Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- RefSeek
- Hamdard University
- EBSCO A-Z
- NSD - Norwegian Centre for Research Data
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 10, Issue 5
High Prevalence of Cancer among Carriers of a Truncated Mutation in Ataxia Telangiectasia Gene
Eman Fares and Fuad Fares*Received: 30-Aug-2021 Published: 20-Sep-2021, DOI: 10.35248/2161-1041.21.10.200
Abstract
Ataxia Telangiectasia (AT) gene plays an important role in DNA damage response and involved in several cellular signaling pathways. Here we report a high prevalence of cancer among a Druze family of ~1000 members affected with AT living in the same village in North Israel. During the last four decades 16 AT patients were found homozygotes carrying a truncated mutation at position 1339 (C>T) that eliminating the production of AT protein. The high number of patients in this family is due to highly inbred with a large number of consanguineous marriages. Therefore, the relatively high number of members of the family is carriers of this mutation. Moreover, in this family more than 75 cancer patients were diagnosed. Thus, it is possible to postulate that carriers of truncated mutations in AT gene are potentially at increased high risk for developing cancer. AT patients and heterozygous carriers of AT mutations are sensitive to radiation therapy. Therefore, treatment of cancer patients carrying mutated AT gene may avoid the use of radiation therapy and radiomimetic drugs. New medications act on ATM independent pathways should be discovered.
Keywords
Ataxia telangiectasia; Cancer prevalence; Truncated mutation; Carriers; Radiotherapy
Introduction
Ataxia Telangiectasia (AT) is a rare autosomal recessive disorder that affects ~1:40,000–1:100,000 children in various ethnic groups. AT is a complex disease that characterized by progressive cerebellar ataxia, ocular telangiectasia, broncho-pulmonary infections, endocrine abnormalities, immunodeficiency, poor growth, gonadal atrophy, delayed development, insulin resistant, diabetes, chromosomal and genomic instability, hypersensitivity to ionic radiation and a predisposition to cancer development. AT patients, have a highly increased incidence of different types of cancer including lymphomas and leukemia [1,2]. Moreover, AT is often referred to as a genome instability or DNA damage response syndrome. Most patients are confined to a wheel chair before 10 years of age and die before the third decade of life. Cells derived from patients with AT demonstrate sensitivity to ionizing irradiation, chromosomal instability, shortened telomeres, premature senescence and a defective response to DNA double strand breaks (DSBs) [3]. AT is caused by mutations in the Ataxia Telangiectasia Mutated (ATM) gene which encodes a protein of the same name. The primary role of the ATM protein is coordination of cellular signaling pathways in response to DNA double strand breaks, oxidative stress and other genotoxic stress. This protein was found to be a member of an expanding family of proteins, all containing a C-terminal region resembling the catalytic domain of phosphatidylinositol 3-kinase (PI 3-kinase) and is involved in cellular response to DNA damage, regulation of telomere length and cell cycle control [4,5].
The diagnosis of A-T is usually suspected by the combination of neurologic clinical features such as: Ataxia, abnormal control of eye movement, postural instability, telangiectasia, frequent infections, IgA deficiency, lymphopenia especially affecting T lymphocytes and increased alpha-fetoprotein levels. A diagnosis of A-T can be confirmed by identification of pathological mutations in the ATM gene. The ATM gene had been localized to chromosome 11q22-23, contains 66 exons and coding to a nuclear 350 KDa protein with homology to PI-3 Kinases [1]. At present, more than 400 different mutations had been identified in this gene causing AT syndrome. The reported mutations are specific to one family or ethnic group [4].
ATM plays an important role in DNA damage response and involved in several cellular signaling pathways. ATM regulated cellular oxidative stress signaling, involved in the control of proteostasis, prevented protein aggregation, played a role in the cytosol in regulating autophagosome and involved in controlling cell cycle progression [6]. Therefore, ATM can be defines as a tumor suppressor that plays an important role in cancer development. Hereditary mutations of genes involved in DNA repair, such as ataxia telangiectasia mutated (ATM), may result in markedly increased susceptibility to a variety of cancers.
