demonstrate CTA-LTM. The snails were then provided ad libitum access to food for a few days to keep them healthy. Subsequently, the snails were mildly food-deprived (e.g., 1 day). These snails exhibited good memory recall [25,26]. We therefore concluded that the optimal internal state for memory recall of CTA is provided by either an injection of insulin or mild food deprivation.
More recently, Totani et al. observed a difference in MIP II mRNA expression levels in the snail CNS, as described above, using real-time polymerase chain reaction (PCR) experiments [27]. The study comprised 3 cohorts of snails. (A) Snails that were heavily food-deprived, trained with the CTA training protocol, and then provided food. These snails were referred to as snails without mild food deprivation. (B) Snails that were heavily food-deprived, trained with the CTA training protocol, provided food, and then mildly food-deprived. These snails exhibited good memory recall and were referred to as the good memory-recall performers (defined as a snail that made 0 - 1 bites/min during the memory recall test in response to presentation of the CS [20]). (C) Snails that were heavily fooddeprived, trained with the CTA training protocol, provided food, and then mildly food-deprived. These snails exhibited poor memory recall and were referred to as the poor memoryrecall performers (defined as a snail that made ≥ 2 bites/min in response to the CS during the memory recall test).
Totani et al. found that MIP II levels were significantly higher in the good memory-recall performers after mild food deprivation, compared with the poor performers and the snails without mild food deprivation (Figure 1).
That is, the level of MIP II, the major MIP, spontaneously changes and increases during mild food deprivation. Therefore, the effects of insulin injection are similar to those of mild food deprivation in Lymnaea CTA [27].
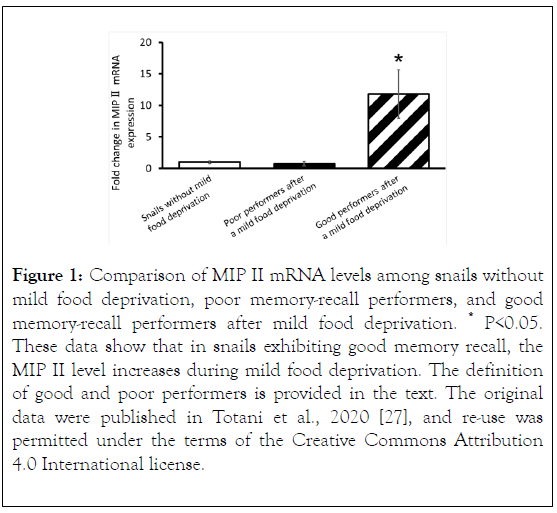
Figure 1. Comparison of MIP II mRNA levels among snails without mild food deprivation, poor memory-recall performers, and good memory-recall performers after mild food deprivation. *P<0.05. These data show that in snails exhibiting good memory recall, the MIP II level increases during mild food deprivation. The definition of good and poor performers is provided in the text. The original data were published in Totani et al., 2020 [27], and re-use was permitted under the terms of the Creative Commons Attribution 4.0 International license.
Expansion Of The Insulin Scenario For Learning And Memory To Other Animals
The most popular studies of the contribution of insulin function to cognition in humans involves a treatment for Alzheimer disease [28]. Clinical studies reveal that intranasal insulin administration provides reliable treatment results in patients with Alzheimer disease. Many studies have evaluated insulin function in invertebrates, such as Caenorhabditis elegans and Drosophila [29,30]. Especially in Drosophila, the relation between the actions of insulin and those of cAMP-regulated transcriptional coactivator (CRTC) has been clarified [31]. CRTC is thought to be activated when the insulin signaling cascade is downregulated in hungry flies [32]. Hungry flies learn conditioned food aversions by 1-trial learning because of the downregulation of the insulin signaling pathway and the upregulation of the CRTC signaling pathway [33]. Because the state of hunger in flies is caused by food deprivation for 9 to 16 h before conditioned food aversion, it may correspond to the state of mild food deprivation in our Lymnaea studies. The relation between the insulin signals and the CRTC signals in Lymnaea has not yet been studied, and thus the molecular mechanisms should be carefully examined in Lymnaea.
