Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 8
Hematological and Histopathological Changes in Artificially Infected Nile tilapia with vibrio species
Begonesh Bekele1, Natarajan P1, Kassaye Balkew Workagegn1* and Devika Pillai22Department of Aquaculture, Kerala University of Fisheries & Ocean Studies, Kochi, India
Received: 23-Jan-2021 Published: 22-Jul-2021, DOI: 10.35248/2155-9546.21.12.648
Abstract
Bacterial pathogens such as vibrio species cause a significantly high economical loss in the aquaculture sector. Thus, the main objective of this study was to isolate and identify Vibrio species and evaluated the hematological, biochemical, and histopathological changes in experimentally infected Nile tilapia with 0.1 ml of 1×106 colonyforming units (CFU)/ml of Vibrio species isolates. The clinical examination of the infected Nile tilapia revealed all fish became less active and the feeding rate decreased, the dorsal portion of the body became darkened. Fin rot appeared especially at the tips of the caudal fin and showed extensive hemorrhages at the ventral body part. The results also showed reduction in hemoglobin, hematocrit, protein, albumin, glucose, globulin, and triglyceride contents in the infected fish than the control group. The major histopathological changes noticed were inflammatory lesions, hemorrhagic dermis and melanomacrophage aggregation in the skin; hepatocellular degeneration, melanomacrophage aggregation, and nuclear hypertrophy in the liver; hypertrophy and fusion of different gill lamellae; myofibril disintegration and necrosis in the muscle tissue; and sub-mucosal oedema and atrophy in the intestine. In conclusion, experimental infection of fish with vibrio bacteria produced significant changes in clinical, hematological, biochemical and histopathological characteristics of Nile tilapia, in which the vibrio bacteria caused significantly high tissue damage and found to be severe in all the tissues examined. Therefore, it is important to develop strategies to control such pathogenic bacteria in order to achieve a sustainable fish production under a wide range of production environments.
Keywords
Biochemistry; Hematology; Histopathology; Nile Tilapia; Vibrio species
Introduction
Nile tilapia, Oreochromis niloticus, is one of the most popular tropical fish and is becoming very important commercial fish as it grows fast, fed at trophic level, grow in a wide range of production systems and highly acceptable by customer. It is widely cultured in ponds, cage, tanks, and raceways. Recently, Nile tilapia production increased significantly and contributes about half of Ethiopian fish production [1] and it is an important source of protein for human consumption. However, Bacterial pathogens such as vibrio species is one of the most common fish diseases among cultivable fishes such as Nile tilapia cause a significantly high economical loss in the aquaculture sector [2]. Vibrio species are gram-negative bacteria commonly isolated from fish farms [3]. It is widely responsible for mortality in aquaculture farms worldwide. The disease is characterized by septicemia, dermal ulceration, and hematopoietic necrosis. The primary mode of infection in fish consists of penetration of bacterium into the host tissue. A detailed investigation of vibrio species concluded that the infection might be due to the hemolytic activity of bacterial infection [4]. Some of the common symptoms of diseases in fish caused by the pathogenic vibrio include intestinal necrosis, anemia, ascetic fluid, petechial hemorrhages in the muscle wall, and liquid accumulation in the air bladder, excess mucus secretion, and blood clots in moribund fishes [5].
The hematological parameters of fish are of great importance in aquaculture as it reflects the physiological, environmental, and handling stress to fish species [6]. The hematological parameters serve as an important tool for disease diagnosis and to reveal the health status of the fish [7,8]. Therefore, understanding about hematological, biochemical, and histopathological changes in cultured fishes have been an important tool for monitoring of outbreaks of bacterial disease in aquaculture as their investigations
have been widely used to describe the health status of fish due to infection and stress in fish owing to environmental contaminants [9]. Therefore, the objectives of this study were to isolate and identify Vibrio species, and to investigate the impacts of Vibrio species on hematological, biochemical, and histopathological alterations in Nile tilapia injected intraperitoneally with Vibrio species originally isolated from naturally infected fish.