Literature Review
High prevalence of cancer among family affected with AT
Here we report a high prevalence of AT patients and cancer cases in a Druze family of ~1000 members living in the same village in the North region of Israel. During the last four decades 16 AT patients were found homozygotes carrying a truncated mutation at position 1339 (C>T) that eliminating the production of ATM protein [7]. The homozygous patients described in this report first showed symptoms of AT at the age of 4-6 years and the average mortality age of patients is 12 years. These facts may indicated that truncation mutations in the ATM gene are associated with an aggressive phenotype of AT. The high number of patients in this family is due to highly inbred with a large number of consanguineous marriages. Moreover, the Druze religion strictly prohibits interreligious marriages. Therefore, we guess the relatively high number of members of the family is carriers of this mutation even higher than that reported before, where germ-line ATM heterozygosity occurs in less than 1% of the population [8]. According to Hardy Weinberg law it is possible to estimate that ~20% of the family members are carriers of the ATM mutated gene. Interestingly, in this affected family with AT, more than 75 cancer patients were diagnosed. Females were diagnosed with ovarian, breast, gastrointestinal and lymphoma cancers; and males were diagnosed with lymphoma, prostate, lung, melanoma, gastrointestinal and pancreatic cancers. It is known that hereditary mutations of genes involved in DNA repair such as ATM gene increased the susceptibility to cancer. Thus, we postulate that ATM carriers are potentially at increased high risk for developing cancer. The number of cancer cases in this family is significantly higher than that of the estimated number by the Israel Ministry of health. It was estimated that approximately 500/100.000 and 303/100.000 of new cases were diagnosed in the Israeli Jewish and Arab populations, respectively. Recently it was reported that the lifetime risk for breast cancer in women who are heterozygous for a hereditary germ-line mutations of the ATM gene is likely greater than 25% [9]. Other studies indicated that heterozygous for ATM gene mutations resulted in 2-3-fold risk of cancer, and a 5- to 9-fold risk of breast cancer in women [10]. The results in the described family indicated that the risk of cancer is higher (~75/1000) than that reported in the Arab population in Israel (~303/100.000) and this due to the high number of ATM carriers. On the other hand, somatic ATM mutations are commonly found in lymphoid malignancies and solid tumors [11]. This may emphasize that carriers of ATM truncated mutations have an increased susceptibility to a variety of cancers. This is in agreement with the fact that ATM has a role in activating cell cycle checkpoints following exposure to DNA damage. Defect in DNA repair results in genomic instability and accumulation of other genetic abnormalities, which is a hallmark of cancer development. Activation of ATM phosphorylates and facilitate the activation of downstream targets effectors such as Breast Cancer-1 (BRCA1), BH3-Interacting Domain death agonist (BID), the effector Chekpoint Kinase-2 (CHK2) and the tumor suppressor gene, p53, which lead to a long-term cell-cycle arrest or apoptosis [12,13].
It is well known that AT patients and heterozygous carriers of ATM mutations are sensitive to radiation therapy. Exposure to radiation dissociates ATM from homodimers into active monomers that interacts with and phosphorylates other sensor proteins such as p53, Chekpoint Kinase-2 (CHK2), BID and BRCA1 that may lead to cell cycle arrest, apoptosis and DNA repair (Figure 1) [14]. In addition, cultured cells from heterozygote carriers of ATM mutations have been reported to have a variable sensitivity to radiation, being more sensitive than normal control cells but less sensitive than homozygous ATM null cells [15].
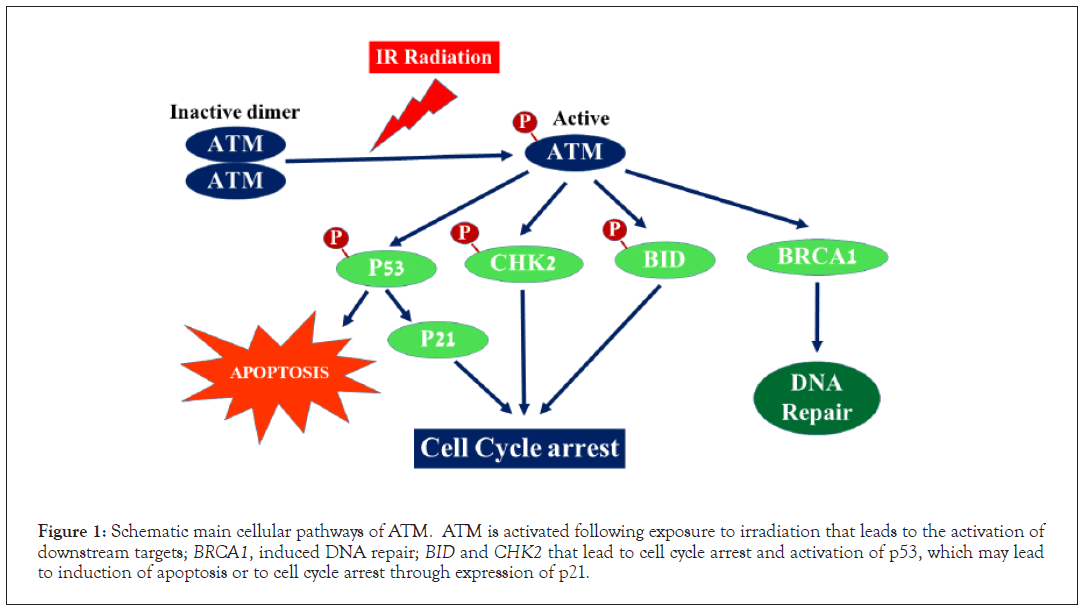
Figure 1: Schematic main cellular pathways of ATM. ATM is activated following exposure to irradiation that leads to the activation of downstream targets; BRCA1, induced DNA repair; BID and CHK2 that lead to cell cycle arrest and activation of p53, which may lead to induction of apoptosis or to cell cycle arrest through expression of p21.