Conclusion
The involvement of insulin function in learning and memory is not restricted to snails, but may occur across phyla. For a comprehensive understanding of the involvement of insulin in learning and memory, subsequent studies should target the cascades from insulin reception to a de novo protein synthesis mechanism. The transcription factors involved in protein synthesis may include cAMP-responsive element binding protein, which is well analyzed in Lymnaea. We hope that this clarification of insulin function in learning and memory opens up new approaches for studying the mechanisms underlying learning and memory.
Acknowledgments
None to report.
Sources of Funding
This work was partly supported by a Waseda University Early Bird Project (BD070Z003200) to Y.T.; and Waseda University Grants for Special Research Projects (2018K-141 and 2020C-135) to E.I.
Conflict of Interest
None to report.
REFERENCES
- Thorpe A, Duve H. Invertebrate neuroendocrinology. Insulin found at last? Nature. 1988; 331: 483-484.
- Smit AB, Vreugdenhil E, Ebberink RH, Geraerts WP, Klootwijk J, Joosse J. Growth-controlling molluscan neurons produce the precursor of an insulin-related peptide. Nature. 1988; 331: 535-538.
- Van Minnen J, Smit AB, Joosse J. Central and peripheral expression of genes coding for egg-laying inducing and insulin-related peptides in a snail. Arch Histol Cytol. 1989; 52: 241-252.
- Azami S, Wagatsuma A, Sadamoto H, Hatakeyama D, Usami T, Fujie M, et al. Altered gene activity correlated with long-term memory formation of conditioned taste aversion in Lymnaea. J Neurosci Res. 2006; 84:1610-1620.
- Kojima S, Yamanaka M, Fujito Y, Ito E. Differential neuroethological effects of aversive and appetitive reinforcing stimuli on associative learning in Lymnaea stagnalis. Zoolog Sci. 1996; 13: 803-812.
- Totani Y, Kotani S, Odai K, Ito E, Sakakibara M. Real-time analysis of animal feeding behavior with a low-calculation-power CPU. IEEE Transact Biomed Eng. 2020; 67: 1197-1205.
- Rosenzweig MR, Bennett EL, Colombo PJ, Lee DW, Serrano PA. Short-term, intermediate-term, and long-term memories. Behav Brain Res. 1993;57:193-198.
- Nakai J, Totani Y, Kojima S, Sakakibara M, Ito E. Features of behavioral changes underlying conditioned taste aversion in the pond snail Lymnaea stagnalis. Invert Neurosci. 2020; 20: 8.
- Li KW, Geraerts WP, Ebberink RH, Joosse J. Purification and sequencing of molluscan insulin-related peptide I (MIP I) from the neuroendocrine light green cells of Lymnaea stagnalis. Mol Cell Endocrinol. 1992; 85:141-150.
- Li KW, Geraerts WP, Joosse J. Purification and sequencing of molluscan insulin-related peptide II from the neuroendocrine light green cells in Lymnaea stagnalis. Endocrinol. 1992; 130: 3427-3432.
- Smit AB, Geraerts PM, Meester I, Van Heerikhuizen H, Joosse J. Characterization of a cDNA clone encoding molluscan insulin-related peptide II of Lymnaea stagnalis. Eur J Biochem. 1991; 199: 699-703.
- Smit AB, Thijsen SF, Geraerts WP, Meester I, Van Heerikhuizen H, Joosse J. Characterization of a cDNA clone encoding molluscan insulin-related peptide V of Lymnaea stagnalis. Brain Res Mol Brain Res. 1992;14: 7-12.
- Smit AB, Van Marle A, Van Elk R, Bogerd J, Van Heerikhuizen H, Geraerts WP. Evolutionary conservation of the insulin gene structure in invertebrates: cloning of the gene encoding molluscan insulin-related peptide III from Lymnaea stagnalis. J Mol Endocrinol. 1993; 11:103-113.
- Smit AB, Spijker S, van Minnen J, Burke JF, De Winter F, van Elk R, Geraerts WP. Expression and characterization of molluscan insulin-related peptide VII from the mollusc Lymnaea stagnalis. Neuroscience 1996; 70: 589-596.