Materials and Methods
Collection naturally infected fish and bacterial enumeration
A total of 60 infected Nile tilapia with body weight ranged from 43.2 to 139 g were collected from Hawassa Agricultural Research Center and transported to Hawassa University laboratory, Ethiopia for both clinical examination and isolation of Vibrio species. Clinical examination of Nile tilapia was performed following the method used by [1,4]. For isolation and identification, fish samples were collected from the fish body surface, gills, liver, intestine, and kidney under aseptic conditions. Isolation of Vibrio species colonies was performed by using the streaked plate method using Mac Conkey and Thiosulfate-Citrate-Bile Salts-sucrose (TCBS) agar and incubated at 37°C for 24hrs. The identification of Vibrio species was carried out using both morphological and biochemical analysis as used by [1,4].
Experimental infection
For pathogenicity test, 30 healthy Nile tilapia with an average body weight of 30+1.3 g was collected from the Hawassa Agriculture Research Breading Center and stocked in a 40-liter holding tank in the laboratory. After 7 days acclimation, the fish were equally divided into two groups, non-infected, as control and infected fish with 0.1 ml Vibrio species isolate suspension containing 1×106 colony-forming unit per milliliter (CFU/mL) [10]. Each group (n=15) of fish was monitored for 14 days with one-third daily water replacement. The fish were fed with pelleted feed at a rate of 3% of body weight of the fish per day.
During the 14 days of rearing, clinical and post mortem examinations were performed in order to evaluate the development of disease symptoms after infection. For hematology studies, blood samples were withdrawn from the dorsal aorta of fish into a syringe containing a drop of 10% EDTA solution. Hemoglobin (Hb) content of blood samples was estimated using Sahlis Hemoglobinometer and Hematocrit (Hct) by centrifuging samples obtained ¾ directly via 75 μl microhematology tubes or blood capillary tube (Hematology centrifuge, SR10000, Thailand). Blood glucose was also estimated using a touch screen glucose reader, Accu-Chek Advantage 2 Roche. For histopathological study, 1 cm3 muscle, gill, liver, and intestine were fixed in Bouin’s fluid (75% picric acid, 25% formalin, and 5% glacial acetic acid). After 24hrs of fixation, the tissues were washed in 70% alcohol and were then processed in an automatic tissue processor for dehydration, clearing, and infiltration. The samples were then embedded in melted paraffin wax, trimmed and sectioned in microtome at 5 μm thickness. The ribbon with sections obtained was placed in a water bath at a temperature of 400°C which was then mounted on a glass slide with a mounting media. After storing the slides in slide racks for a day, the slides were treated with xylol to remove the paraffin wax from the sections. After clearing the sections in alcohol grades and then in xylol and benzene, the sections were stained with hematoxylin and eosin. The stained sections on slides were then mounted with coverslip using DPX. The stained slides were kept for dry and were examined under a stereo microscope, and photographs of stained sections were taken using microphotography. Finally, data were analyses using SPSS software version 20 windows were used to analyze the data with the level of significance at p<0.05.
Results
Clinical examinations
The clinical examination of the infected Nile tilapia revealed some clinical symptoms presented in Figure 1. After 12 hrs of injection of Vibrio species, all fish fingerlings became less active and the feeding rate of fish decreased. After 24 hrs of injection, the dorsal portion of the body became darkened, and mild hyperemia of the pectoral and ventral fin bases was observed. Later, hyperemia extended to the pectoral and ventral fin bases as well as at the margins of the vent. Fin rot appeared especially at the tips of the caudal fin and showed extensive hemorrhages at the ventral body part, and the fish became lethargic and remained at the bottom of the aquarium. There was a loss of scales and excess mucus secretion around the body surface (Figure 1).

Figure 1: Artificially infected Nile tilapia showing hemorrhagic, ulceration and lesions on the skin surface, rot fins, and exophthalmia on the abdominal parts.
Hematological and serum biochemical characters
Hematological and biochemical findings of infected and uninfected fish were analyzed and presented in Table 1. The fish injected with Vibrio species showed significantly (P<0.05) lower hemoglobin, hematocrit, glucose, triglyceride, total protein and albumin content than the control group. However, globulin was not significantly different among the two groups. The post mortem examination of infected fish revealed pale gills, fluid accumulation in intestine, pale liver and enlarged gall bladder filled with black secretion (Figure 2).