Chemotherapy toxicity is increased in patients with heterozygous germ-line ATM mutations [11]. Probably this is due to the defective DNA repair and genomic instability. This raises theoretical concern with radiation exposure of ATM heterozygous carriers. Moreover, it was hypothesized that carrying ATM mutations may result in chemotherapy resistance. In order to avoid therapeutic radiation and chemotherapy resistance therapy of cancer patients it is highly recommended detecting sporadic ATM mutations in cancer biopsies. These observations are similar to that reported before and indicated that carriers of germ line mutations in BRCA1 and BRCA2 genes have very high risks of breast cancer and ovarian cancer [16]. Therefore, Carriers of BRCA and/or ATM should undergo annual mammographic screening for early detection of breast cancer.
Conclusion
In conclusion, these observations may provide estimates of cancer risk based on screening of ATM mutation carrier status. Therefore, it is highly recommended to add ATM gene mutations to commercially available gene panel assays for carrier’s detection. Considering ATM carriers could be important for prevention and early detection of cancer. Moreover, detection of ATM inherited germ mutation or somatic mutation in cancer biopsies may be important for designing new therapeutic strategies of cancer. Therefore, we hypothesized that treatment of cancer patients carrying mutated ATM should avoid the use of radiation therapy and radiomimetic drugs. Furthermore, new medications act on ATM independent pathways should be discovered.
Acknowledgments
We are grateful to all families for their contributions and providing the data for preparation of this report. We also thank the stuff of Molecular Genetic Laboratory at Carmel Medical Center, Haifa, Israel, for their collaboration.
Author Contribution
Eman Fares: Performed research, analyzed the data and participate in writing the paper.
Fuad Fares: Design the research and participate in writing the paper.
Funding Support
No specific funding was disclosed
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- Reiman A, Srinivasan V, Barone G, Last JI, Wootton LL, Davies EG, et al. Lymphoid tumours and breast cancer in ataxia telangiectasia; substantial protective effect of residual ATM kinase activity against childhood tumours. Br J Can. 2011;105(4):586-591.
- Nissenkorn A, Levy-Shraga Y, Banet-Levi Y, Lahad A, Sarouk I, Modan-Moses D. Endocrine abnormalities in ataxia telangiectasia: Findings from a national cohort. Pediatr Res. 2016;79(6):889-894.
- Derheimer FA, Kastan MB. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS letters. 2010;584(17):3675-3681.
- Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268(5218):1749-1753.
- Lavin F, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15(1):177-202.
- Stagni V, Cirotti C, Barilà D. Ataxia-telangiectasia mutated kinase in the control of oxidative stress, mitochondria, and autophagy in cancer: a maestro with a large orchestra. Fron Oncol. 2018;16:8-73.
- Fares F, Axelord Ran S, David M, Zelnik N, Hecht Y, Khairaldeen H, et al. Identification of two mutations for ataxia telangiectasia among the Druze community. Prenat Diagn. 2004;24(5):358-362.
- Rothblum-Oviatt C, Wright J, Lefton-Greif MA, McGrath-Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: A review. Orphanet J Rare Dis. 2016;11(1):1-21.
- Jerzak KJ, Mancuso T, Eisen A. Ataxia–telangiectasia gene (ATM) mutation heterozygosity in breast cancer: A narrative review. Curr Oncol. 2018;25(2):176-180.
- Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161 families affected by ataxia–telangiectasia. N Engl J Med. 1991;325(26):1831-1836.
- Choli M, Kipps T, Kurzrock R. ATM Mutations in cancer: Therapeutic implications. Mol Cancer Ther. 2016.
- Stagni V, Cirotti C, Barilà D. Ataxia-telangiectasia mutated kinase in the control of oxidative stress, mitochondria, and autophagy in cancer: A maestro with a large orchestra. Fron Oncol. 2018;8:73.
- Shiloh Y, Ziv Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14(4):197-210.
- Bernstein JL, WECARE study collaborative group, concannon P. ATM, radiation, and the risk of second primary breast cancer. Int J Rad Biol. 2017;93(10):1121-1127.
- Kiuru A, Kämäräinen M, Heinävaara S, Pylkäs K, Chapman K, Koivistoinen A, et al. Assessment of targeted and non-targeted responses in cells deficient in ATM function following exposure to low and high dose X-rays. PLoS One. 2014;9(3):e93211.
- Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402-2416.
Citation: Fares E, Fares F (2021) High Prevalence of Cancer among Carriers of a Truncated Mutation in Ataxia Telangiectasia Gene. Hereditary Genet. 10:200.
Copyright: © 2021 Fares E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