- Meester I, Ramkema MD, Van Minnen J, Boer HH. Differential expression of four genes encoding molluscan insulin-related peptides in the central nervous system of the pond snail Lymnaea stagnalis. Cell Tissue Res 1992; 269: 183-188.
- Smit AB, Van Kesteren RE, Li KW, Van Minnen J, Spijker S, Van Heerikhuizen H, et al. Towards understanding the role of insulin in the brain: lessons from insulin-related signaling systems in the invertebrate brain. Prog Neurobiol. 1998; 54: 35-54.
- Hatakeyama D, Ito I, Kojima S, Fujito Y, Ito E. Complement receptor 3-like immunoreactivity in the light green cells and the canopy cells of the pond snail, Lymnaea stagnalis. Brain Res. 2000; 865:102-106.
- Murakami J, Okada R, Sadamoto H, Kobayashi S, Mita K, Sakamoto Y, et al. Involvement of insulin-like peptide in long-term synaptic plasticity and long-term memory of the pond snail Lymnaea stagnalis. J Neurosci 2013; 33: 371-383.
- Roovers E, Vincent ME, Van Kesteren E, Geraerts WP, Planta RJ, Vreugdenhil E, et al. Characterization of a putative molluscan insulin-related peptide receptor. Gene 1995;162:181-188.
- Sugai R, Azami S, Shiga H, Watanabe T, Sadamoto H, Kobayashi S, et al. One-trial conditioned taste aversion in Lymnaea: good and poor performers in long-term memory acquisition. J Exp Biol. 2007; 210:1225-1237.
- Mita K, Okuta A, Okada R, Hatakeyama D, Otsuka E, Yamagishi M, et al. What are the elements of motivation for acquisition of conditioned taste aversion? Neurobiol Learn Mem. 2014; 107:1-12.
- Mita K, Yamagishi M, Fujito Y, Lukowiak K, Ito E. An increase in insulin is important for the acquisition conditioned taste aversion in Lymnaea. Neurobiol Learn Mem 2014; 116:132-138.
- Totani Y, Aonuma H, Oike A, Watanabe T, Hatakeyama D, Sakakibara M, et al. Monoamines, insulin and the roles they play in associative learning in pond snails. Front Behav Neurosci. 2019;13: 65.
- Kojima S, Sunada H, Mita K, Sakakibara M, Lukowiak K, Ito E. Function of insulin in snail brain in associative learning. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015; 201: 969-981.
- Ito E, Yamagishi M, Hatakeyama D, Watanabe T, Fujito Y, Dyakonova V, et al. Memory block: a consequence of conflict resolution. J Exp Biol. 2015; 218:1699-1704.
- Ito E, Totani Y, Oike A. Necessity knows no law in a snail. Eur Zoolog J. 2017; 84: 457-464.
- Totani Y, Nakai J, Dyakonova VE, Lukowiak K, Sakakibara M, Ito E. Induction of LTM following an insulin injection. eNeuro 2020; 7: ENEURO.0088-20.2020.
- Bhattamisra SK, Shin LY, Saad HIBM, Rao V, Candasamy M, Pandey M, et al. Interlink between insulin resistance and neurodegeneration with an update on current therapeutic approaches. CNS Neurol Disord Drug Targets. 2020.
- Kim SY, Webb AE. Neuronal functions of FOXO/DAF-16. Nutr Healthy Aging. 2017; 4:113-126.
- Nässel DR, Zandawala M. Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog Neurobiol. 2019; 179:101607.
- Hirano Y, Saitoe M. Hunger and memory; CRTC coordinates long-term memory with the physiological state, hunger. Commun Integr Biol. 2013;6: e25152.
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol 2011; 12:141-151.
- Sadamoto H, Sato H, Kobayashi S, Murakami J, Aonuma H, Ando H, et al. CREB in the pond snail Lymnaea stagnalis: cloning, gene expression, and function in identifiable neurons of the central nervous system. J Neurobiol. 2004; 58: 455-466.
Citation: Totani Y, Nakai J, Ito E (2020) Impact of Insulin on Memory Recall. J Data Mining Genomics Proteomics. 11:225. DOI: 10.35248/2153-0602.20.11.225.
Copyright: © 2020 Totani Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