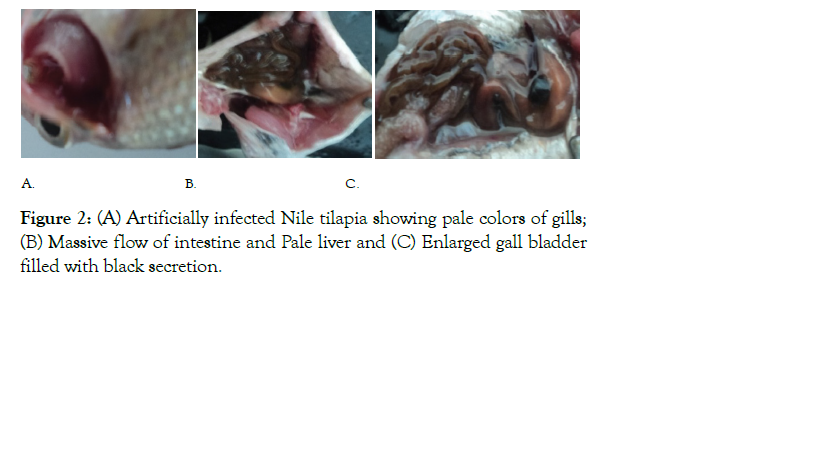
Figure 2: (A) Artificially infected Nile tilapia showing pale colors of gills; (B) Massive flow of intestine and Pale liver and (C) Enlarged gall bladder filled with black secretion.
| Parameters | Uninfected group | Infected group |
|---|---|---|
| Hematocrit (%) | 53.6 ± 0.1a | 33.4 ± 0.9b |
| Hemoglobin (mg/dl) | 6.33 ± 0.4a | 3 ± 0.4b |
| Glucose (mg/dl) | 55.1 ± 2.8a | 43.5 ± 0.9b |
| Triglyceride (mg/dl | 4.7 ± 0.2a | 3.5 ± 0.2b |
| Total serum protein (mg/dl | 7.63 ± 0.4a | 4 ± 0.2b |
| Albumin (mg/dl) | 6 ± 0.5a | 2.8 ± 0.1b |
| Globulin (mg/dl) | 1.44 ± 0.1a | 1.3 ± 0.1a |
Table 1: Comparison of hematological & serum biochemical parameters between infected and non-infected fish.
Histopathological changes after experimental infection
The histopathological changes of the examined internal organs (skin, gill, muscle, liver, and intestine) of the fish are presented in Figures 3-7. The present study revealed, the histopathological changes of skin of infected fish showed focal sloughing of the epidermis, congested, and hemorrhagic dermis, aggregates of melano-macrophage cells (Figure 3). While in the case of gill tissue infected with Vibrio species showed sloughing of the lamellar
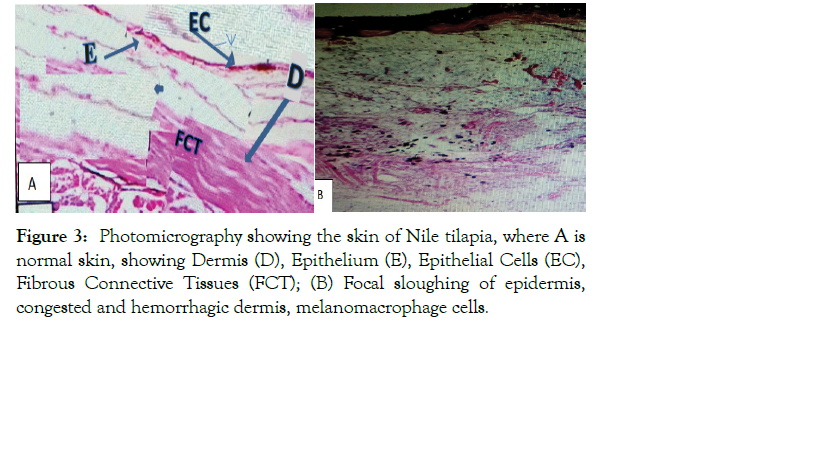
Figure 3: Photomicrography showing the skin of Nile tilapia, where A is normal skin, showing Dermis (D), Epithelium (E), Epithelial Cells (EC), Fibrous Connective Tissues (FCT); (B) Focal sloughing of epidermis, congested and hemorrhagic dermis, melanomacrophage cells.
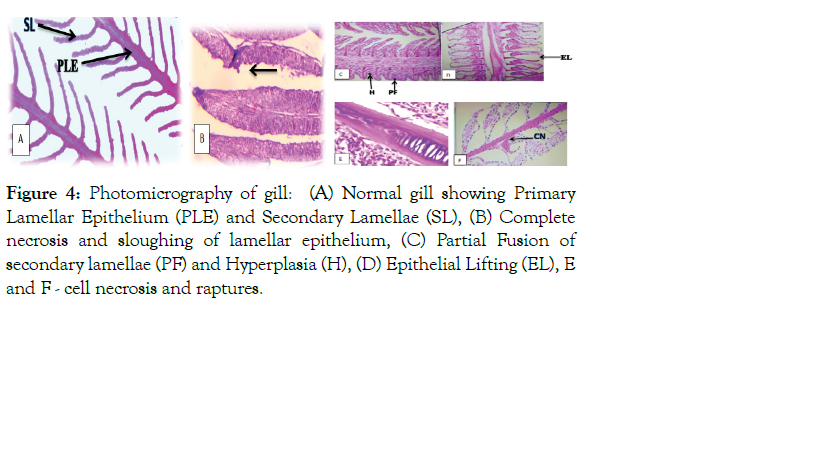
Figure 4: Photomicrography of gill: (A) Normal gill showing Primary Lamellar Epithelium (PLE) and Secondary Lamellae (SL), (B) Complete necrosis and sloughing of lamellar epithelium, (C) Partial Fusion of secondary lamellae (PF) and Hyperplasia (H), (D) Epithelial Lifting (EL), E and F - cell necrosis and raptures.
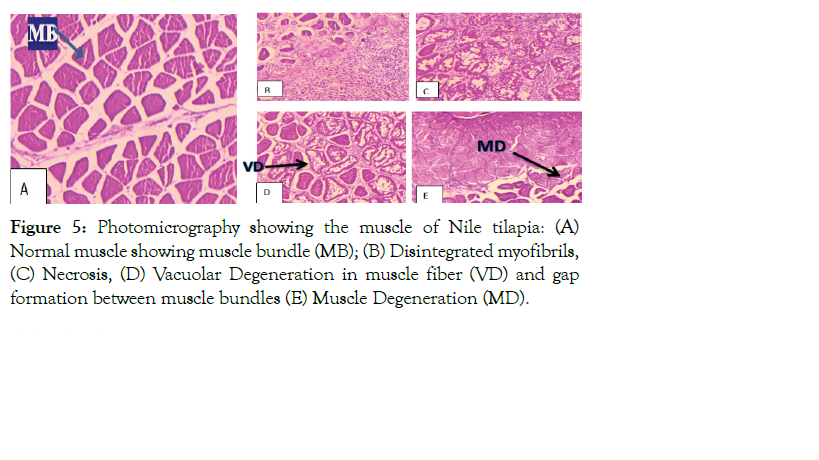
Figure 5: Photomicrography showing the muscle of Nile tilapia: (A) Normal muscle showing muscle bundle (MB); (B) Disintegrated myofibrils, (C) Necrosis, (D) Vacuolar Degeneration in muscle fiber (VD) and gap formation between muscle bundles (E) Muscle Degeneration (MD).
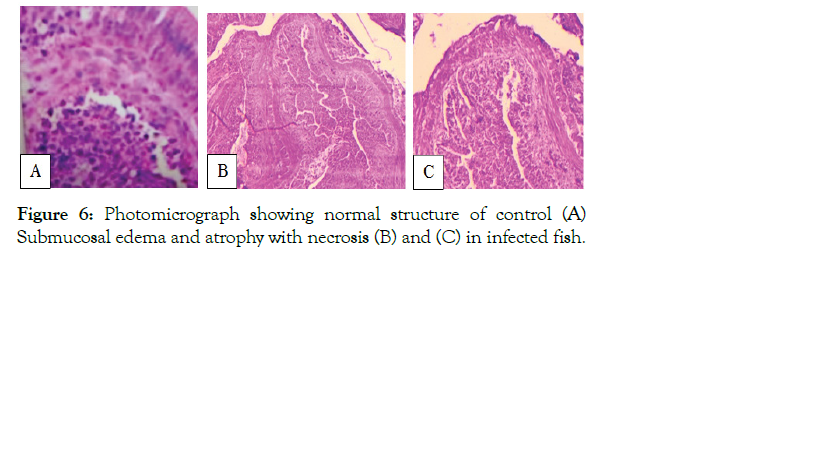
Figure 6: Photomicrograph showing normal structure of control (A) Submucosal edema and atrophy with necrosis (B) and (C) in infected fish.
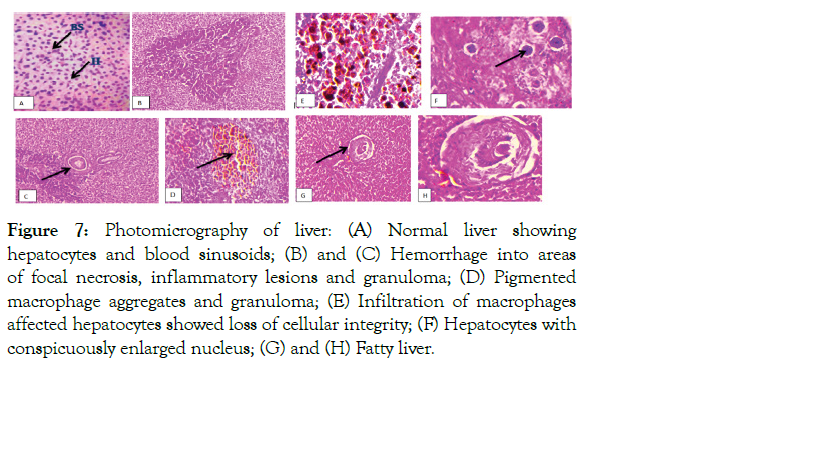
Figure 7: Photomicrography of liver: (A) Normal liver showing hepatocytes and blood sinusoids; (B) and (C) Hemorrhage into areas of focal necrosis, inflammatory lesions and granuloma; (D) Pigmented macrophage aggregates and granuloma; (E) Infiltration of macrophages affected hepatocytes showed loss of cellular integrity; (F) Hepatocytes with conspicuously enlarged nucleus; (G) and (H) Fatty liver.
epithelium, severe hyperplasia leading to inter-lamellar fusion, epithelial cell damage, and cell necrosis and rupture (Figure 4). Unlike infected fish, the skin and gills of control fish showed a normal architecture (Figures 3 and 4).
The present study demonstrated that muscle of infected fish showed intermyofibrillar space, disintegrated myofibrils, necrosis, and vacuolar degeneration in muscle fiber and gap formation between muscle bundles which ultimately led to muscle degeneration (Figure 5). Similarly, the results of the present study revealed that the intestine of infected fish exhibited sub mucosal edema and atrophy with necrosis (Figure 6), while demonstrated that liver and muscle of normal fish exhibits a normal architecture, and there was no pathological alterations (Figures 5 and 6).
The histopathological examination of liver tissue sampled from infected fish also showed extensive hemorrhagic areas of focal necrosis, presence of a number of inflammatory lesions and granuloma, infiltration of macrophages, affected hepatocytes with enlarged nucleus and loss of cellular integrity, the appearance of epithelioid-like cells, pigmented macrophage aggregates, and granuloma. The liver composed of hepatocytes which are polygonal cells with a central spherical nucleus and a densely stained nucleus (Figure 7).
Discussion
Aquaculture is a new and rapidly growing sector in Ethiopia and gets special attention in the country to increase food and nutrition security. During intensification, it subjects to different environmental stressors such as water quality and bacterial infections. Vibrio species is one of the most pathogenic bacteria causing large cultured fish mortality. In the present study, Nile tilapia was collected from the fish breeding station and examined for the presence of Vibrio species, and then clinical and post mortem examination was carried out.
Clinical examination
The clinical examination of infected Nile tilapia showed respiratory problem, erratic swimming, loss of equilibrium, darkened skin, hemorrhages on their body surface, symptoms of fins rot and abdominal dropsy, which is in line with the report of Abd El-Aziz, El-Hady, Minami, Younes [10-13], in which most of these clinical symptoms were observed on the fish infected with Vibrio species. The respiratory problem and loss of equilibrium observed on the infected could be due to the decrease of gill surface epithelium, hyperplasia of the epithelium, swelling, and fusion of gill lamellae and muscle dysfunction [14]. Darkened skin, hemorrhages on their body surface, symptoms of fins rot and abdominal dropsy could be attributed to the intensity of bacteria and its concurrent interaction with the tissues of the fish [10,11].
Hematology and biochemistry
The lower hemoglobin and hematocrit percentage in the infected fish were could be due to the Vibrio species infection in which it is an indication of severe anemia. Such a response might be due to the disruption in erythrocyte production [15]; and destruction of hematopoietic organs [16]. Similar results were also reported by Haney who observed decreased hemoglobin and hematocrit in different infected fish species with Vibrio and Aeromonas species [17]. Yu observed significant reduction in hematocrit and hemoglobin contents which are indicators of anemia in Nile tilapia infected with Vibrio species. Similarly, the declining in the serum protein level in the infected fish could be due to the injury of liver and kidney that may be reduced ability of synthesis and absorption of protein. Maqsood, Al Dohail, Yu and El-Sharaby also reported that pathogenic bacteria such as Vibrio and Aeromonas species have reduced serum protein levels in different fish species [14,18-20]. Glucose and globulin level of infected Nile tilapia in the present study was significantly low compared with the control group as also reported by Barham.
The postmortem examination of the infected Nile tilapia were also showed pale colors of gills and liver, massive intestinal fluid, and enlarged gall bladder filled with black fluid which agreed well with the findings of Abd El-Aziz and Youne [10,11]. Korun and Timur also reported that European Seabass infected with Vibrio species showed paleness of liver and accumulation of fluid in the intestine [21].
Histopathological examination
The histopathological changes of infected fish skin, such as the presence of focal sloughing of the epidermis, the congested and hemorrhagic dermis, aggregates of melanomacrophage cells, observed was in line with the findings of Abd El-Aziz and El- Sharaby, who reported that the presence of melanomacrophage indifferent layers of skin and underlining muscles could be due to bacterial infection [11,14]. Similarly, the histopathological changes of infected fish gill tissue such as sloughing of the lamellar epithelium, severe hyperplasia leading to inter-lamellar fusion, epithelial cell damage and cell necrosis and rupture observed was in line with the findings of Peretra who reported lamellar epithelial detachment [22], lamellar fusion and inflammation in the gills of infected Nile tilapia. El-Sharaby and Sami also reported hematocytes necrosis and aggregation in Vibrio and Aeromonas species infected fishes gill tissue [14,23].
The histopathological changes of infected fish muscles such as intermyofibrillar space disintegrated myofibrils, necrosis, and vacuolar degeneration in muscle fiber and muscle degeneration observed in the present study were in agreement with Afifi who reported perivascular cutting or degradation of abdominal muscles and connective tissue [24]. The results of the present study also revealed intestinal submucosal edema and atrophy with necrosis in which the results were agreed well with the findings of El-Sharaby and Sami, who reported hematocytes aggregation, necrosis, and hemorrhage in the intestine of Vibrio species and Aeromonas infected fishes [14,23].
The alterations of infected fish liver tissue such as extensive hemorrhagic areas of focal necrosis, presence of a number of inflammatory lesions and granuloma, infiltration of macrophages, hepatocytes with enlarged nucleus and loss of cellular integrity, pigmented macrophage aggregates, observed in the present study were in agreement with the findings of Diggles & Koron and Timur [21,25]. Braunbeck stated that the changes in the size and shape of hepatocyte cell nucleus could be due to increased effects of pathogenic activities [26]. Kranz also stated that macrophage aggregation can be developed in infected fish as a response to pathogenic influence [27]. Hepatic lesions also increase the density of melanomacrophage aggregates in the liver [28]. Large hepatocytes lesion and aggregation in the liver and other related organs indicated that these organs are probably the target organs for cell aggregation and bacterial pathogenesis as also stated by Abdelhamed and Sami [23,29]. It is believed that melanomacrophage aggregates observed in the liver of tilapia in this study may be due to the chronic stress caused by the bacterial injection. The presence of histopathological lesions in the internal organs clearly explains the septicemic nature of the Vibrio species infection.
Many studies showed that infective bacteria could produce toxin and extracellular products by different pathogens that may have hemolytic and proteolytic activities as they have toxic effects which further cause hemorrhage, degradation, and necrosis of fish tissues [6,24]. The toxic effect on the epithelial cells leads to irritation and excessive mucous secretion which are defense mechanisms against toxins. Also, it ruptures subcutaneous blood vessels resulting in the infiltration of red blood cells and leukocytes into the surrounding tissues as well as escape of the plasma protein that causes edema, congestion and hemorrhage at the site of infection as reported by Easa [30,31].
Conclusion
In conclusion, experimental infection with vibrio bacteria produced significant changes in clinical, hematological, biochemical histopathological characteristics of Nile tilapia, in which the vibrio bacteria caused tissue damage and found to be severe in all the tissues examined. Therefore, it is important to develop strategies to control such pathogenic bacteria in order to achieve sustainable fish production under a wide range of production environments.
REFERENCES
- Bekele B, Workagegn KB, Natarajan P. Prevalence and antimicrobial susceptibility of pathogenic bacteria in Nile Tilapia, Oreochromis niloticus L. Int J Aquac Fish Sci. 2019;5(4):022-6.
- El-Sayed M, Algammal A, Abouel-Atta M, Mabrok M, Emam A. Pathogenicity, genetic typing, and antibiotic sensitivity of Vibrio alginolyticus isolated from Oreochromis niloticus and Tilapia zillii. Rev Med Vet. 2019;170(4-6):80-6.
- Chatterjee S, Haldar S. Vibrio related diseases in aquaculture and development of rapid and accurate identification methods. J Marine Sci Res Dev. 2012;1(1):1-7.
- Austin B, Austin DA. Aeromonadaceae representative (Aeromonas salmonicida). In Bacterial Fish Pathogens. Springer, Dordrecht. 2012;pp:147-228.
- Haldar S, Maharajan A, Chatterjee S, Hunter SA, Chowdhury N, Hinenoya A, et al. Identification of Vibrio harveyi as a causative bacterium for a tail rot disease of sea bream Sparus aurata from research hatchery in Malta. Microbiol Res. 2010;165(8):639-48.
- Austin B, Austin DA. Aeromonadaceae representatives Aeromonas salmonicida. Bacterial Fish Diseases: Diseases in Farmed and Wild Fish. Ellis Horwood, Chichester. 1987;pp:86–170.
- Řehulka J. Aeromonas causes severe skin lesions in rainbow trout (Oncorhynchus mykiss): clinical pathology, haematology, and biochemistry. Acta Veterinaria Brno. 2002;71(3):351-60.
- Martins ML, Tavares-Dias M, Fujimoto RY, Onaka EM, Nomura DT. Haematological alterations of Leporinus macrocephalus (Osteichtyes: Anostomidae) naturally infected by Goezia leporini (Nematoda: Anisakidae) in fish pond. Arq Bras Med Vet Zootec. 2004;56:640-6.
- Inocente Filho C, Müller EE, Pretto-Giordano LG, Bracarense AP. Histological findings of experimental Streptococcus agalactiae infection in Nile tilapias (Oreochromis niloticus). Brazilian J Vet Pathol. 2009;2(1):12-5.
- Younes AM, Fares MO, Gaafar AY, Mohamed LA. Isolation of Vibrio alginolyticus and Vibrio vulnificus strains from cultured Oreochromis niloticus around Qarun Lake, Egypt. Glob Vet. 2016;16(1):1-5.
- Abdel-Aziz M, Eissa AE, Hanna M, Abou Okada M. Identifying some pathogenic Vibrio/Photobacterium species during mass mortalities of cultured Gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) from some Egyptian coastal provinces. Int J Vet Sci Med. 2013;1(2):87-95.
- El-Hady MA, El-khatib NR, Abdel-Aziz ES. Microbiological studies on Vibrio species isolated from some cultured fishes. Anim Health Res J. 2015;3(1):12-9.
- Minami T, Iwata K, Shimahara Y, Yuasa K. Vibrio harveyi infection in farmed greater amberjack Seriola dumerili. Fish Pathol. 2016;51(1):1-7.
- El-Sharaby SM, Abd-Elgaber M, Tarabees R, Khalil RH, Ali MN, El-Ballal S. Bacteriological and histopathological studies on Vibrio species isolated from naturally infected freshwater fish in Delta Region, Egypt. Adv Anim Vet Sci. 2018;6(1):17-26.
- Omoregie E. Changes in the haematology of the Nile tilapia, Oreochromis niloticus Trewavas under the effect of crude oil. Acta Hydrobiol Pol Acad Sci. 1998;40:287-92.
- Sampath K, Velamman S, Kennedy IJ, James R. Haematological changes and their recovery in Oreochromis mossambicus as a function of exposure period and sublethal levels of Ekalux. Acta Hydrobiol. 1993;1(35):73-83.
- Haney DC, Hursh DA, Mix MC, Winton JR. Physiological and hematological changes in chum salmon artificially infected with erythrocytic necrosis virus. J Aquat Anim Health. 1992;4(1):48-57.
- Yu JH, Han JJ, Park SW. Haematological and biochemical alterations in Korean catfish, Silurus asotus, experimentally infected with Edwardsiella tarda. Aquac Res. 2010;41(2):295-302.
- Maqsood S, Samoon MH, Singh P. Immunomodulatory and growth promoting effect of dietary levamisole in Cyprinus carpio fingerlings against the challenge of Aeromonas hydrophila. Turk J Fish Aquat Sci. 2009;9(1).
- Al-Dohail MA, Hashim R, Aliyu-Paiko M. Evaluating the use of Lactobacillus acidophilus as a biocontrol agent against common pathogenic bacteria and the effects on the haematology parameters and histopathology in African catfish Clarias gariepinus juveniles. Aquac Res. 2011;42(2):196-209.
- Korun J, Timur G. Marine vibrios associated with diseased sea bass (Dicentrarchus labrax) in Turkey. J Fishscicom. 2008;2(1):66-76.
- Pereira DP, Santos DM, Neta AV, Cruz CF, Neta RN. Morphological changes in fish gills of Oreochromis niloticus (Pisces, Cichlidae) as biomarkers of aquatic pollution in the Lagoon of Jansen, São Luís, State of Maranhão (Brazil). Biosci J. 2014;30(4):1213-21.
- AlYahya SA, Ameen F, Al-Niaeem KS, Al-Sa'adi BA, Hadi S, Mostafa AA. Histopathological studies of experimental Aeromonas hydrophila infection in blue tilapia, Oreochromis aureus. Saudi J Biol Sci. 2018;25(1):182-5.
- Afifi SH, Al-Thobiati S, Hazaa MS. Bacteriological and histopathological studies on Aeromonas hydrophila infection of Nile tilapia (Oreochromis niloticus) from fish farms in Saudi Arabia. Ass Veter Med J. 2000;42(84):195-205.
- Diggles BK, Carson J, Hine PM, Hickman RW, Tait MJ. Vibrio species associated with mortalities in hatchery-reared turbot (Colistium nudipinnis) and brill (C. guntheri) in New Zealand. Aquac. 2000;183(1-2):1-2.
- Braunbeck T, Storch V, Bresch H. Species-specific reaction of liver ultrastructure in zebrafish (Brachydanio rerio) and trout (Salmo gairdneri) after prolonged exposure to 4-chloroaniline. Arch Environ Contam Toxicol. 1990;19(3):405-18.
- Kranz H. Changes in splenic melano-macrophage centres of dab Limanda limanda during and after infection with ulcer disease. Dis Aquat Organ. 1989;6(3):167-73.
- Pacheco M, Santos MA. Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol Environ Saf. 2002;53(3):331-47.
- Abdelhamed H, Ibrahim I, Baumgartner W, Lawrence ML, Karsi A. Characterization of histopathological and ultrastructural changes in channel catfish experimentally infected with virulent Aeromonas hydrophila. Frontiers in microbiology. 2017;8:1519.
- Easa ME, Akeula MA, El-Nimr MM. Enzootic of fin rot among carp fingerlings in Egyptian fishpond. J Egypt Vet Med Assoc. 1985;45(1):83-93.
- Workagegn KB. Establishment of a base population for long-term genetic improvement of Nile tilapia in Ethiopia. 2019.
Citation: Bekele B, Natarajan P, Workagegn KB, Pillai D (2021) Hematological and Histopathological Changes in Artificially Infected Nile tilapia with vibrio species. J Aquac Res Development. 12:648
Copyright: © 2021 Bekele B, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

